Abstract
The factors preventing healing in venous leg ulcers are still not fully understood. Iron‐mediated tissue damage has been hypothesised, yet anecdotally anaemia is also thought to have a negative effect on wound healing. This article summarises the current evidence for these theories and their likely effects in the context of venous ulceration. A comprehensive search of the literature was conducted. Studies suggest that a number of forms of iron including haemosiderin and ferritin are implicated in progression of venous disease, ulcer formation, and impaired healing, which is thought to be primarily free radical mediated. There is a paucity of evidence for the role of iron deficiency and anaemia on ulcer healing; however, there is likely to be a highly complex interplay between the damaging effects of iron on local tissues and the negative effects of anaemia‐mediated tissue hypoxia. Studies looking at options to increase local oxygen delivery such as topical haemoglobin suggest that this may have an impact on some aspects of healing, but findings are generally inconclusive. There is growing evidence that locally elevated iron levels may have a detrimental effect on ulcer healing and formation; however, more robust research is needed.
Keywords: anaemia, healing, iron, venous ulcers
1. INTRODUCTION
It is estimated that 4.5% of the adult population in the United Kingdom have a wound at any one time, with an annual NHS cost of £6 billion. A study by Guest et al in 2015 estimated that 19% of all wounds are venous leg ulcers, and some studies suggest that up to 30% of venous leg ulcers remain unhealed at 24 weeks causing significant pain and psychological distress to the patients but the factors preventing healing are still not fully understood.1, 2
In chronic venous disease (CVD), venous stasis allows extravasation of erythrocytes into the surrounding tissues. These erythrocytes are degraded by interstitial macrophages, releasing iron that becomes bound to proteins to form ferritin. When ferritin becomes saturated with iron, it undergoes partial degradation by oxidation reactions to produce haemosiderin, which is widely accepted as the cause of the pigmentation seen in the limb of a patient with chronic venous disease (Figure 1); however, the role of these iron compounds in venous disease progression is not clear.3
Figure 1.
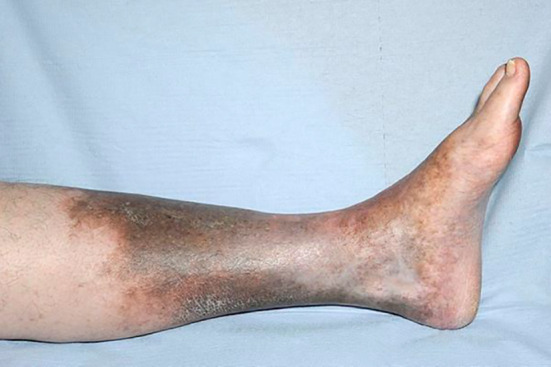
Haemosiderin deposition in a leg with chronic venous disease
Iron has a crucial role in a number of cellular functions and helps maintain homeostasis. It is a fundamental part of the haemoglobin molecule facilitating oxygen carriage to tissues as well as mediating immune function and regulating DNA and replication, particularly important in areas of high cell turnover such as the skin.4
As a transition metal, iron is able to undergo redox reactions donating or accepting electrons to cycle between Fe2+ and Fe3+ ion states, but as a result of this, it can easily produce reactive oxygen species with unpaired electrons (Figure 2), which oxidise lipids, proteins, nucleic acids, and connective tissue causing damage.4, 5 It also increases the action of matrix metalloproteinases (zinc‐dependent endopeptidases), which degrades collagen, elastin, and laminin breaking down extracellular matrix, thus damaging tissue integrity.6, 7
Figure 2.
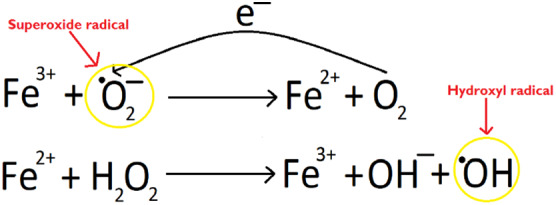
Schematic representation of the Fenton and Haber‐Weiss reaction generating free oxygen species in the presence of iron. Free radicals are circled in yellow
In humans, the majority of free iron is stored bound to proteins in complexes such as apoferrin, transferrin lactoferrin, ferritin, and haemoglobin so that free radical production is limited.4, 6 The balance of free iron to bound iron ions is kept in strict homeostasis through a number of regulatory elements including iron regulatory proteins, iron‐responsive elements, and haemoxygenase‐1 mediating production of ferritin and transferrin receptors.8
Despite theories surrounding iron‐mediated tissue damage causing ulceration and impaired wound healing, there is also evidence to suggest that anaemia or systemic iron deficiency can also have a negative effect on wound healing. Early animal studies suggested reduced wound strength in rats with chronic iron deficiency and rabbits with anaemia,9, 10 but a study by Trueblood et al assessed rat colon wound tensile strength in both acute anaemia following bloodletting and chronic iron deficiency caused by nutritional deficit found no such relationship.11 Studies of the effect of anaemia on wound healing in humans are small and often similarly conflicting. This review aims to summarise the current evidence in the literature for these theories and provide an overall understanding of the interaction between local iron damage and systemic iron deficiency on the pathophysiology of venous leg ulcers.
2. METHODS
A literature search of MEDLINE® (1988‐5/8/2018), EMBASE (1988‐5/8/2018), and the Cochrane Library (5/8/1988‐6/8/2018) was conducted to identify suitable articles from the past 30 years. Search domains used included wound healing, ulcers and skin, iron, ferritin, transferrin, haemosiderin, haemoglobin, and anaemia combined using Boolean operators; reference lists were also checked for appropriate articles not identified in the initial search. When compiling the article, evidence was considered from randomised control trials, case‐control trials, cohort or observational studies, case series, or case reports with a primary focus on the effect of iron (or its ions or compounds) or haemoglobin on venous leg ulcers or chronic venous disease in humans. Studies were excluded if the aetiology of the ulceration was not clearly venous.
3. RESULTS
After initial screening of search results, eight studies were identified as appropriate for review. A number of iron parameters were studied using different techniques. 252 subjects were studied across five case‐control and three observational cohort studies. A summary of included studies is available in Table 1.
Table 1.
Summary of included studies
| Author | Year | Study type | Number of patients | Aim | Main findings |
|---|---|---|---|---|---|
| Ackerman et al | 1988 | Observational cohort study | 16 | To measure iron levels in tissue surrounding venous ulcers | Iron level at the ulcer edge was consistently higher than those samples taken from elsewhere on the ulcerated limb |
| Stucker et al | 2000 | Case‐control study | 53 | To identify the difference in the rate of fall of transcutaneous oxygen tension in limbs with venous ulceration compared with healthy controls | Transcutaneous oxygen saturation fell significantly faster in the control group than in those with venous ulceration in 55‐45 mmHg range of transcutaneous oxygen (1.9 mmHg vs 0.8 mmHg; P < .05) |
| Wenk et al | 2001 | Observational cohort study | 17 | To compare the levels of iron in acute and chronic wound fluid and plasma. To assess the ability of a deferoxamine‐cellulose dressing to bind iron ions in solution | Iron levels lower in acute and postsurgical wound fluid compared with chronic wound fluid. Deferoxamine‐cellulose dressing does bind to iron ions in solution |
| Allhorn et al | 2003 | Case‐control study | 17 | To detect the levels of haem, iron, and α1 macroglobulin in wound fluid from chronic ulcers | Iron identified on biopsies from chronic venous leg ulcers but not in the acute wounds. Higher levels of iron in plasma of patients with chronic wounds compared with healthy controls |
| Zamboni et al | 2005 | Case‐control study | 44 | To investigate the potential consequences of leg haemosiderin deposits on both iron metabolism and activation of MMPs | Significantly higher level of iron in the leg compared with arm of patients with CVD |
| Caggiati et al | 2008 | Case‐control study | 28 | To detect levels of haemosiderin and melanin in biopsies from patients with chronic venous insufficiency | All patient samples showed higher levels of melanin than controls. Haemosiderin found in areas of lipodermatosclerosis or heavy pigmentation |
| Yeoh‐Ellerton and Stacey | 2003 | Case‐control study | 33 | To determine differences in iron and iron protein (ferritin and transferrin) levels in chronic venous ulcers and acute wounds | Chronic leg ulcer wound fluid had significantly higher levels of ferritin than acute lesions (median 2.96 vs 1.10 mg/L; P < .0001); however, chronic ulcers had lower levels of transferrin than acute wounds (median 13 μmoL/L vs 18.5 μmoL/L; P < .001) |
| Caggiati et al | 2010 | Observational cohort study | 44 | To evaluate skin haemosiderin deposition in relation to the presence and severity of skin changes in CVD legs | Haemosiderin present on biopsy of lipodermatosclerosis. None identified in visually normal skin. Occasional haemosiderin identified in tissue adjacent to ulceration |
A study by Stucker et al in 2000 sought to clarify whether variations in the oxygen consumption of the tissues of the limb were related to active venous disease. 39 subjects with varying degrees of chronic venous disease were studied against a group of 14 healthy controls. By administering oxygen to supersaturate haemoglobin and then causing temporary arterial occlusion to prevent further oxyhaemoglobin being delivered to the area, researchers stated that this allowed the study of the oxygen equilibrium between that dissolved in plasma and tissues and that bound to haemoglobin at different partial pressures. 12 There was no significant difference in the rate of fall of transcutaneous oxygen saturation and therefore oxygen consumption at the ankle between controls and those with venous disease above 120 mmHg (P < .05); however, in the 55‐45 mmHg range, the transcutaneous oxygen saturation fell significantly faster in the control group than in those with venous ulceration (1.9 mmHg vs 0.8 mmHg P < .05). No such differences were found in the forearm or calf at the same partial pressure. This suggests that there may be higher levels of haemoglobin to release oxygen in the tissues surrounding venous ulceration compared with tissues distant from ulceration, and the research team hypothesised that a lack of oxygen in the tissues of chronic venous disease was not the driving force for ulceration.
The findings of this study need to be interpreted with caution as their conclusions are based on the principle that no oxygen dissociation from haemoglobin will occur in the tissues above 120 mmHg based on the oxygen dissociation curve; however, due to other potential environmental changes in the tissues surrounding a wound, the oxygen dissociation curve could potentially shift, affecting oxygen release and this is not discussed. It is also not clear from the test case presented what degree of variation in transcutaneous oxygen saturations can be within normal limits so that further data on this method would be helpful to put their findings in context as would a larger, adequately powered study.
A study by Wenk et al in 2001 identified a difference in iron content of exudate between chronic and acute wounds. The exudate of nine patients with chronic venous leg ulcers was compared with that of four patients post‐mastectomy, four patients with bullous pemphigoid, and one post myoscarcoma excision. Atomic absorption spectroscopy found that iron levels were significantly lower in acute blisters and post‐surgical wound fluid than in chronic venous leg ulcer fluid 1.15 vs 0.87 vs 3.71 μm/g protein, respectively, P < .02.7 This method was however limited by requiring a minimum of 200 μL of wound fluid, which may underestimate the amount of iron in a wound that produces minimal exudate.
A similar study by Allhorn et al in 2003 collected wound fluid, plasma, and wound edge biopsies from chronic venous ulcers of 12 patients. Biopsies were also taken from the edges of acute wounds or healthy tissue for comparison. Iron deposits were seen in biopsies from chronic venous leg ulcers but not in the acute wounds; however, due to small study numbers, the reliability of this finding is questionable. No comparative data were presented for chronic vs acute wound fluid; however, plasma from those with chronic ulcers was found to have 7.3 μM of iron compared with 13.9 μM in the plasma of healthy controls.13 This suggests that iron deficiency may be associated with chronic venous ulcers, but much larger observational data sets are required to support or disprove this hypothesis.
In two studies conducted by Caggiati et al, punch biopsies were taken from legs with varying degrees of chronic venous disease to assess the degree of haemosiderin deposition. In both studies, the majority of patients with CVD but visually normal skin did not have haemosiderin evident on biopsy (46/49 and 22/25, respectively). In patients with pigmented skin (CEAP 4), only those classified as having severe pigmentation had haemosiderin deposition on biopsy (3/12). In lipodermatosclerotic tissue, haemosiderin was evident in all samples as did all those biopsies from ulcerated tissue.14, 15
Zamboni et al in 2005 conducted a study in 30 patients with unilateral chronic venous disease and 14 controls comparing the plasma iron levels from the arm and affected leg. This demonstrated a significantly higher level of iron in the leg compared with arm of affected patients (75 mg/100 mL vs 69 mg/100 mL; P = .0001). No significant difference was found between iron levels in the legs and arms of the control group (67 mg/100 mL vs 62 mg/100 mL), but levels were consistently higher in both the arms and legs of the patients with venous disease compared with controls (P < .004). When stratified for CEAP classification, those with stage 5–6 disease had significantly higher iron levels in the leg than those with CEAP 4 (86 vs 65 mg/100 mL; P < .0004). Other iron parameters were also significantly higher in the legs than in the arms of affected patients, %total iron binding capacity (TIBC) (24 vs 22%; P = .002), and ferritin (125 mg/100 mg vs 119 mg/100 mL; P = .0001); however, TIBC and transferrin did not show any significant difference.16
Ackerman et al in 1988 analysed the skin iron deposition in patients with venous leg ulcers using X‐ray spectroscopy and found that the mean iron level at the ulcer edge was consistently higher than those samples taken from elsewhere on the ulcerated limb (250 vs 128 mcg of iron in 1 mg wet tissue), but the result was not statistically significant (P < .25). The authors also did not find any correlation between the degree of local iron deposition and severity or duration of the adjacent ulcer.17
Ellerton and Stacey in 2003 took samples from 21 venous leg ulcer patients and 12 acute mastectomy wounds. Chronic leg ulcer wound fluid had significantly higher levels of ferritin than acute lesions (median 2.96 vs 1.10 mg/L; P < .0001); however, chronic ulcers had lower levels of transferrin than acute wounds (median 13 μmoL/L vs 18.5 μmoL/L; P < .001). No significant difference was found in the concentration of absolute iron in the wound fluid from acute and chronic wounds. In biopsies from normal tissue, very little ferritin staining was evident and it was confined to the dermis, whereas in biopsies from the chronic wound edges, much more extensive staining was shown. Similarly very little ferric iron was identified in biopsies of normal skin, or the epidermis of the ulcer edge, although higher levels were found in the dermis of the edge and base of the ulcer compared with intact skin.18
4. DISCUSSION
Anaemia is defined by the World Health Organization as haemoglobin <130 g/L in adult men, <120 g/L in adult non‐pregnant women, and <110 g/L in pregnant women.19 There are many different mechanisms for anaemia including bone marrow failure, deficiencies such as iron or B12, or blood loss although “anaemia” and “iron deficiency” are terms often incorrectly used synonymously. It is also increasingly recognised that iron deficiency can occur without associated low haemoglobin, and for this reason we consider both forms of deficiency when considering the effect on wound healing.20 The variety of terminology and definitions used for anaemia and the different parameters by which iron can be measured make interpretation of data challenging due to heterogeneity of the pathologies studied.
Only a handful of studies are available in the literature looking at the potential relationship between iron and venous ulceration, and as described here, existing studies are generally limited by small sample sizes and inadequate power. The interpretation of the results presented here is also limited by the lack of baseline data provided on “normal variation” of iron and its compounds in wound fluid, tissue, or plasma of the general population to enable results to be accepted as significant rather than a reflection of heterogeneity of the patient group studied or a feature of normal physiological variation. Similarly, the variation in methods of iron detection used in the different studies makes it difficult to draw direct comparisons between results; this may in part account for why Wenk et al in 2001 using atomic absorption spectroscopy found significantly higher iron levels in chronic venous leg ulcer fluid than acute wound fluid, whereas Yeoh‐Ellerton and Stacey in 2003 used both the ferrozine assay and inductively coupled plasma atomic emission spectrometry to detect iron but no significant difference was found in iron levels despite finding higher ferritin in chronic leg ulcer fluid compared with acute wounds.
Despite the limitations of these studies, the findings do support the hypothesis that iron and its compounds are found in higher concentrations in areas of ulceration and chronic venous disease though further conclusive proof is still needed. When sampling wound fluid, Wenk et al in 2001 found statistically significantly higher iron levels in wound fluid from chronic venous ulcers than from acute wounds; Yeoh‐Ellerton and Stacey in 2003 were unable to find such a pattern, but did identify higher ferritin (a compound of iron) in the wound fluid. However, ferritin is an acute phase protein so this may be a consequence of inflammation found in any chronic wound rather than as a feature of iron‐related venous ulceration.
On tissue biopsy, Allhorn in 2003 found that samples from wound edges contained iron, whereas biopsies from acute wounds had no such finding; the study by Ackerman et al in 1988 identified similar findings of higher iron levels at the ulcer edge compared with elsewhere on the ulcerated limb which were statistically significant. The findings by Caggiatti et al in 2008 and 2010 that haemosiderin was present on biopsy from ulcerated, severely pigmented, or lipodermatosclerotic skin but not present in the biopsies from the limbs with chronic venous disease without pigmentation suggests that haemosiderin deposition may be a later feature of the disease but not instrumental in the formation of initial ulceration or tissue damage.
When sampling plasma for iron levels, Allhorn in 2003 found that iron levels were lower in those with chronic ulceration compared with those with healthy controls. Interestingly Zamboni's study found the average level of iron in plasma was higher in the control group than those with CVD; however, this was not analysed any further nor tested for statistical significance to enable a more robust conclusion. Zamboni went further and identified that plasma levels were higher in the affected leg compared with arm of those with chronic venous disease. Another study by Zamboni found that patients with chronic venous disease (c4‐C6) who also had C282Y gene mutation (a common mutation found in haemochromatosis, a condition characterised by systemic iron overload) had a significant increase in the risk of venous ulceration (OR 6.69, P = .01) compared with controls without the gene.21 These findings again support the theory that localised iron levels may impact on ulceration and chronic venous disease.
Whether systemic iron deficiency or anaemia has a negative impact on venous leg ulceration is not clear from the available evidence currently. However, historically there has been a belief that anaemia causes impaired wound healing via reduced tissue oxygenation based on early animal studies. Following on from these, Jonsson et al in 1991 measured oxygen tension postoperatively via a subcutaneous probe and identified that increased oxygen tension in the wound was associated with increased collagen deposition at days 5 and 7. Interestingly, collagen deposition appeared independent of blood loss in surgery or postoperative haematocrit, suggesting that tissue oxygenation is independent of anaemia, although heterogeneity of the wounds studied does make it difficult to draw robust conclusions.22
A small prospective study of diabetic foot disease found that anaemia or iron deficiency with a normal haemoglobin was present in 21/27 patients studied. However, due the small sample size and short follow‐up period, it is not possible to draw any conclusions regarding the impact on wound healing in these patients.23 A larger observational study of diabetic patients also identified anaemia as a risk factor for the formation of diabetic foot ulcers (P = .004); however, results were not stratified for severity of disease, duration of underlying diabetes, or renal failure, all of which may have an impact on wound healing and diabetic foot disease.24
If systemic anaemia does indeed convey a negative effect on wound healing and ulceration, this needs to be considered as a simple therapeutic target. Correction of anaemia can be attempted in a number of ways including blood transfusions, iron infusions, and direct stimulation of erythropoiesis in the bone marrow using erythropoietin; however, no research is currently available on the effect of any of these treatments on healing in venous leg ulcers. In addition to correcting anaemia systemically, products are available claiming to increase haemoglobin in and around the wound itself by topical application, but there is only one single blind randomised control trial to support its use. This study by Arenbergerova et al in 2013 found a wound size reduction of 53.4% in the treatment group (P < .0001, 90% power); however, the study also recorded an average increase in wound surface area by 21% at week 13 in the control group despite receiving standard care (P < .01). This increase in average wound area despite gold standard compression is unexpected and as such raises some questions about the research methodology and reliability of results.25
If local iron overload does indeed have a deleterious effect, then a method of chelating iron within the wound to prevent ionic iron releases reactive oxygen species. Deferoxamine is an iron chelator already established as a systemic treatment for iron overload in conditions such as haemochromatosis. An in vitro study by Taylor et al in 2005 exposed a polyurethane sheet coated with deferoxamine to iron sulphate solution. Upon environmental scanning electron microscopy, iron ions could be demonstrated bound to the polyurethane sheet5; similarly Wenk et al in 2001 exposed cellulose coupled with deferoxamine to iron solution and found significant iron uptake.7 These findings support the theory that deferoxamine‐impregnated dressings may be an effective solution to localised iron overload in chronic wounds such as venous leg ulcers.
5. LIMITATIONS
There is relatively little good quality evidence available analysing the relationship between systemic or local iron and venous leg ulceration, the majority of studies are observational in nature with small sample sizes reducing the reliability of results and increasing the risk of type 1 and type 2 errors. In addition to the individual study limitations already discussed in this article, there is also a risk of this review being affected by publication bias; however, the authors attempted to minimise this by identifying relevant grey literature such as abstracts from conferences in initial searches as well as reviewing the reference lists of all potential studies to identify any not found in the original described search. As many of the studies included here demonstrate negative findings, the authors feel that the effect of any reporting bias is relatively small. The study designs do not provide enough methodological information to enable formal assessment of bias, but while the evidence available is of comparatively poor quality, it is the best available currently to inform future research.
6. CONCLUSION
There is growing evidence that locally elevated levels of iron and its subtypes may have a negative effect on ulcer healing as well as increasing the likelihood of venous ulceration developing; however, more robust research is needed to fully understand the complex interplay between iron and the physiology of venous disease and healing. Our team have designed a novel pilot research study to assess the prevalence of anaemia and the relationship between plasma and wound fluid levels of iron and its subtypes in patients with venous leg ulcers, and any association this may have with rate of healing and this prospective study is now underway.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Ferris AE, Harding KG. An overview of the relationship between anaemia, iron, and venous leg ulcers. Int Wound J. 2019;16:1323–1329. 10.1111/iwj.13192
REFERENCES
- 1. Guest J, Ayoub N, McIwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5:e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker CN, Finlayson KJ, Shuter P, Edwards HE. Risk factors for delayed healing in venous leg ulcers: a review of the literature. Int J Clin Pract. 2015;69(9):967‐977. [DOI] [PubMed] [Google Scholar]
- 3. Trenam CW, Dabbagh AJ, Blake DR, Morris CJ. The role of iron in an acute model of skin inflammation induced by reactive oxygen species (ROS). Br J Dermatol. 1992;126(3):250‐256. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=1313278. [DOI] [PubMed] [Google Scholar]
- 4. Lansdown AB. Iron: a cosmetic constituent but an essential nutrient for healthy skin. Int J Cosmet Sci. 2001;23(3):129‐137. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed8&NEWS=N&AN=32575460. [DOI] [PubMed] [Google Scholar]
- 5. Taylor JE, Laity PR, Hicks J, et al. Extent of iron pick‐up in deforoxamine‐coupled polyurethane materials for therapy of chronic wounds. Biomaterials. 2005;26(30):6024‐6033. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=15885771. [DOI] [PubMed] [Google Scholar]
- 6. Singh AV, Subhashree L, Milani P, Gemmati D, Zamboni P. Interplay of iron metallobiology, metalloproteinases, and FXIII, and role of their gene variants in venous leg ulcer. Int J Low Extrem Wounds. 2010;9(4):166‐179. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=21118859. [DOI] [PubMed] [Google Scholar]
- 7. Wenk J, Foitzik A, Achterberg V, et al. Selective pick‐up of increased iron by deferoxamine‐coupled cellulose abrogates the iron‐driven induction of matrix‐degrading metalloproteinase 1 and lipid peroxidation in human dermal fibroblasts in vitro: a new dressing concept. J Invest Dermatol. 2001;116(6):833‐839. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=11407968. [DOI] [PubMed] [Google Scholar]
- 8. Pourzand C, Watkin RD, Brown JE, Tyrrell RM. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96(12):6751‐6756. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=10359784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bains JW, Crawford DT, Ketcham AS. Effect of chronic anemia on wound tensile strength: correlation with blood volume, total red blood cell volume and proteins. Ann Surg. 1966;164(2):243‐246. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med1&NEWS=N&AN=5915934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchmiller‐Crair TL, Kim CS, Won NH, Chopourian HL, Shaw KS, Fonkalsrud EW. Effect of acute anemia on the healing of intestinal anastomoses in the rabbit. J Trauma. 2001;51(2):363‐368. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=11493801. [DOI] [PubMed] [Google Scholar]
- 11. Trueblood HW, Nelsen TS, Oberhelman HAJ. The effect of acute anemia and iron deficiency anemia on wound healing. Arch Surg. 1969;99(1):113‐116. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med1&NEWS=N&AN=5787617. [DOI] [PubMed] [Google Scholar]
- 12. Stucker M, Falkenberg M, Reuther T, Altmeyer P, Lubbers DW. Local oxygen content in the skin is increased in chronic venous incompetence. Microvasc Res. 2000;59(1):99‐106. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=10625576. [DOI] [PubMed] [Google Scholar]
- 13. Allhorn M, Lundqvist K, Schmidtchen A, Akerstrom B. Heme‐scavenging role of alpha1‐microglobulin in chronic ulcers. J Invest Dermatol. 2003;121(3):640‐646. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=12925227. [DOI] [PubMed] [Google Scholar]
- 14. Caggiati A, Rosi C, Casini A, et al. Skin iron deposition characterises lipodermatosclerosis and leg ulcer. Eur J Vasc Endovasc Surg. 2010;40(6):777‐782. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=20880725. [DOI] [PubMed] [Google Scholar]
- 15. Caggiati A, Rosi C, Franceschini M, Innocenzi D. The nature of skin pigmentations in chronic venous insufficiency: a preliminary report. Eur J Vasc Endovasc Surg. 2008;35(1):111‐118. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=17920308. [DOI] [PubMed] [Google Scholar]
- 16. Zamboni P, Scapoli G, Lanzara V, et al. Serum iron and matrix metalloproteinase‐9 variations in limbs affected by chronic venous disease and venous leg ulcers. Dermatol Surg. 2005;31(6):644‐649. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=41035973. [DOI] [PubMed] [Google Scholar]
- 17. Ackerman Z, Seidenbaum M, Loewenthal E, Rubinow A. Overload of iron in the skin of patients with varicose ulcers. Possible contributing role of iron accumulation in progression of the disease. Arch Dermatol. 1988;124(9):1376‐1378. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=3415280. [PubMed] [Google Scholar]
- 18. Yeoh‐Ellerton S, Stacey MC. Iron and 8‐isoprostane levels in acute and chronic wounds. J Invest Dermatol. 2003;121(4):918‐925. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=14632213. [DOI] [PubMed] [Google Scholar]
- 19. Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309‐1316. [DOI] [PubMed] [Google Scholar]
- 20. Soppi ET. Iron deficiency without anemia – a clinical challenge. Clin Case Rep. 2018;6(6):1082‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zamboni P, Tognazzo S, Izzo M, et al. Hemochromatosis C282Y gene mutation increases the risk of venous leg ulceration. J Vasc Surg. 2005;42(2):309‐314. [DOI] [PubMed] [Google Scholar]
- 22. Jonsson K, Jensen JA, Goodson WH, Scheuenstuhl H, West J, Williams Hopf H, Hunt T. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214(5):605‐613. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=1953114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright JA, Oddy MJ, Richards T. Presence and Characterisation of Anaemia in Diabetic Foot Ulceration. Anemia. 2014;1‐8. 10.1155/2014/104214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hokkam E. Assessment of risk factors in diabetic foot ulceration and their impact on the outcome of the disease. Prim Care Diabetes. 2009;3:219‐224. [DOI] [PubMed] [Google Scholar]
- 25. Arenberger P, Elg F, Petyt J, Cutting K. Expected outcomes from topical haemoglobin spray in non‐healing and worsening venous leg ulcers. J Wound Care. 2015;24(5):228‐236. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med8&NEWS=N&AN=25970759. [DOI] [PubMed] [Google Scholar]


