Abstract
Cold atmospheric plasma (CAP) is a group of various chemical active species, such as ozone and nitric oxide, generated by working gas. CAP was demonstrated to have an effect on tissue regeneration and wound healing. We conducted this study to evaluate the efficacy and safety of CAP as a novel therapy for diabetic wounds in vitro and in vivo. The plasma consists of ionised helium gas that is produced by a high‐voltage and high‐frequency power supply. Eight‐week‐old male db/db mice and C57BL mice were treated with helium gas (control group), 90s' CAP (low‐dose group), and 180s' CAP (high‐dose group). Mice were treated and observed for 2 weeks. Skin samples from around the wound and blood samples were collected. Our in vitro analysis included scratch wound‐healing assays by using human HaCaT immortalised human epidermal cells. After 14 days of treatment, CAP could obviously promote diabetic wound healing. Wound closure rates were significantly higher in the low‐dose group and high‐dose groups compared with the control group. Meanwhile, compared with the control group, the protein expression of IL‐6, tumour necrosis factor‐α, inducible nitric oxide synthase, and superoxide dismutase in two CAP groups significantly decreased, while the protein expression of vascular endothelial growth factor and transforming growth factor‐β in two CAP groups significantly increased (all P < .05); these data show good agreement with the change in mRNA level (all P < .05). In vitro, scratch wound‐healing assays showed that plasma treatment could effectively ensure healing within 3 minutes of exposure (all P < .05). In addition, no difference was found in histological observations of normal skin and the level of serum alanine transaminase, aspartate aminotransferase, blood urea nitrogen, creatinine, and white blood cells among the CAP groups and control group. CAP treatment for 3 minutes every day improves wound healing in diabetic mice by suppressing inflammation, reducing oxidative stress, and enhancing angiogenesis, involving several proteins signalling, and it is safe for the liver and kidney.
Keywords: angiogenesis, cold atmospheric plasma, diabetes mellitus, diabetic wounds
1. INTRODUCTION
Diabetes mellitus (DM) is a prevalent disease, especially among the elderly. It was demonstrated that, in the United States, DM affects over 25.8 million people (8.3% of the total population), and 1.9 million new cases (aged 20 years or older) are diagnosed every year.1 In adults, 90% to 95% of patients with diabetes have type 2 DM, which results in various chronic complications and an enormous medical cost burden.2 Among adults in China, the newest estimated overall prevalence of diabetes and prediabetes was 10.9% and 35.7%, respectively.3
Diabetes is the most common disease associated with lower limb amputation, resulting in nearly half of the cases of non‐traumatic amputation in Romanian, Spain, and Germany.4, 5, 6 The 5‐year survival rate of diabetic foot (DF) patients with lower‐extremity amputation was only 50%, even worse than that of many kinds of cancers.7
Cold atmospheric plasma (CAP), also called non‐thermal atmospheric‐pressure plasma, was characterised by various chemical active species, such as nitric oxide (NO), atomic oxygen (O), and ozone (O3). CAP was generated by electron impact excitation of the working gas at atmospheric pressure and mild temperature. Because of its possible treatment effect on chronic wounds, CAP attracted much interest with regard to treating patients with DF.8 Nowadays, the biomedical applications of CAP mainly include sterilisation, disinfection, and medical therapies.8 The involved mechanism focused on inflammation suppression, cell proliferation, and reactive oxygen species.9, 10 It was reported that CAP promoted proliferation and growth factor secretion of endothelial cells and fibroblasts in vitro.10, 11 Considering the possible effectiveness of CAP in wound therapy and its associated cells, we carried out this research to clarify the potential mechanism of CAP in wound healing and evaluate the efficacy and safety of CAP in vitro and in vivo in diabetic mice.
2. MATERIALS AND METHODS
2.1. Animals
Animal procedures were performed strictly according to the regulations of animal care of the National Institutes of Health (NIH) concerning the Principles of Laboratory Animal Care (NIH, 1985), followed by approval from the Institutional Animal Care and Use Committee. Eight‐week‐old male db/db mice (C57BL/KsJ‐leprdb/leprdb) were commercially obtained from the Model Animal Research Centre of Nanjing University (Nanjing, China) and were subsequently maintained at 12:12‐hour light/dark cycle, with free access to standard water and diet.
2.2. Plasma set‐up
The plasma source consisted of a gun‐like hand‐held unit, which was used for the generation of the plasma jet, delivered at atmospheric pressure, a RF power supply (system power: 42 W at 220 V, 50 Hz; size: 292 × 225 × 136 mm), and a gas supply unit. The plasma nozzle (ID: 4 mm and OD: 6 mm) was made of a quartz glass tube. An internal electrode and a grounded metal electrode were coaxially mounted in the centre and on the outer wall of the quartz tube, respectively. The internal electrode consisted of a metal rod (Ø = 1 mm) and a capillary quartz tube (ID: 1 mm and OD: 2 mm). The feeding gases for this study were 99.999% pure helium (He), with a 3 lit/min gas flow rate.
When the source was operating, high‐voltage and high‐frequency electricity (8.5 kV and 17 kHz, respectively) was applied between the two electrodes, where the plasma was generated because of the collision between electronics and helium molecules. With the helium flow, the plasma expanded into the surrounding air outside the nozzle, where O2 and N2 were involved in the collision and generated the plasma jet consisting of reactive oxygen and nitrogen species. The distance between wound surface and nozzle was set as 20 mm, rendering direct contact between wound and plasma plumes. The plasma was exposed for 90 s and 180 s for two plasma treated groups. The apparatus is illustrated in Figure 1.
Figure 1.
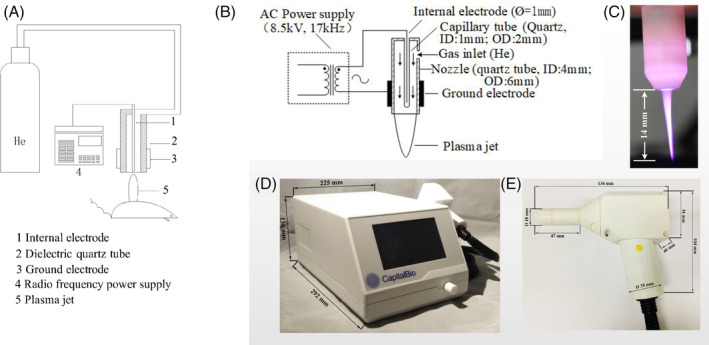
Structure and example diagrams of plasma set‐up. Schematic diagram of plasma A, set‐up and B, nozzle; instance diagrams of C, plasma jet; D, radio frequency power supply; and E, plasma nozzle
2.3. Study designs
All db/db mice were assigned to three groups (Control and 90s’ treated and 180s’ treated groups; n = 10) and exposed to He, plasma (treated 90s), and plasma (treated 180s), respectively, for 14 consecutive days. After stretching and fixing the mice using adhesive tape to expose their dorsal skin, 6 mm‐diameter full‐thickness punch biopsy wounds were inflicted on the dorsal skin of male db/db mice by biopsy punch (Acuderm Inc., Fort Lauderdale, Florida). Afterwards, wounds were dressed with Tegaderm (Nexcare, 3M), followed by changing every second day. The measurement of wound closure rates was conducted by tracing the wound area every second day on an acetate paper. In addition, Image‐Pro Plus 5.1 software (Media Cybernetics) was used for quantification of gross wound closure, and wound healing was shown as the proportion of the original wound area that had healed according to the formula [1 − (wound area day x/wound area day 0)] × 100. Wounds samples were resected from mice on days 3, 7, and 15. Similarly, skin wound tissue was cautiously resected, followed by collection of granulation tissues within the wound and within a 10‐mm margin around the wound. At the end of 15 days, mice were euthanised using isoflurane anaesthesia, followed by collection of blood specimens from right ventricles using syringes containing ethylene diamine tetraacetic acid (EDTA) and subsequent centrifugation (10 000g at 4°C) for 10 minutes for white blood cell (WBC) count, blood urea nitrogen (BUN), aspartate aminotransferase (AST), serum alanine transaminase (ALT), and creatinine (Cr) detection (7600‐020, Hitachi Inc., Tokyo, Japan).
2.4. Histopathological and immunohistochemical analysis
We used qualitative and semi‐quantitative methods, including histopathological and immunohistochemical analyses, to evaluate inflammatory response in the skin tissue samples. Briefly, the formalin‐fixed, paraffin‐embedded specimens were cut into 3 μm sections and stained with haematoxylin and eosin (H&E) and immunohistochemical staining, including interleukin‐6(IL‐6), superoxide dismutase (SOD), tumour necrosis factor‐α (TNF‐α), catalase (CAT), inducible nitric oxide synthase (iNOS), transforming growth factor‐β (TGF‐β), and vascular endothelial growth factor (VEGF), according to the description by MetaMorph software. Both primary and secondary antibodies were purchased from Sigma (St. Louis, MO), followed by observation under a microscope (BX53, Olympus, Japan). The histological image was independently evaluated by two investigators, with intensive expertise of evaluation of the presence or absence of inflammatory cells, such as neutrophils, macrophages, lymphocytes, plasmocytes, and so on. The above characteristics were shown as intensity: mild (absence of inflammatory cells), moderate (presence of a small number of inflammatory cells), and serious: (presence of a large number of inflammatory cells). Moreover, the medical image analysis system (Image‐Pro Plus 5.1) was used to assess the stained section, followed by quantification of DAB intensity (brown areas).
2.5. Immunofluorescence analysis
Samples were incubated with normal serum at 37°C for 30 minutes for blocking and were subsequently reacted with anti‐IL‐6 (Abcam, England), anti‐TNF‐α (Abcam, England), anti‐VEGF (proteintech), anti‐iNOS (Abcam, England), anti‐TGF‐β (Abcam, England), and anti‐SOD (Santa Cruz) antibodies for 1 hour at room temperature. Afterwards, specimens were incubated with goat anti‐rabbit secondary antibodies (Life Technologies) in the dark for 1 hour, followed by visualisation under a fluorescence microscope (Axio Observer A1, Carl Zeiss, Jena, Germany).
2.6. Western blot analysis
Protein samples were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrohoresis (SDS‐PAGE) and transferred onto Polyvinylidene Fluoride (PVDF) membranes. The membranes were blocked with 5% skimmed milk and were subsequently reacted with primary antibodies against IL‐6, TNF‐α, VEGF, iNOS, TGF‐β, SOD, CAT, and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (Abcam) at 4°C overnight, incubated with HRP‐conjugated goat anti‐rabbit or anti‐mouse secondary antibodies. Finally, the membranes were washed with Tris buffered saline Tween (TBST), visualised by electrochemiluminescence (ECL), and subjected to autoluminography for 1 to 5 minutes.
2.7. Fluorescence quantitative polymerase chain reaction
After resection, the wound tissue was immediately stored in liquid nitrogen and, subsequently, at −70°C until further use. TRIzol reagent (Invitrogen) was used for the extraction of total RNA. SYBR Green PCR Master (TaKaRa, Japan) was used to perform fluorescence quantitative polymerase chain reaction after adding first‐stand cDNA using a StepOnePlus Sequence Detection System (Biosystems, California) to determine the mRNA expression of IL‐6, TNF‐α, VEGF, iNOS, TGF‐β, SOD, and CAT. The2‐△△Ct methods were used to calculate the relative expression of mRNA after normalisation to GAPDH expression. The primer sequences were shown in Table 1.
Table 1.
Primers used in the real‐time PCR assay
| Name | Sequence (5′ to 3′) | |
|---|---|---|
| IL‐6 | F | CAAAGCCAGAGTCCTTCAG |
| R | GATGGTCTTGGTCCTTAGC | |
| TNF‐α | F | GGGTGTTCATCCATTCTC |
| R | CCCAGCATCTTGTGTTTC | |
| VEGF | F | TACTGCCGTCCGATTGAG |
| R | CCTATGTGCTGGCTTTGG | |
| iNOS | F | ACATCGACCCGTCCACAGTAT |
| R | CAGAGGGGTAGGCTTGTCTC | |
| TGF‐β | F | CCACCTGCAAGACCATCGAC |
| R | CTGGCGAGCCTTAGTTTGGAC | |
| SOD | F | AACCAGTTGTGTTGTCAGGAC |
| R | CCACCATGTTTCTTAGAGTGAGG | |
| CAT | F | GGAGGCGGGAACCCAATAG |
| R | GTGTGCCATCTCGTCAGTGAA | |
| GAPDH | F | ATCACTGCCACCCAGAAG |
| R | TCCACGACGGACACATTG |
2.8. Wound‐healing assay (scratch assay) in vitro
The migratory behaviour of the HaCaT cell was examined by wound‐healing assay according to previous analysis.12 A scratch was made using a 10‐μL pipette tip, leaving a cell‐free gap (“defined as wound”), followed by rinsing again to remove the detached cells. The plasma‐activated medium (PAM) was defined as a medium treated by CAP for 1, 2, 3, 4, 5, and 6 minutes, which were added into the wells, as well as untreated medium for control, in three replicates. The cells were incubated at 37°C. The photos of the scratches in the control and PAMs were taken immediately 24 hours and 48 hours after the addition of the PAM or untreated medium. Each analysis was performed in duplicate.
2.9. Statistical analysis
SPSS v. 22.0 (SPSS Inc., Chicago, Illinois) was used for statistical analysis. GraphPad Prism 5.0 (GraphPad Software, Inc.) was used to plot figures. Data were shown as mean ± SD for continuous variables. For measurement data, a Kruskal‐Wallis test or ANOVA was used for the comparison of differences among groups if appropriate. A two‐tailed P value < .05 was considered statistically significant.
3. RESULTS
3.1. CAP‐enhanced diabetic wound healing in db/db mice
The results showed that the wound areas of the db/db mice in the control group closed significantly slower than those in the CAP treatment group (Figure 2A,B). The administration of plasma significantly improved wound recovery rates compared with db/db controls starting from day 7 to day 15. There was no significant difference in the overall wound‐healing rate between the two plasma treating groups, although the db/db mice in the 180s’ treating group had a more decreased wound area than the 90s’ treating group from day 2 to day 7 (Figure 2B). These results demonstrated that plasma treatment improved wound healing in diabetic mice without a difference in exposure time.
Figure 2.
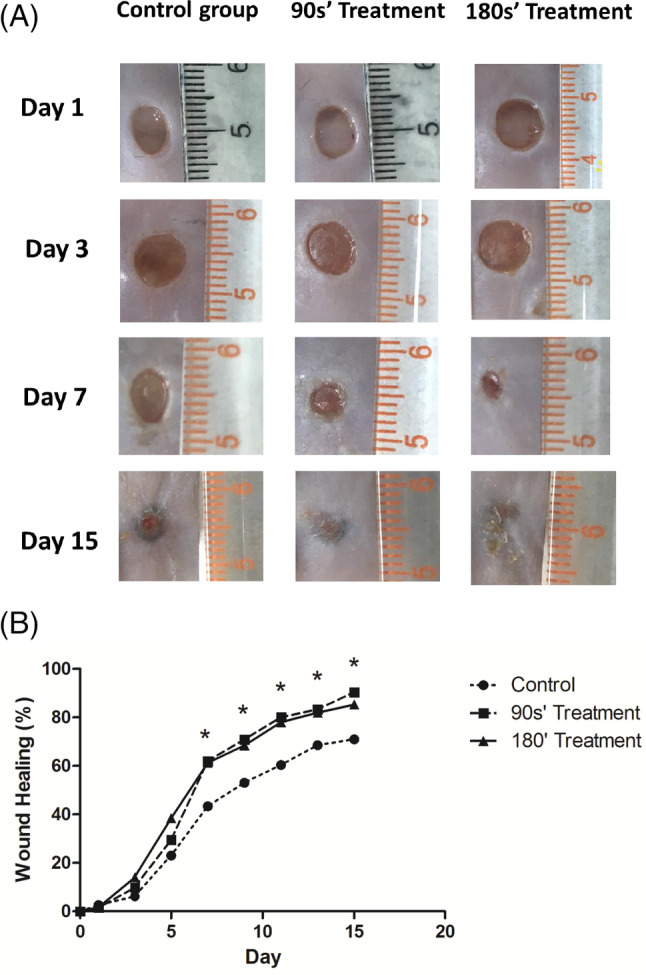
CAP treatment accelerated wound healing in db/db mice. A, Photographs show the wound performance after 1, 3, 7, and 15 days of treatment in three groups. B, Comparisons of wound‐healing rates in three groups were expressed by the percentage of closed wound area. *P < .05 180’ treatment vs control
3.2. CAP inhibited inflammation and inflammatory cytokines by H&E and IHC staining
As shown in Figure 3, after 7 days treatment, H&E sections indicated that the wounds treated with plasma were well‐healing with dense cell‐deposited matrix with the mild and moderate inflammation, while the inflammation in control group was still serious. Qualitative inspection of the immunohistochemical result showed significantly decreased levels of IL‐6, TNF‐α, iNOS, and SOD and increased levels of VEGF and TGF‐β in the wound tissue of plasma‐treated mice. High plasma exposure could also enhance this effect (Figure 3D).
Figure 3.
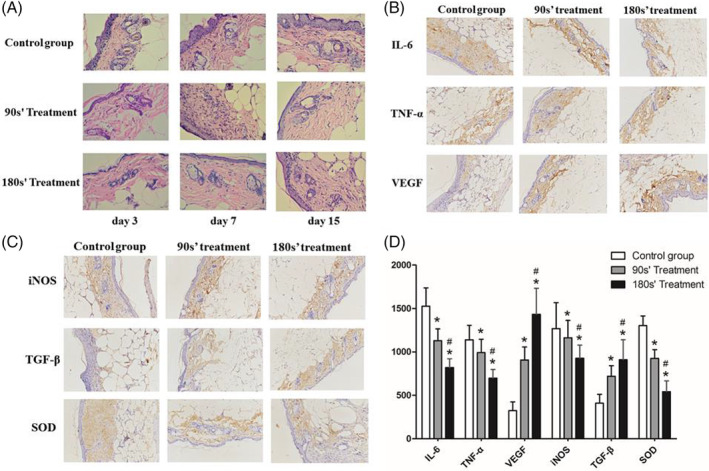
Skin tissue around the wound area was collected at days 3, 7, and 15 after treatment. A, Representative photomicrographs of HE staining at magnifications 400. B, IHC staining of IL‐6, TNF‐α, and VEGF in each group. C, IHC staining of iNOS, TGF‐β, and SOD in each group. D, Quantitative analysis of IL‐6, TNF‐α, VEGF, iNOS, TGF‐β, and SOD in each group. *P < .05 vs control, #P < .05 vs. 90s’ treatment
3.3. Immunofluorescence assay in wound tissue
Immunofluorescence analysis demonstrated that the expression of IL‐6, TNF‐α, iNOS, and SOD were decreased, while that of VEGF and TGF‐β increased after 7 days treatment of plasma. The decrease or increase range was exposure‐dependent. Mice in the 180s’ plasma treatment group had lower expression of IL‐6, iNOS, and TNF‐α and higher expression of VEGF than the 90s’ plasma treatment group. DAPI (4, 6‐diamino‐2‐phenylindole) counterstaining helped to identify non‐damaged nuclei (Figure 4).
Figure 4.
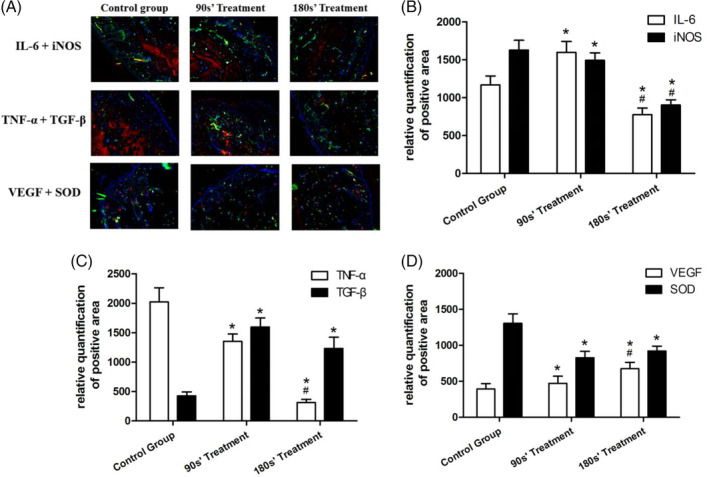
Immunofluorescence assay in wound tissue. B‐D, Continuous data are presented as mean ± SD. *P < .05 vs control, #P < .05 vs 90s’ treatment
3.4. Protein and mRNA expression in wound skin tissue by western blot and real‐time PCR
Figures 5, 6, 7, 8 show the IL‐6, TNF‐α, iNOS, SOD, VEGF, and TGF‐β proteins and mRNA expression levels in wound tissues in three groups. There were great differences between IL‐6, TNF‐α, iNOS, SOD, VEGF, and TGF‐β protein expression levels among control and plasma‐treated groups (all P < .01). The wound tissue VEGF and TGF‐β protein expressions were significantly increased (Figure 7), while the IL‐6, TNF‐α, iNOS, and SOD protein decreased (Figures 5 and 6) in plasma‐treated groups compared with the control group (All P < .05). Mice in the 180s’ treatment group also had significantly higher VEGF and TGF‐β protein compared with the 90s’ treatment group, with lower IL‐6, TNF‐α, iNOS, and SOD protein. Moreover, the relative quantification of VEGF and TGF‐β mRNA were significantly increased, and those of IL‐6, TNF‐α, iNOS, and SOD were decreased after plasma treatment (all P < .05, Figure 8).
Figure 5.
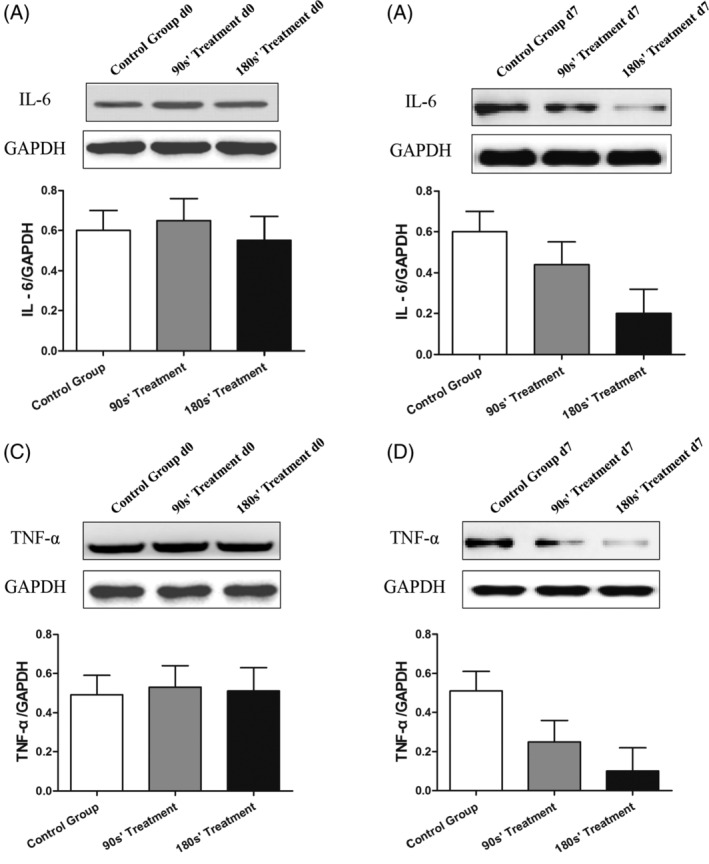
Protein expression of IL‐6 and TNF‐α in wound skin tissue by Western Blot. *P < .05 vs control, #P < .05 vs 90s’ treatment
Figure 6.
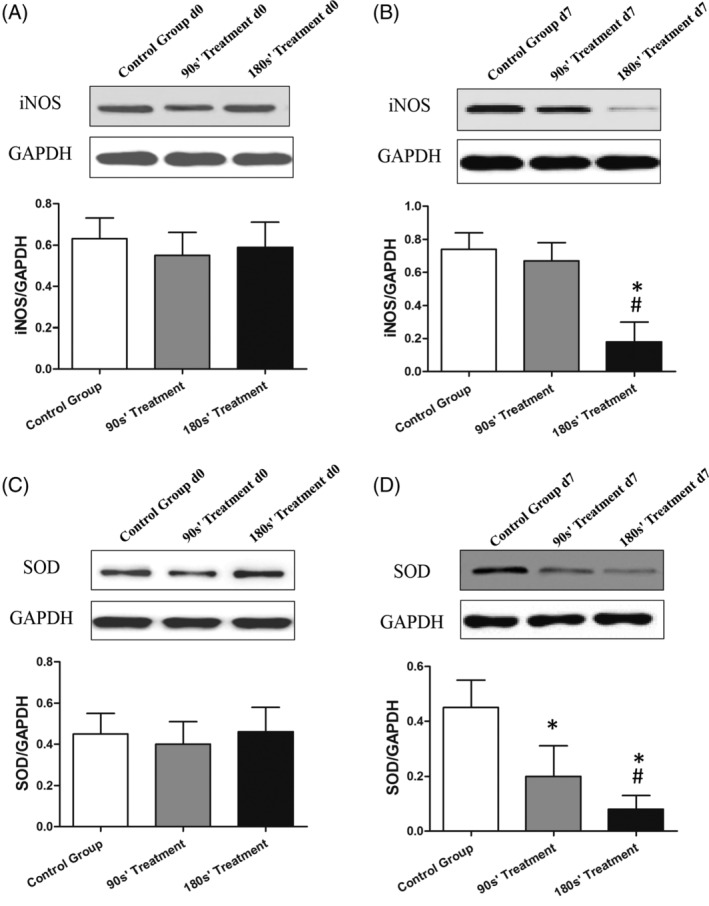
Protein expression of iNOS and SOD in wound skin tissue by Western Blot. *P < .05 vs control, #P < .05 vs 90s’ treatment
Figure 7.
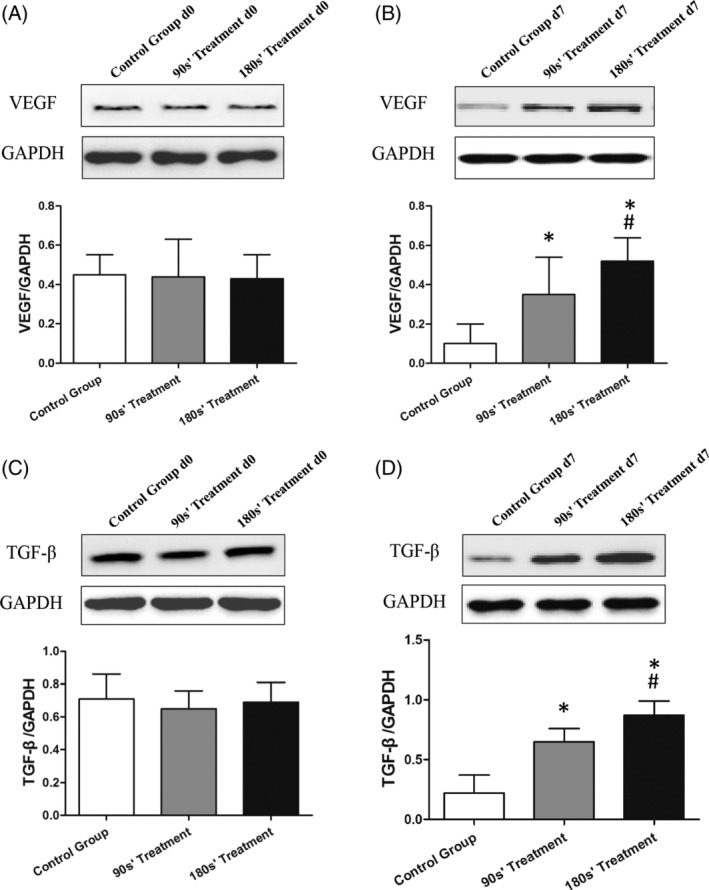
Protein expression of VEGF and TGF‐β in wound skin tissue by Western Blot. *P < .05 vs control, #P < .05 vs 90s’ treatment
Figure 8.
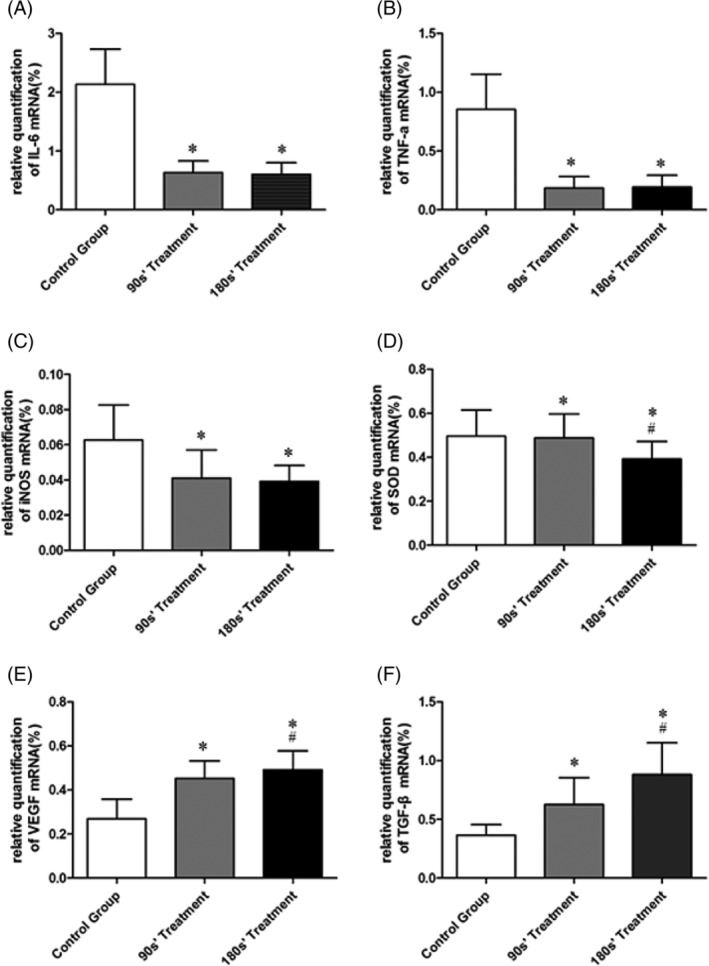
mRNA expression in wound skin tissue by real‐time PCR. *P < .05 vs control, #P < .05 vs 90s’ treatment
3.5. Safety of plasma in treating diabetic wound
After 14 days of CAP exposure, there was no significant difference in WBC and serum ALT, AST, BUN, and Cr levels among the three groups (Table 2). Compared with the control group, the WBC, ALT, AST, BUN, and Cr levels in the two treatment groups were also not significantly different.
Table 2.
Safety evaluation of plasma treatment among two CAP‐treated groups and control group
| Control group | 90s’ treatment | 180s’ treatment | P | |
|---|---|---|---|---|
| WBC (×1012/L) | 9466.7 ± 1282.9 | 8883.3 ± 485.6 | 9400.0 ± 576.6 | NS |
| ALT (U/L) | 8.3 ± 2.3 | 9.3 ± 3.5 | 8.67 ± 5.03 | NS |
| AST (U/L) | 115.33 ± 75.38 | 184.0 ± 29.5 | 71.0 ± 44.7 | NS |
| BUN (mmol/L) | 1.4 ± 0.7 | 2.5 (1.3, 4.7) | 6.10 ± 3.6 | NS |
| Cr (mg/dL) | 1.7 ± 0.7 | 1.7 ± 0.6 | 1.5 ± 0.6 | NS |
Note: Continuous data are presented as mean ± SD, or median (5th‐95th percentile) as appropriate.
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; WBC, white blood cell.
3.6. CAP treatment accelerates wound healing in vitro
In this migration assay, it was observed that the migration rate of HaCaT cells in a PAM‐activated medium was significantly accelerated. The results showed that the PAM activated by CAP for 1, 2, and 3 minutes had a significant effect on HaCaT cell migration ability compared with the control at 24 and 48 hours (Figure 9). However, longer time‐activated PAM went against the migration to the scratch. These data suggested moderate‐dose treatment with CAP‐accelerated cell migration and enhanced wound healing in vitro.
Figure 9.
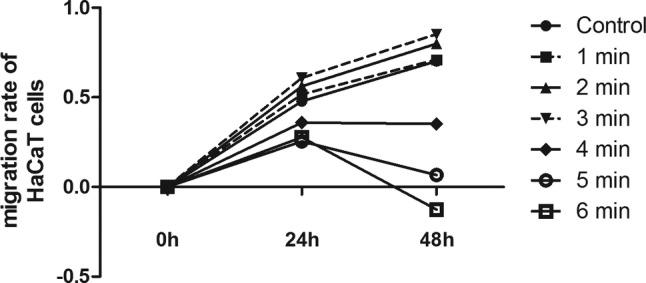
CAP treatment accelerated wound healing in vitro
4. DISCUSSION
Wound healing in diabetes patients is difficult and essential to the treatment of DF, which has a great influence on patients' life. In this study, we investigate the efficacy and safety of CAP as a novel therapy for diabetic wounds in vitro and in vivo. To our knowledge, this was the first study that combined animal model and cell experiments to assess the effect of CAP on regulating diabetic wound healing and involved mechanisms, as well as finding the best exposure time of CAP on treating diabetic skin wounds.
The associated factors affecting diabetic wound healing varied, mainly including serious infection, unbalanced inflammation, defected tissue repair capability, and damaged angiogenesis. Previous researchers have tried their best and created many techniques to promote wound healing and avoid amputation of patients with DF. In the present study, the efficacy of CAP as a physical therapeutic method for enhancing the healing of diabetic wounds in the mice model and human epidermal cells were evaluated. We found that the wound areas of the db/db mice in control group closed significantly slower than those in the plasma‐treated group. The administration of plasma (90s and 180s) significantly improved wound recovery rates compared with controls from day 7 to 15. In vitro, the migration rate of HaCaT cells in a plasma‐activated medium was significantly accelerated compared with the control at 24 and 48 hours. The best treatment time was detected and proved to be 3 minutes.
Plasma is the fourth state of matter and combined completely or partly ionised particles.13 CAP was found to inactivate different microorganisms very effectively and was widely used in medical equipment sterilisation.8 Even faced with the methicillin‐resistant Staphylococcus aureus, plasma treatment was reported to have great bactericidal effect.14 In addition to sterilisation, our research showed that CAP treatment could inhibit inflammation, reduce oxidative stress, and enhance angiogenesis of the diabetic wound, eventually speeding up the rate of wound healing in db/db mice. When discussing the effect of CAP on wound inflammation, we chose a traditional inflammation marker IL‐6 and TNF‐α to reflect the inflammation level of mice wounds. After 7 days CAP treatment, it was demonstrated that wounds were well‐healing with milder inflammation and decreased expression level of IL‐6 and TNF‐α compared with control group by section analysis and Western Blot (all P < .01). Besides, oxidative stress was evaluated using iNOS and SOD levels, while angiogenesis was evaluated using VEGF and TGF‐β levels in mice wound. We found that the wounds in plasma treatment groups had a significantly decreased level of iNOS and SOD and increased level of VEGF and TGF‐β than the control group (all P < .01). The decrease or increase range was proportional to the exposure time, which showed CAP treatment as an being exposure‐dependent (all P < .05). Consistent with our study, other researchers also confirmed that the plasma exposure induced neovascularisation after 7 days of treatment in burn wounds and chronic wounds.15, 16 However, only a few experiments analysed whether plasma administration affects oxidative stress.
There were several kinds of reactive ions, molecules, and free radicals, such as atomic oxygen (O), hydroxyl (OH), nitrogen molecule (N2), super oxide anion (O2−), and NO, in the plasma.17 It was reported that these reactive agents played an important role in the interaction between the plasma and cells.9 Our results demonstrated that CAP treatment could accelerate the migration rate of HaCaT cells in PAM‐activated medium. The PAM activated by CAP for 1, 2, and 3 minutes had a significant effect on HaCaT cell migration ability compared with the control at 24 and 48 hours. However, longer time‐activated PAM (including 4, 5, 6 minutes) went against the migration to the scratch. In fact, the effects of plasma were extensively investigated in vitro by using different types of cells in a monolayer. Wound‐related cells include keratinocytes, fibroblasts, and epithelial and endothelial cells, but also inflammatory cells, especially in terms of chronic infected wounds.18 Despite the use of different cell types or different plasma sources, most of the studies supported the conclusion that short plasma treatment times/low plasma doses had stimulating effects (increase of proliferation and migration, induction of DNA repair) and that long plasma treatment times/high plasma doses induce lethal effects (cell death by apoptosis, stop of proliferation, DNA damage, cell cycle arrest).9 Effects on cells because of the different kinds of radiation could be excluded if cells were only exposed to a plasma‐treated medium (indirect treatment).9 It was summarised that plasma treatment produced reactive radicals and UV radiation, which changed cell viability, induced cell apoptosis, modified cell adhesion, and altered cell migration.19, 20, 21 Meanwhile, good compatibility of plasma on skin has been reported. Plasma treatment of rats’ wounded skin did not have any toxic effects on the skin.22
In this study, we also evaluated the safety of CAP in treating db/db mice skin wound. First, the 180s plasma treatment did not cause visible damage to db/db mice's skin wound, such as turning red, burning, or blistering. Besides, after 14 days of plasma treatment, compared with the control group, the levels of WBC, ALT, AST, BUN, and Cr in the 90s and 180s CAP treatment groups were not significantly different. These results suggest that CAP did not have an obvious impact on systemic inflammation and liver and kidney function in diabetic mice. Innovation of new technology requires assessing not only its effectiveness but also its security. The safety of medical equipment should especially be given much more attention to prevent side effects. Considering the various functions of CAP, attempts have been made to incorporate technologies associated with CAP into medical practice for various situations.23, 24, 25 CAP's feasibility for use has been demonstrated in haemostasis, wound care, and anti‐cancer therapy.9, 26 Now, the commercial viability of CAP had been studied at many companies and created some relevant products such as MicroPlaSter manufactured by ADTEC Plasma Technology Co.27 A series of studies describing the clinical benefit of plasma equipment in controlling bacterial infection in chronic wounds and eradicating infected microorganisms provides new clues for solving issues related to the increase in bacteria that are resistant to multiple antibiotics.23, 24
In summary, this is the first study that combined animal and cell experiments and analysed the underling mechanisms of CAP for treating diabetic skin wounds with regard to three aspects of inflammation, oxidative stress, and angiogenesis. Besides, we also simply evaluated the safety of CAP as a kind of therapy in systemic inflammatory response and organ functions. Our in vitro and in vivo results showed that the CAP treatment could inhibit inflammation, decrease oxidative stress, and enhance angiogenesis, eventually speeding up wound union in db/db mice and human HaCaT immortalised human epidermal cells. After 3 minutes of daily exposure to plasma for 14 consecutive days, no relevant side effects in db/db mice were detected. DF is one of the most severe chronic complications of DM worldwide.28 Because of delayed or impaired wound healing, DF may result in serious outcomes, such as leg or foot amputation.29 These complications deteriorate the quality of life of patients and increase the burden of patient's family and social medical care.29 Considering the serious damages of DF in patients with type 2 diabetes and the high efficiency of plasma in treating diabetic skin wound, it is of great significance to take CAP treatment into consideration when dealing with a bacterial infection in chronic wounds, especially in bacteria that are resistant to multiple antibiotics. Although the potential mechanisms need deeper understanding, CAP treatment could provide us with a novel therapy in clinical diabetic wound management.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors are thankful for the support of CapitalBio Corporation and the National Engineering Research Center for Beijing Biochip Technology. This study was supported by the National Natural Science Fund of China (Grant Nos 81270397 and 81770802 for Fang Liu), Shanghai Science & Technology Pillar Program in the Field of Medicine and Agriculture (15411953100 for Fang Liu), and Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine Grant Support (20152232 for Fang Liu).
He R, Li Q, Shen W, et al. The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. Int Wound J. 2020;17:851–863. 10.1111/iwj.13341
R. He, Q. Li, and W. Shen contributed equally to this study.
Funding information Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine Grant Support, Grant/Award Number: 20152232; National Natural Science Fund of China, Grant/Award Numbers: 81270397, 81770802; Shanghai Science & Technology Pillar Program in the Field of Medicine and Agriculture, Grant/Award Number: 15411953100
Contributor Information
Dong Wang, Email: dongwang@capitalbio.com.
Fang Liu, Email: f-liu@sjtu.edu.cn.
REFERENCE
- 1. Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta: United States Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2. Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115(1):114‐126. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bondor CI, Veresiu IA, Florea B, Vinik EJ, Vinik AI, Gavan NA. Epidemiology of diabetic foot ulcers and amputations in Romania: results of a cross‐sectional quality of life questionnaire based survey. J Diabetes Res. 2016;2016:5439521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Font‐Jimenez I, Llaurado‐Serra M, Roig‐Garcia M, De Los Mozos‐Perez B, Acebedo‐Urdiales S. Retrospective study of the evolution of the incidence of non‐traumatic lower‐extremity amputations (2007‐2013) and risk factors of reamputation. Prim Care Diabetes. 2016;10(6):434‐441. [DOI] [PubMed] [Google Scholar]
- 6. Heyer K, Debus ES, Mayerhoff L, Augustin M. Prevalence and regional distribution of lower limb amputations from 2006 to 2012 in Germany: a population based study. Eur. J Vasc Endovasc Surg. 2015;50(6):761‐766. [DOI] [PubMed] [Google Scholar]
- 7. Morbach S, Furchert H, Groblinghoff U, et al. Long‐term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35(10):2021‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyamoto K, Ikehara S, Sakakita H, Ikehara Y. Low temperature plasma equipment applied on surgical hemostasis and wound healings. J Clin Biochem Nutr. 2017;60(1):25‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haertel B, von Woedtke T, Weltmann KD, Lindequist U. Non‐thermal atmospheric‐pressure plasma possible application in wound healing. Biomol Ther. 2014;22(6):477‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shao PL, Liao JD, Wong TW, Wang YC, Leu S, Yip HK. Enhancement of wound healing by non‐thermal N2/Ar micro‐plasma exposure in mice with fractional‐CO2‐laser‐induced wounds. PLoS One. 2016;11(6):e0156699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non‐thermal plasma through fibroblast growth Factor‐2 release. Ann Biomed Eng. 2010;38(3):748‐757. [DOI] [PubMed] [Google Scholar]
- 12. Arndt S, Unger P, Wacker E, et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One. 2013;8(11):e79325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359‐369. [DOI] [PubMed] [Google Scholar]
- 14. Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. Potential cellular targets and antibacterial efficacy of atmospheric pressure non‐thermal plasma. Int J Antimicrob Agents. 2014;43(2):154‐160. [DOI] [PubMed] [Google Scholar]
- 15. Ye F, Kaneko H, Nagasaka Y, et al. Plasma‐activated medium suppresses choroidal neovascularization in mice: a new therapeutic concept for age‐related macular degeneration. Sci Rep. 2015;5:7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pekshev AV, Shekhter AB, Vagapov AB, Sharapov NA, Vanin AF. Study of plasma‐chemical NO‐containing gas flow for treatment of wounds and inflammatory processes. Nitric Oxide. 2018;73:74‐80. [DOI] [PubMed] [Google Scholar]
- 17. Vasilieva T, Chuhchin D, Lopatin S, Varlamov V, Sigarev A, Vasiliev M. Chitin and cellulose processing in low‐temperature electron beam plasma. Molecules. 2017;22(11):1908‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging. 2010;2(9):627‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia‐Alcantara E, Lopez‐Callejas R, Morales‐Ramirez PR, et al. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch Med Res. 2013;44(3):169‐177. [DOI] [PubMed] [Google Scholar]
- 20. Kakudo N, Morimoto N, Kushida S, Ogawa T, Kusumoto K. Platelet‐rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol. 2014;47(2):83‐89. [DOI] [PubMed] [Google Scholar]
- 21. Haertel B, Hahnel M, Blackert S, Wende K, von Woedtke T, Lindequist U. Surface molecules on HaCaT keratinocytes after interaction with non‐thermal atmospheric pressure plasma. Cell Biol Int. 2012;36(12):1217‐1222. [DOI] [PubMed] [Google Scholar]
- 22. Fathollah S, Mirpour S, Mansouri P, et al. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci Rep. 2016;6:19144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isbary G, Morfill G, Schmidt HU, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010;163(1):78‐82. [DOI] [PubMed] [Google Scholar]
- 24. Isbary G, Heinlin J, Shimizu T, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167(2):404‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heinlin J, Isbary G, Stolz W, et al. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol. 2011;25(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 26. Isbary G, Shimizu T, Li YF, et al. Cold atmospheric plasma devices for medical issues. Expert Rev Med Devices. 2013;10(3):367‐377. [DOI] [PubMed] [Google Scholar]
- 27. Isbary G, Shimizu T, Zimmermann JL, Thomas HM, Morfill GE, Stolz W. Cold atmospheric plasma for local infection control and subsequent pain reduction in a patient with chronic post‐operative ear infection. New Microbes New Infect. 2013;1(3):41‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowering CK. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can Fam Physician. 2001;47:1007‐1016. [PMC free article] [PubMed] [Google Scholar]
- 29. Nabuurs‐Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health‐related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia. 2005;48(9):1906‐1910. [DOI] [PubMed] [Google Scholar]


