Abstract
Converging interactions between ascending proprioceptive afferents and descending corticospinal tract projections are critical in the modulation and coordination of skilled motor behaviors. Fundamental to these processes are the functional inputs and the mechanisms of integration in the brain and spinal cord between proprioceptive and corticospinal tract information. In this review, we first highlight key connections between corticospinal tract motor circuit and spinal interneurons that receive proprioceptive inputs. We will also address corticospinal tract access to the presynaptic inhibitory system in the spinal cord and its role in modulating proprioceptive stimuli. Lastly, we will focus on the corticospinal neuron influences on the dorsal column nuclei complex, an integration hub for processing ascending somatosensory information.
Keywords: corticospinal neurons, proprioceptive sensory neurons, spinal cord, motor cortex
1. Introduction
The coordination of peripheral muscle activation for movement control is predicated upon the interplay between proprioceptive sensory feedback information (e.g. muscle activity and body position) and motor output [1, 2]. Descending motor commands from supraspinal structures exert an additional level of integration onto proprioceptive afferent inputs for the appropriate execution of motor function [3, 4]. Much progress has been made on how proprioceptive and descending motor inputs influence one another during movement control at various levels of the brain and spinal cord and remains a central topic of interest in neuroscience.
The corticospinal tract (CST) system which originates in layer V sensorimotor cortex, is a multifunctional pathway that controls skilled and voluntary limb movement, but also mediates sensory functions including the modulation of proprioceptive inputs [3–6]. In primates, cats and rodents, CST axon projections from primary somatosensory cortex (S1) and primary motor cortex (M1), terminate in the dorsal and ventral spinal cord respectively, accessing the different spinal interneuron and select motoneuron populations [7–12]. Importantly, proprioceptive information (i.e. sensory-motor reflex circuits) which is processed in the dorsal spinal cord and a major source for presynaptic inhibition, is also likely modulated by descending CST projections during active movements [3, 13, 14]. In addition, corticospinal axon collaterals innervate the brainstem dorsal column nuclei (DCN) complex, an integration and distribution hub for ascending mechanosensory inputs which includes proprioception [15, 16].
The understanding that sensory and descending motor functions are not separate and that integration between the two is fundamental to movement control has been well established in the motor control field for many decades. However, what has changed in recent years is that we are now elucidating the underlying circuitry mediating these essential control functions with remarkable specify. Here we review the converging interactions between proprioceptive inputs (i.e. mechanosensory neurons connected to muscles, tendons and joints) and descending CST projections in the modulation and coordination of movement control. First, we address the organization and functional connectivity between proprioceptive and sensorimotor CST circuits at the spinal interneuron level. We will also review the role of CST influences on the presynaptic inhibitory system in modulating proprioceptive information. Furthermore, we will look at the dorsal column nuclei (DCN) complex, an integration processing center for ascending proprioceptive and descending sensorimotor inputs.
2. Interneuron spinal network integration of the proprioceptive sensory-motor circuit and sensorimotor CST system
In primates including humans, the CST system operates through direct monosynaptic cortico-motoneuronal connections between CST axons and motoneurons, to facilitate skilled movements of the hands and fingers [3, 4, 6, 17–19]. Additionally, indirect cortico-motoneuronal connections, via segmental and propriospinal interneurons in the spinal cord (i.e. intermediate zone and dorsal horn), as observed in most mammalian species including non-primates, are also fundamentally critical for distal movements, as well as other aspects of skilled motor control [6, 20]. The diversity of spinal interneurons enables the integration of converging CST motor output and proprioceptive sensory afferents, thus contributing to the broad array of skilled motor behaviors [21]. Studies in the primate and cat using classical anatomical and electrophysiological techniques has laid the foundation for circuit analysis in the spinal cord on the interactions between sensory and descending inputs [4, 13, 22]. The advent of powerful cellular and molecular tools in the rodent however, has provided many new insights on the organization and function between proprioceptive and sensorimotor CST circuits at the interneuron spinal level in facilitating movement control.
A recent study by Ueno and colleagues [7**], generated a detailed connectivity map between CST neurons in sensory and motor cortex and various spinal interneuron populations, using numerous tracing and viral techniques, as well as, several Cre-reporter lines. One of the major interneuron groups connected to CST axons from motor cortex is the premotor excitatory propriospinal V2a Chx10 interneurons which synapse onto motoneurons and receive proprioceptive-sensory inputs via feedforward disynaptic inhibition (Figure 1A) [23]. Notably, neuronal silencing of Chx10+ interneurons showed that these interneurons contribute to the execution of skilled reaching during a pellet retrieval task, suggesting that Chx10+ interneurons relay commands from CST neurons to motoneurons [7**]. These propriospinal V2a+ neurons which also receive direct and indirect supraspinal input, are involved in an internal copy of rapid feedback circuit critical for precise skilled reaching (Figure 1A) [23]. Moreover, connectivity mapping revealed CST connections from sensory and motor cortex onto Atoh1+ and Isl1+ interneurons located in laminae IV and V, which receive proprioceptive sensory inputs from group-1 afferents (Figure 1B) [24*]. Atoh1+ interneurons which target the cerebellum, have been shown to be involved in fine motor coordination of the hindlimb [25], whereas Isl1+ interneurons, form excitatory synapses onto motoneurons, and are critical for integrating crucial sensory feedback for paw grip (Figure 1B) [26].
Figure 1.
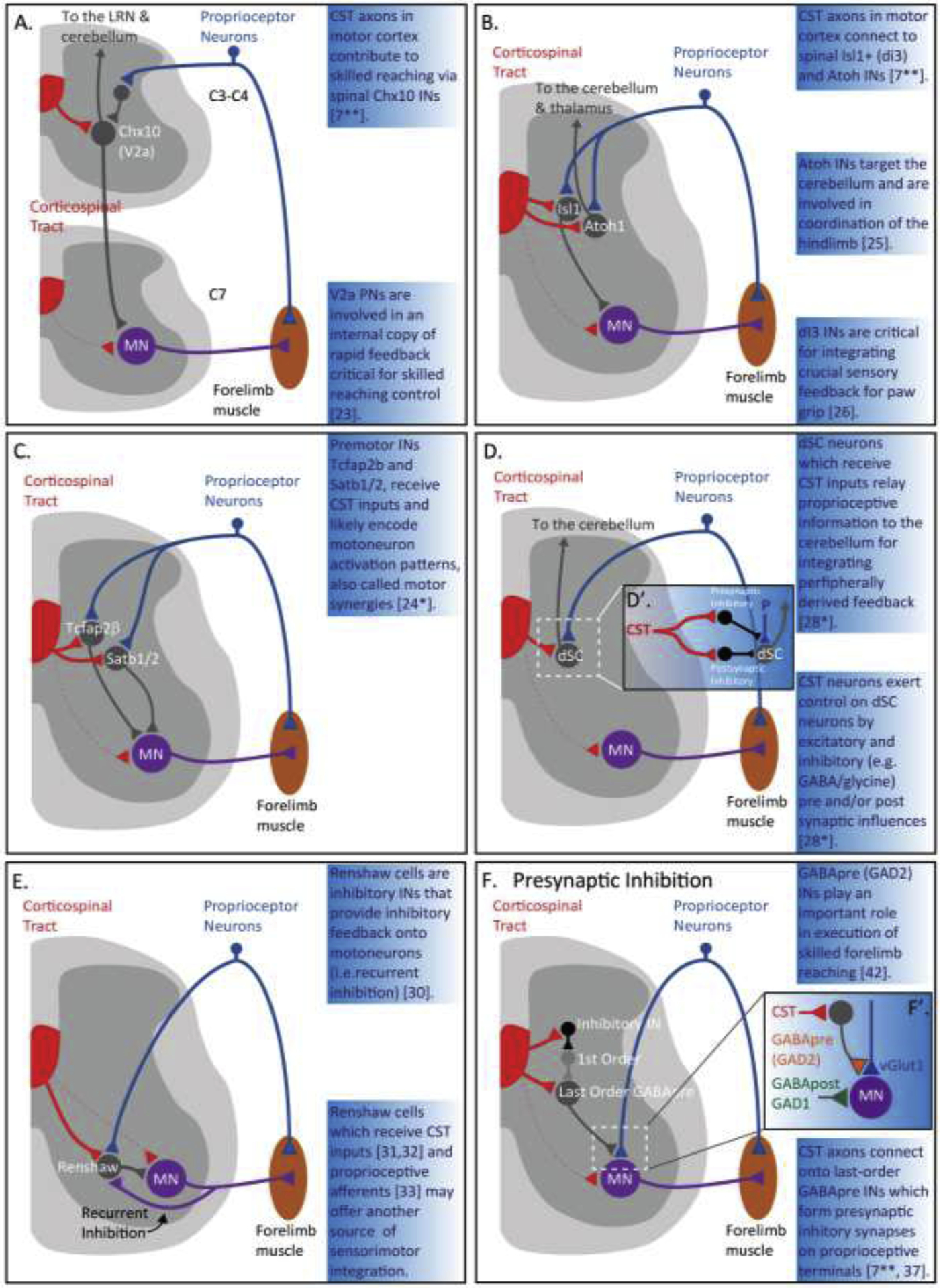
Spinal integration of the proprioceptive sensory-motor circuit and the sensorimotor corticospinal tract system.
Connectivity of spinal circuitry between convergent corticospinal tract (CST) axons (red) and proprioceptive inputs (blue) onto spinal interneurons (gray) (A–E). Rodents form rare direct monosynaptic connections between CST axons (dotted red) and motoneurons (purple).
(A) Connectivity of motor CST axons (red) onto propriospinal Chx10 (V2a) interneurons (gray) in cervical spinal cord levels 3 and 4 (C3–C4) [7**]. Propriospinal V2a interneurons relay information to the lateral reticulate nucleus (LRN) and cerebellum and are critical for skilled reaching [23].
(B) Connectivity of CST axons (red) from sensorimotor cortex to spinal Isl1 and Atoh interneurons (gray) [7**]. Atoh interneurons target the cerebellum and thalamus and are involved in coordination of the hindlimb [25]. dI3 interneurons are critical for integrating for integrating sensory feedback for paw grip [26].
(C) Connectivity of CST inputs (red) onto Tcfap2β and Sat1/2 premotor neurons (gray) located in laminae IV and V [24*]. These premotor neurons may encode for motoneuron activation for motor synergy programs [24*].
(D) Connectivity of CST inputs (red) onto dorsal spinocerebellar (dSC) neurons, known as Clarkes column dSC neurons (gray) [28*]. dSC neurons receive direct excitatory or indirect inhibitory (D’; see inset) inputs via inhibitory interneurons (e.g. GABA and glycine) [28*]. dSC relay proprioceptive information to the cerebellum for peripherally derived sensory feedback [28*].
(E) Connectivity of CST inputs (red) [31, 32] and proprioceptive afferents (blue) [33] onto Renshaw cells (gray) a class of spinal inhibitory interneurons that receive recurrent collaterals from alpha motoneurons (purple) [30]. Renshaw cells project back to alpha motoneurons and provide inhibitory feedback (i.e. GABA, glycine) and is called recurrent inhibition [30].
(F) Connectivity of CST inputs (red) onto last-order GABApre inhibitory interneurons (gray) which modulate the proprioceptive sensory-motor reflex circuit and produce primary afferent depolarization (PAD) [7**, 37, 38*]. (F’; see inset) Last order GABApre interneurons form axo-axonic synapses onto proprioceptive afferent terminals, expressing the GABA synthesizing enzyme GAD2 (also known as GAD65) [38*, 42]. GABApost interneurons which synapse directly on motoneurons, express GAD1 (also known as GAD67) [38*, 42]. (F) CST axons (red) can also depress PAD, by activating inhibitory interneurons (black), which in turn, inactivate first-order PAD interneurons (light gray), enhancing Ia afferents [46, 47].
dSC – dorsal spinocerebellar; LRN – lateral reticular nucleus; MN – motoneuron; IN – interneuron; PN – propriospinal; CST – corticospinal tract; P – proprioceptive neuron; GAD65/67 – glutamic acid decarboxylase; vGlut1 – vesicular glutamate transporter; GABA – gamma-aminobutryric acid.
The innervation of CST neurons onto these interneurons populations raises important questions on how these spinal networks engage proprioceptive afferent and descending sensorimotor CST information to control various skilled movements. In a similar region of the spinal cord as Atoh1+ and Isl1+, a study by Levine and colleagues [24*], characterized a population of motor synergy encoder (MSE) spinal neurons critical for voluntary and reflexive movement patterns. They identified a heterogeneous population of excitatory and inhibitory Tcfap2β+ and Satb1/2+ premotor interneurons located in the medial deep dorsal horn, which receive focal inputs from proprioceptive afferents and CST neurons (Figure 1C). The authors proposed that these interneurons, which are a major source of monosynaptic input to motoneurons, may possibly encode the motoneuron activation patterns for motor synergy programs. A more recent paper by Paixão and colleagues [27] showed that Zic2 neurons which integrate sensory feedback information and are required for corrective motor movements, also receive direct monosynaptic CST inputs from layer V motor cortex.
Another class of spinal neurons involved in sensorimotor integration are the dorsal spinocerebellar (dSC) neurons, located in the thoracic and lumbar nucleus and are known as Clarkes column (Figure 1D) [28*]. Clarkes column dSC neurons are a convergent target for proprioceptive sensory and descending cortical information, which relay proprioceptive information to the cerebellum for integrating and anticipating peripherally derived feedback. CST also neurons exert control over Clarkes column dSC neurons by direct excitatory and/or indirect inhibitory inputs (i.e. glycinergic and GABAergic interneurons), with the capacity to transfer predictive commands, as well as, modulate proprioceptive input for fine motor control (Figure 1D, D’) [28].
Renshaw cells [29] located in the ventral horn, are another class of spinal inhibitory interneurons which receive recurrent collaterals from alpha motoneurons (Figure 1E) [30]. The Renshaw cells which release glycine or GABA, project back to alpha motoneurons and provide inhibitory feedback (called recurrent inhibition) thus limiting motoneuron output. Studies in human have shown that CST neurons suppress Renshaw cells resulting in facilitation of reflex muscle responses [31]. Moreover it was shown in mouse that Renshaw cells receive putative direct connections from CST axons [32], as well as proprioceptive inputs (Figure 1E) [33]. Thus, direct CST and proprioceptive access of Renshaw cells, may offer another source of integration where specific spinal motor pools are potentially subject to inhibitory control.
3. Presynaptic inhibitory influences on the proprioceptive sensory-motor circuit and the CST spinal circuitry
The anatomical origins of CST projections from somatosensory cortex to the spinal cord dorsal horn are likely involved in the modulation of proprioceptive inputs generated by movement control through presynaptic inhibitory mechanisms [3, 13, 14, 22, 34]. The dorsal horn, an important region of presynaptic inhibition, has been shown to possess a diverse array of inhibitory interneurons populations that receive inputs from the CST [35, 36]. One class of inhibitory interneurons that are connected to CST circuits are the GABAergic interneurons, termed GABApre, which modulate the proprioceptive sensory-motor reflex circuit (Figure 1F, 1F’) [7**, 37, 38*]. Last-order GABAergic interneurons form presynaptic inhibitory synapses on proprioceptive afferent terminals and produce what is known as primary afferent depolarization (PAD), modulating proprioceptive control and shaping motoneuron responses [13, 22, 39–41].
A study by Fink and colleagues [42] showed that these GABApre interneurons play an important role in smooth execution of skilled forelimb movement by activating PAD and resulting in presynaptic inhibition of proprioceptive feedback in the spinal cord (Figure 1E, 1E’). This presynaptic inhibitory system with PAD interneurons can be evoked by proprioceptive and local inputs, but can also be recruited by the CST following stimulation of sensorimotor cortex, suggesting a crucial role for appropriate skilled motor control [22, 43–45]. Furthermore, CST axons can depress PAD, switching off presynaptic inhibition via inhibitory interneurons which inactivate first-order PAD interneurons, thus enhancing Ia afferents (Figure 1F) [46, 47].
It has also been shown that glutamate retrograde signals serve to regulate the inhibitory/excitatory signaling across the proprioceptive sensory-motor reflex circuit [48]. The CST system, which is glutamatergic, can release glutamate (i.e. VGlut1 and/or VGlut2) and regulate GABApre interneurons [19, 49, 50]. Additionally, CST axons could exert presynaptic inhibition on Clarkes column dSC neurons by the recruitment of GABApre interneurons [28*]. Corticospinal tract access to the presynaptic inhibitory system therefore enables an additional level of sensorimotor integration by selectively controlling proprioceptive information, sculpting motor neuron output and thus facilitating proper control of motor movement.
4. The dorsal column nuclei complex: an integration hub for processing ascending proprioceptive and descending sensorimotor CST information.
In addition to sensorimotor processing during active movement at the spinal cord level, the brainstem dorsal column nuclei complex (DCN-complex) provides further integration between the corticospinal (CS) neurons and proprioceptive inputs (Figure 2A) [15, 16]. The DCN complex which is recognized as a relay processing center for diverse ascending mechanosensory information, send projection targets throughout the brain and spinal cord, and also receive descending modulatory inputs from the CS neurons in sensorimotor cortex [51–53]. Previous work in primates and cat have shown that CS neurons originating in lamina V of primary motor cortex [(M1); area 4] and area 3a, send projecting axons to cervical spinal cord, as well as collaterals towards the proprioceptive-dominated regions of the rostral and ventral portion of the DCN (Figure 2A) [51, 54–59]. These parallel CST influences onto cervical neuron populations and the DCN level suggest an important role for skilled forelimb movement control [15]. Indeed, lesion experiments of the DCN in monkeys revealed deficits in skilled forelimb performance with limited defects in hindlimb function [60].
Figure 2.
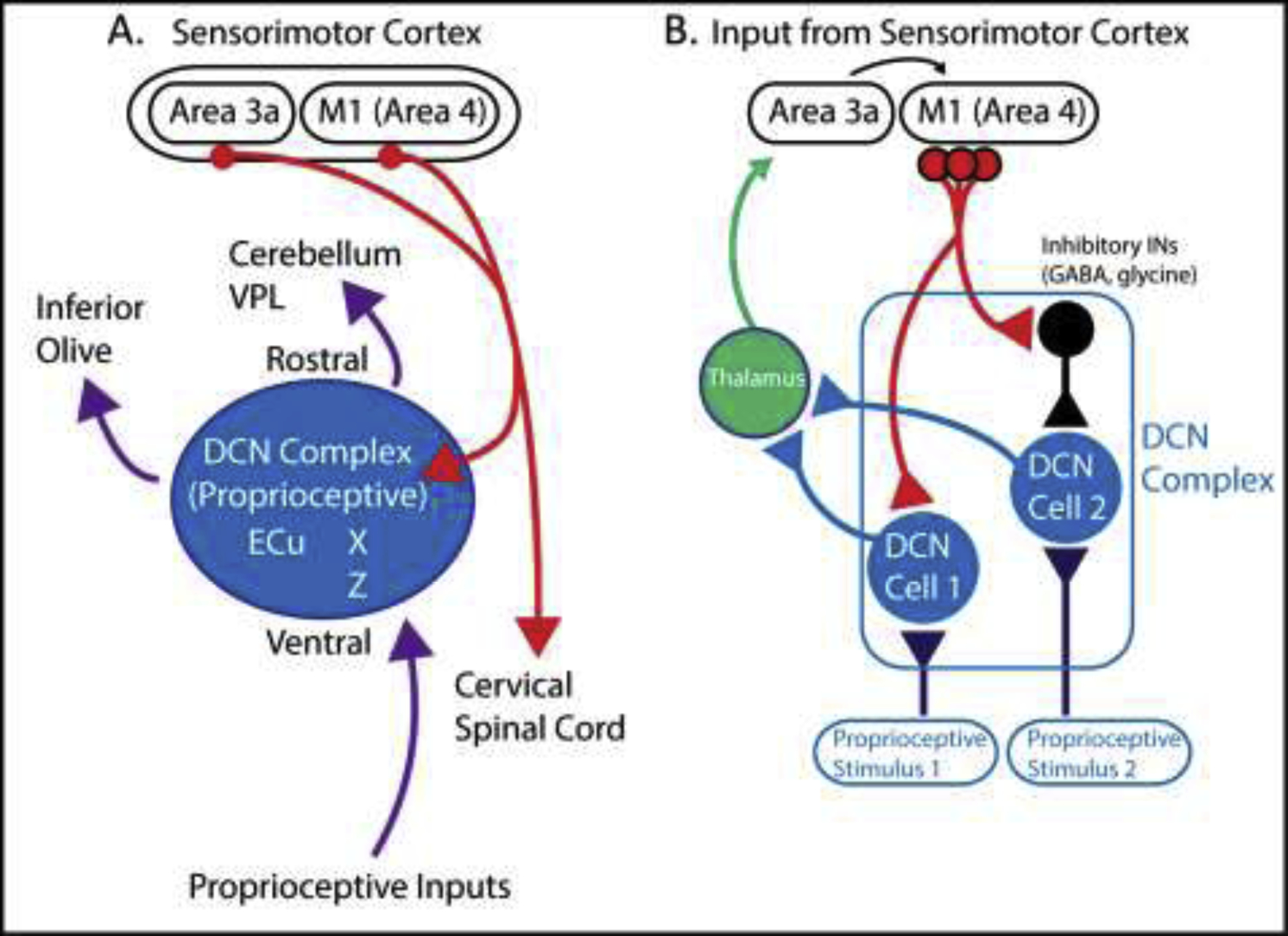
The dorsal column nuclei complex: an integration center for processing ascending proprioceptive information and descending sensorimotor corticospinal inputs. (A) Schematic diagram showing corticospinal (CS) neurons (red) originating in M1 (area 4) and area 3a sending axon collaterals towards proprioceptive regions (rostral and ventral portion) of the dorsal column nuclei complex (DCN) (blue) as well as cervical spinal cord (see Figure 1) [51–59]. Furthermore, purple arrows shows proprioceptive inputs to the DCN complex (blue) and proprioceptive outputs to the cerebellum, inferior olive and ventral posterior lateral (VPL) nucleus of the thalamus.
(B) CS inputs (red) make both direct excitatory (i.e. glutamatergic) and/or indirect post inhibitory effects through inhibitory INs (i.e. GABA & glycine) (black) on DCN cells (blue) within the DCN complex [15, 16, 61–63]. CST inputs enhance relevant proprioceptive stimuli through excitatory interneurons and inhibit non-relevant proprioceptive stimuli via inhibitory interneurons [64*]. Furthermore, ascending proprioceptive information (dark blue) processed in the DCN, are relayed to thalamus (green), and modulated by cortical feedback from proprioceptive cortex (area 3a) and M1 (area 4).
VPL – ventral posterior lateral nucleus of the thalamus; X - nucleus X; Z – nucleus Z; ECu – External cuneate nuclei; DCN – dorsal column nuclei; M1 – primary motor cortex
Corticospinal inputs from sensorimotor cortex onto the DCN-complex also induce both direct excitatory (i.e. glutamatergic) effects, and indirect post inhibitory effects (i.e. GABAergic), via inhibitory INs within the DCN-complex (Figure 2B) [61–63]. For example, in cat, ascending proprioceptive input processed in the DCN and relayed to the thalamus, are modulated by cortical feedback from area 3a and M1 [64*]. Descending CST collaterals exert an excitatory influence directly onto DCN neurons that process relevant muscular inputs (i.e. the same joint), and at the same time will activate GABA/glycine INs, thus inhibiting DCN cells that receive non-relevant muscle inputs (i.e. from different joints) (Figure 2B) [64*]. This suggests that inputs from sensorimotor cortex to the DCN complex plays an important role in “selecting” the relevant and non-relevant ascending proprioceptive information during active movements. This relatively ubiquitous gating mechanism is also observed in other sensory systems, such as in layer 6 corticothalamic cells, which regulate sensory processing by modulating thalamic neuron activity [65]. For an extensive review on the DCN complex, see Loutit, Vickery & Potas, 2020 [16].
5. Conclusion
Converging influences between proprioceptive inputs and descending corticospinal tract projections are critical for skilled movement control. Proprioceptive feedback relayed to the spinal cord, the brainstem DCN complex and cortical motor centers, can engage the CST system through interneuron populations for feedforward processing of proprioceptive stimuli during active movements. At the spinal cord level, CST connection patterns onto excitatory and inhibitory interneuron populations in the dorsal horn modulate proprioceptive feedback signals at the local circuit level, as well as ascending projections to cortex for the coordination of skilled movements. CST regulation of PAD through GABApre interneurons also serves to selectively control for desired or disruptive proprioceptive sensory input. Furthermore, descending CST axon collaterals from M1 and 3a on the DCN complex, can discriminate between appropriate proprioceptive stimuli, by inhibiting non-relevant DCN cells and by selectively enhancing relevant DCN cells via cortical feedback. Thus, regulation of proprioceptive feedback is fundamental to sensorimotor processing where the diversity of integration at various levels of the central nervous system enables a wide repertoire of skilled motor behaviors. The gradual enhancement of powerful genetic tools and the exceptional engineering of targeted silencing or activation of neuron cell types, will pave the way for future insights in the converging integration of proprioceptive and descending motor pathways underlying skilled movement control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
conflict - review
The authors have no competing interests to declare.
References
- 1.Tuthill JC and Azim E, Proprioception. Curr Biol, 2018. 28(5): p. R194–R203. [DOI] [PubMed] [Google Scholar]
- 2.Azim E and Seki K, Gain control in the sensorimotor system. Curr Opin Physiol, 2019. 8: p. 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemon RN, Descending pathways in motor control. Annu Rev Neurosci, 2008. 31: p. 195–218. [DOI] [PubMed] [Google Scholar]
- 4.Lemon RN and Griffiths J, Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve, 2005. 32(3): p. 261–79. [DOI] [PubMed] [Google Scholar]
- 5.Lemon R, Recent advances in our understanding of the primate corticospinal system. F1000Res, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alstermark B and Isa T, Circuits for skilled reaching and grasping. Annu Rev Neurosci, 2012. 35: p. 559–78. [DOI] [PubMed] [Google Scholar]
- 7.**.Ueno M, et al. , Corticospinal Circuits from the Sensory and Motor Cortices Differentially Regulate Skilled Movements through Distinct Spinal Interneurons. Cell Rep, 2018. 23(5): p. 1286–1300 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study generated a detailed a connectivity map between corticospinal neurons in sensory and motor cortex and spinal interneurons (i.e. Chx10, Isl1, dSC, GABApre) using various mouse genetic, tracing and viral techniques. Notably inhibiting Chx10 resulted in impairements in skilled reaching.
- 8.Bareyre FM, Haudenschild B, and Schwab ME, Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. J Neurosci, 2002. 22(16): p. 7097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armand J, et al. , Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. J Neurosci, 1997. 17(1): p. 251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulter JD and Jones EG, Differential distribution of corticospinal projections from individual cytoarchitectonic fields in the monkey. Brain Res, 1977. 129(2): p. 335–40. [DOI] [PubMed] [Google Scholar]
- 11.Martin JH, Differential spinal projections from the forelimb areas of the rostral and caudal subregions of primary motor cortex in the cat. Exp Brain Res, 1996. 108(2): p. 191–205. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, et al. , Deconstruction of Corticospinal Circuits for Goal-Directed Motor Skills. Cell, 2017. 171(2): p. 440–455 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canedo A, Primary motor cortex influences on the descending and ascending systems. Prog Neurobiol, 1997. 51(3): p. 287–335. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Lopez Y, et al. , Sensorimotor Integration by Corticospinal System. Front Neuroanat, 2016. 10: p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino J, Martinez L, and Canedo A, Sensorimotor Integration at the Dorsal Column Nuclei. News Physiol Sci, 1999. 14: p. 231–237. [DOI] [PubMed] [Google Scholar]
- 16.Loutit AJ, Vickery RM, and Potas JR, Functional organization and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub. J Comp Neurol, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Brinkman J and Kuypers HG, Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain, 1973. 96(4): p. 653–74. [DOI] [PubMed] [Google Scholar]
- 18.Martin JH, The corticospinal system: from development to motor control. Neuroscientist, 2005. 11(2): p. 161–73. [DOI] [PubMed] [Google Scholar]
- 19.Porter R, Lemon R, and Physiological Society (Great Britain), Corticospinal function and voluntary movement. Monographs of the Physiological Society. 1993, Oxford: Clarendon Press. xvii, 428 p. [Google Scholar]
- 20.Yoshida Y and Isa T, Neural and genetic basis of dexterous hand movements. Curr Opin Neurobiol, 2018. 52: p. 25–32. [DOI] [PubMed] [Google Scholar]
- 21.Alaynick WA, Jessell TM, and Pfaff SL, SnapShot: spinal cord development. Cell, 2011. 146(1): p. 178–178 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudomin P and Schmidt RF, Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res, 1999. 129(1): p. 1–37. [DOI] [PubMed] [Google Scholar]
- 23.Azim E, et al. , Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature, 2014. 508(7496): p. 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.*.Levine AJ, et al. , Identification of a cellular node for motor control pathways. Nat Neurosci, 2014. 17(4): p. 586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterized a population of motor synergy encoder (MSE) spinal neurons that may represent a central node for voluntary and reflexive movement. They showed Tcfap2β and Sat1/2 premotor neurons which receive inputs from proprioceptive sensory and CST inputs, may encode for motoneuron activity for motor synergy programs.
- 25.Yuengert R, et al. , Origin of a Non-Clarke’s Column Division of the Dorsal Spinocerebellar Tract and the Role of Caudal Proprioceptive Neurons in Motor Function. Cell Rep, 2015. 13(6): p. 1258–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui TV, et al. , Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron, 2013. 78(1): p. 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paixao S, et al. , Identification of Spinal Neurons Contributing to the Dorsal Column Projection Mediating Fine Touch and Corrective Motor Movements. Neuron, 2019. 104(4): p. 749–764 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.*.Hantman AW and Jessell TM, Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nature Neuroscience, 2010. 13(10): p. 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that the dorsal spinocerebellar (dSC) neurons, known as Clarkes column dSC neurons, receive direct excitatory and/or indirect inhibitory inputs from descending CST axons with the capacity to modulate proprioceptive input. Clarkes column neurons relay proprioceptive information to the cerebellum for anticipating sensory feedback.
- 29.Renshaw B, Central effects of centripetal impulses in axons of spinal ventral roots. J Neurophysiol, 1946. 9: p. 191–204. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez FJ and Fyffe RE, The continuing case for the Renshaw cell. J Physiol, 2007. 584(Pt 1): p. 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzocchio R, Rossi A, and Rothwell JC, Depression of Renshaw recurrent inhibition by activation of corticospinal fibres in human upper and lower limb. J Physiol, 1994. 481 (Pt 2): p. 487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Acunzo P, et al. , A conditional transgenic reporter of presynaptic terminals reveals novel features of the mouse corticospinal tract. Front Neuroanat, 2014. 7: p. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mentis GZ, et al. , Primary afferent synapses on developing and adult Renshaw cells. J Neurosci, 2006. 26(51): p. 13297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolpert DM, Ghahramani Z, and Flanagan JR, Perspectives and problems in motor learning. Trends Cogn Sci, 2001. 5(11): p. 487–494. [DOI] [PubMed] [Google Scholar]
- 35.Abraira VE, et al. , The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell, 2017. 168(1–2): p. 295–310 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepien AE, Tripodi M, and Arber S, Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron, 2010. 68(3): p. 456–72. [DOI] [PubMed] [Google Scholar]
- 37.Russ JB, et al. , Corticospinal tract insult alters GABAergic circuitry in the mammalian spinal cord. Front Neural Circuits, 2013. 7: p. 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.*.Betley JN, et al. , Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell, 2009. 139(1): p. 161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed the connectivity and synaptic differentiation of GABAergic interneurons that mediate presynaptic inhibition on their sensory targets. They showed that GABApre interneurons that form axo-axonic contacts with proprioceptive sensory terminals express the GABA synthesizing enzyme, GAD2 (also known as GAD65).
- 39.Eccles JC, Kostyuk PG, and Schmidt RF, Central pathways responsible for depolarization of primary afferent fibres. J Physiol, 1962. 161: p. 237–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eccles JC, Kostyuk PG, and Schmidt RF, The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. J Physiol, 1962. 162: p. 138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lidierth M and Wall PD, Dorsal horn cells connected to the lissauer tract and their relation to the dorsal root potential in the rat. J Neurophysiol, 1998. 80(2): p. 667–79. [DOI] [PubMed] [Google Scholar]
- 42.Fink AJ, et al. , Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature, 2014. 509(7498): p. 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter D, Lundberg A, and Norrsell U, Primary Afferent Depolarization Evoked from the Sensorimotor Cortex. Acta Physiol Scand, 1963. 59: p. 126–42. [DOI] [PubMed] [Google Scholar]
- 44.Eccles JC, Eccles RM, and Magni F, Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol, 1961. 159: p. 147–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundberg A, Supraspinal Control of Transmission in Reflex Paths to Motoneurones and Primary Afferents. Prog Brain Res, 1964. 12: p. 197–221. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg A and Vyklicky L, Inhibitory Interaction between Spinal Reflexes to Primary Afferents. Experientia, 1963. 19(5): p. 247–&. [DOI] [PubMed] [Google Scholar]
- 47.Rudomin P, et al. , Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol, 1983. 50(4): p. 743–69. [DOI] [PubMed] [Google Scholar]
- 48.Mende M, et al. , Sensory-Derived Glutamate Regulates Presynaptic Inhibitory Terminals in Mouse Spinal Cord. Neuron, 2016. 90(6): p. 1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giuffrida R and Rustioni A, Glutamate and aspartate immunoreactivity in corticospinal neurons of rats. J Comp Neurol, 1989. 288(1): p. 154–64. [DOI] [PubMed] [Google Scholar]
- 50.Persson S, et al. , Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J Comp Neurol, 2006. 497(5): p. 683–701. [DOI] [PubMed] [Google Scholar]
- 51.Kuypers HG and Tuerk JD, The Distribution of the Cortical Fibres within the Nuclei Cuneatus and Gracilis in the Cat. J Anat, 1964. 98: p. 143–62. [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez L, Lamas JA, and Canedo A, Pyramidal tract and corticospinal neurons with branching axons to the dorsal column nuclei of the cat. Neuroscience, 1995. 68(1): p. 195–206. [DOI] [PubMed] [Google Scholar]
- 53.Rustioni A and Hayes NL, Corticospinal tract collaterals to the dorsal column nuclei of cats. An anatomical single and double retrograde tracer study. Exp Brain Res, 1981. 43(3–4): p. 237–45. [DOI] [PubMed] [Google Scholar]
- 54.Cheema S, Rustioni A, and Whitsel BL, Sensorimotor cortical projections to the primate cuneate nucleus. J Comp Neurol, 1985. 240(2): p. 196–211. [DOI] [PubMed] [Google Scholar]
- 55.Bentivoglio M and Rustioni A, Corticospinal neurons with branching axons to the dorsal column nuclei in the monkey. J Comp Neurol, 1986. 253(2): p. 260–76. [DOI] [PubMed] [Google Scholar]
- 56.Cheema S, Whitsel BL, and Rustioni A, The corticocuneate pathway in the cat: relations among terminal distribution patterns, cytoarchitecture, and single neuron functional properties. Somatosens Res, 1983. 1(2): p. 169–205. [DOI] [PubMed] [Google Scholar]
- 57.Gordon G and Jukes MG, Descending Influences on the Exteroceptive Organizations of the Cat’s Gracile Nucleus. J Physiol, 1964. 173: p. 291–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuypers HG, An anatomical analysis of cortico-bulbar connexions to the pons and lower brain stem in the cat. J Anat, 1958. 92(2): p. 198–218. [PMC free article] [PubMed] [Google Scholar]
- 59.Kuypers HG, Hoffman AL, and Beasley RM, Distribution of cortical “feedback” fibers in the nuclei cuneatus and gracilis. Proc Soc Exp Biol Med, 1961. 108: p. 634–7. [DOI] [PubMed] [Google Scholar]
- 60.Vierck CJ Jr., Comparison of forelimb and hindlimb motor deficits following dorsal column section in monkeys. Brain Res, 1978. 146(2): p. 279–94. [DOI] [PubMed] [Google Scholar]
- 61.Aguilar J, et al. , New corticocuneate cellular mechanisms underlying the modulation of cutaneous ascending transmission in anesthetized cats. J Neurophysiol, 2003. 89(6): p. 3328–39. [DOI] [PubMed] [Google Scholar]
- 62.Andersen P, et al. , Mechanisms of Synaptic Transmission in the Cuneate Nucleus. J Neurophysiol, 1964. 27: p. 1096–116. [DOI] [PubMed] [Google Scholar]
- 63.Canedo A, Martinez L, and Marino J, Tonic and bursting activity in the cuneate nucleus of the chloralose-anesthetized cat. Neuroscience, 1998. 84(2): p. 603–17. [DOI] [PubMed] [Google Scholar]
- 64.*.Leiras R, et al. , Processing afferent proprioceptive information at the main cuneate nucleus of anesthetized cats. J Neurosci, 2010. 30(46): p. 15383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that in the cat, ascending proprioceptive input to the dorsal column nuclei (DCN) complex and relayed to the thalamus, are modulated by cortical feedback from area 3a and M1. Descending corticospinal collaterals form direct excitatory and/or inhibitory connections onto DCN complex neurons, amplifying relevant proprioceptive stimuli or inhibiting non-relevant stimuli during movement.
- 65.Crandall SR, Cruikshank SJ, and Connors BW, A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron, 2015. 86(3): p. 768–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


