Abstract
The antimicrobial efficacy of antiseptics used in wound management is tested in vitro under standardised conditions according to DIN EN 13727, with albumin and sheep erythrocytes used as organic challenge. However, these testing conditions do not adequately simulate the wound bed environment. Thus, the aim of this study was to compare the efficacy of different antiseptics such as octenidine dihydrochloride (OCT), chlorhexidine digluconate (CHX), polyhexamethylene biguanide (PHMB), and povidone‐iodine under challenge with human wound exudate instead of standardised organic load in an in vitro setting according to DIN EN 13727. Moreover, protein contents, pH, and temperature were compared with standardised testing conditions. The tested antiseptic agents were reduced to different extents based on their bactericidal efficacy, when challenged with human wound exudate compared with standardised conditions. Overall, 0.10% OCT showed the highest effects reaching full efficacy after 30 seconds. CHX and PHMB were the least efficient. Next to the protein content, other components of wound exudate, such as the microflora, seem to influence the efficacy of antiseptics. In summary, the optimisation of in vitro testing conditions in future applications, to more adequately simulate the wound bed environment, will allow a more realistic picture on the potential performance of antiseptics in clinical practice.
Keywords: antiseptics, chronic wounds, DIN EN 13727, organic challenge, wound exudate
1. INTRODUCTION
Wounds are often infected by a variety of different pathogens, and antiseptics are frequently used to reduce this bioburden. Specifically chronic wounds are often colonised by a pool of different pathogens, promoting the formation of drug‐resistant biofilms and delaying wound healing.1, 2 Furthermore, methicillin‐resistant Staphylococcus aureus (MRSA) have been found to be increasingly present in chronic wounds substantially complicating their management.3, 4 Antimicrobial antiseptics are particularly suitable for eliminating these disturbing factors for wound healing.
Important features of antiseptics include a broad spectrum of activity against various microorganisms and biofilms, immediate onset, long‐lasting biocidal activity, potency in the presence of organic challenges, and a good tolerability.5 Thus, the efficacy of antiseptics is tested under both in vitro and in vivo conditions.
Prior to testing an antiseptic in a clinical setting, in vitro tests are conducted under standardised conditions according to European Standards (EN) or methods by the German Society for Hygiene and Microbiology. Phase 1 of standardised testing according to DIN EN 1040 solely assesses the bactericidal efficacy of an antiseptic agent without the addition of organic challenges.6 However, antiseptics are often confronted with various organic challenges found in the wound bed, such as blood and protein, which can reduce their efficacy.7 Thus, in phase 2 according to DIN EN 13727, organic challenges of the wound bed are simulated by adding albumin and sheep erythrocytes to the test solution.8 However, the standardised test conditions of phase 2 are based on theoretical considerations only.7
In general, wound exudate is characterised by high protein content and consists of many organic components such as platelets, fibrin, and plasma proteins.9 The specific composition of wound exudates varies depending on the state of the wound. Different parameters influence the condition in the wound bed, many of which are still unknown.9, 10, 11 Moreover, compared with the bacterial isolates obtained under conventional culture techniques, a significantly greater bacterial diversity can be expected directly in chronic ulcer wounds.5 Thus, a realistic simulation of clinical conditions is challenging and in vitro tests may not adequately reflect the wound bed environment, in which the antiseptics will be used in routine clinical practice.
Although past studies compared the bactericidal efficacy of different antiseptics using standard tests with and without addition of standardised organic load, this study for the first time assesses the bactericidal efficacy of different antiseptics under more realistic conditions by using human wound exudate as organic challenge in quantitative suspension tests based on DIN EN 13727.7, 12 The aim of this study was to investigate the effect of human wound exudate on the bactericidal efficacy of the antiseptics such as octenidine dihydrochloride (OCT), chlorhexidine digluconate (CHX), polyhexamethylene biguanide (PHMB), and povidone‐iodine (PVP‐I) compared with their performance under standardised testing conditions with albumin and sheep erythrocytes. Moreover, to assess whether the standardised testing conditions according to DIN EN 13727 sufficiently reflect realistic wound conditions, the protein contents in human wound exudates as well as wound pH and temperature were measured and compared with the standardised testing conditions according to DIN EN 13727.
2. MATERIALS AND METHODS
2.1. Patient population
All patients of 18 years or older with chronic leg ulcer (ulcus cruris) who had signed the informed consent form and data privacy statement could be included in the study. Patients with underlying infectious diseases (eg, human immunodeficiency virus [HIV], hepatitis C), malignant neoplasms in the wound area, and pregnant or breastfeeding women were excluded from the study. If the patients were undergoing antibiotic therapy or treatment with topical antimicrobials within 3 days prior to study entry they were also excluded.
2.2. Collection of wound exudate
For the collection of wound exudate, three different methods were applied, depending on the wound and the appropriate techniques of wound management. All wound exudate samples were stored at −20°C until further use or at 6°C, when used on the same day. Prior to further testing, wound exudates were warmed up to room temperature.
2.2.1. 24‐hour‐sponge method
By the first method, samples were taken during regular dressing changes after the sponge (Mepilex [Mölnlycke Health Care GmbH, Düsseldorf, Germany] or Biatain Ibu [Coloplast GmbH, Hamburg, Germany]) had remained on the wound for at least 24 hours. After removal and transfer into a sterile container, the sponge was soaked with maximum 5 ml protease inhibitor solution (cOmplete Protease Inhibitor Cocktail tablets, Roche, Germany). Wound exudates were collected by cutting the sponge into appropriate sizes with sterile scissors and squeezing the sponge with a sterile stainless steel garlic press or potato press depending on dressing size.
2.2.2. Ultrasonic suspension method
Ultrasonic‐assisted wound (UAW) treatment was performed for selective wound debridement during regular wound care using a high‐power low‐frequency ultrasound device (Sonoca, Söring, Norderstedt, Germany). The ultrasonic suspension was collected in a kidney dish, aliquoted, and stored until further use.
2.2.3. Vacuum exudate method
Wound exudate was collected during vacuum‐assisted closure (VAC) therapy. The collected vacuum exudate was aliquoted and stored until further use. In addition, wound exudate was also extracted from the sponge, which was applied on the wound surface during VAC therapy, using the 24‐hour sponge method described earlier.
2.3. Determination of wound characteristics
2.3.1. Wound exudate protein content
Wound exudate protein content was determined by using a bicinchoninic acid (BCA) assay (Pierce BCA Protein Assay, Fisher Scientific GmbH, Schwerte, Germany) according to the manufacturers' instructions.
2.3.2. Wound temperature
Wound temperature was measured in the centre of the wound using a digital infrared thermometer (Testo AG, Lenzkirch, Germany) after removal of the dressing.
2.3.3. Wound pH
The pH was measured directly in the wound after dressing removal and in wound exudate prior to quantitative suspension testing using pH indicator strips (Merck, Darmstadt/Germany).
2.4. Total germ count in wound exudates
Serial dilutions of wound exudates (in tryptone salt broth: 1.0 g/L tryptone, 8.5 g/L NaCl, pH 7.0 ± 0.2) were plated on casein peptone soybean flour peptone (CASO) agar (Oxoid Deutschland GmbH, Wesel, Germany) and incubated for 48 to 72 hours at 36 ± 1°C. All visible colony‐forming units (cfu) were counted independent of their species.
2.5. Quantitative suspension tests according to DIN EN 13727:2009
Quantitative suspension tests were performed at room temperature (21.5‐23.4°C) according to the dilution neutralisation method of DIN EN 13727 (5.5.2).8
2.5.1. Comparative tests
Comparative tests were performed according to DIN EN 13727:2009 (5.5.2.2) protocol8 under low burden conditions (clean conditions) with 0.3 g/L bovine serum albumin (bovine serum albumin fraction V [Serva Electrophoresis GmbH, Heidelberg, Germany] diluted in tryptone salt broth [1.0 g/L tryptone, 8.5 g/L NaCl, pH 7.0 ± 0.2]) or high burden conditions (dirty conditions) with 3.0 g/L bovine serum albumin (bovine serum albumin fraction V [Serva Electrophoresis GmbH] diluted in tryptone salt broth) and 3.0 ml/L sheep erythrocytes (Fiebig‐Nährstofftechnik, Idstein‐Niederauroff, Germany) using the test organism Staphylococcus aureus ATCC 33592.13
Control procedures were performed according to DIN EN 13727:2009 (5.5.2.3‐5.5.2.5) as well.8
2.5.2. Testing of wound exudate
After a 2‐minute equilibration of one part wound exudate (containing accompanying flora) and one part methicillin‐resistant Staphylococcus aureus ATCC 3359213 (1.5‐5.0 × 108 cfu/ml cultivated according to DIN EN 12353:2011)14 suspension, the wound exudate test solution was mixed with eight parts of antimicrobial solution (1.25 × final test concentration).
The following final concentrations of antimicrobial solutions were tested:
0.05% OCT (pH 5.8) and 0.10% OCT (pH 5.6)
0.02% PHMB (pH 5.3), 0.04% PHMB (pH 5.2), 0.10% PHMB (pH 5.3) and 0.15% PHMB (pH 5.3)
0.05% CHX (pH 4.5) and 0.20% CHX (pH 5.0) as well as
7.40% PVP‐I (pH 1.8) and 9.70% PVP‐I (pH 1.7)
Aliquots of 1 ml were obtained after 15 and 30 seconds and 1, 2, 5, 15, and 30 minutes of contact time and then mixed with the appropriate amount of neutralisation solution (for OCT, PHMB, and CHX: 30.0 g/L Tween 80, 6.0 g/L lecithin, 60.0 g/L saponin, 1.0 g/L histidine, 1.0 g/L tryptone, 8.5 g/L NaCl; for PVP‐I: 30.0 g/L Tween 80, 6.0 g/L lecithin, 1.0 g/L histidine, 5.0 g/L sodium thiosulfate, 1.0 g/L tryptone, and 8.5 g/L NaCl). After 5 minutes of neutralisation, serial dilutions (100, 10−1, and 10−2) were prepared and 0.5 ml of each dilution was plated on agarose plates (for suspension tests with OCT and PHMB in the dilution 100, neutralisation agar was used: 40.0 g/L CASO agar [Oxoid Deutschland GmbH], 3.0 g/L agar, 30.0 g/L Tween 80, 6.0 g/L lecithin, 60.0 g/L saponin, and 1.0 g/L histidine) and incubated as described earlier. Duplicate plates were made of each dilution level.
The corresponding control procedures were performed according to DIN EN 13727:2009 (5.5.2.3‐5.5.2.5).8 Volumes were adjusted to the amount of collected wound exudate.
2.5.3. Calculation of logarithmic reduction factor
Calculations of logarithmic reduction factors (lg RF) were performed according to DIN EN 13727:20098 using the following formula (lg N0 = number of living cfu/ml at the beginning of contact time; lg Na = number of living cfu/ml at the end of contact time):
2.6. Ethics approval
This clinical study (study number: PV3980) was performed at the German University Medical Center Hamburg‐Eppendorf (Comprehensive Wound Center) and approved by the ethics committee of the General Medical Council Hamburg/Germany. The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. All patients had signed the informed consent form and data privacy statement before study inclusion.
3. RESULTS
3.1. Patient characteristics
A total of 30 patients (10 female, 20 male) with chronic leg ulcers and a mean age of 68 years (41‐90 years) were included in this clinical study at the German University Medical Centre Hamburg‐Eppendorf (Comprehensive Wound Centre) between November 15, 2011, and March 15, 2012.
3.2. Wound characteristics
The assessed wound characteristics (temperature, pH) are displayed in Table 1. In comparison with the laboratory conditions according to DIN EN 13727,8 the mean wound temperature of 32.4°C (±1.5) was higher than the temperature in the standardised testing conditions (20.0°C [±1.0]). No difference could be observed between the pH measured directly in the wound and the pH measured in wound exudate.
Table 1.
Wound characteristics compared with the DIN EN 13727:2009 standard test
| Wound characteristics (N = 28)a | Mean ± SD | Median (min‐max) | DIN EN 13727:2009; mean ± SD |
|---|---|---|---|
| Temperature (°C) | 32.4 ± 1.5 | 32.6 (28.8‐34.9) | 20.0 ± 1.0 |
| Wound pH | 8.1 ± 0.5 | 8.0 (7.0‐9.5) | Not defined |
| Exudate pH | 8.1 ± 0.5 | 8.0 (7.0‐9.5) | Not defined |
Abbreviations: max, maximum; min, minimum.
Two samples of wound exudate existed for patient No. 13, which were collected via 24‐hour sponge method and ultrasonic suspension.
Table 2 summarises the mean protein contents determined in wound exudate samples for each collection method and in total. The lowest mean protein content was observed in wound exudate collected via ultrasonic suspension (0.37 ± 0.55) and the highest protein content was measured in wound exudates collected using the 24 hours‐sponge method (1.85 ± 1.06). For quantitative suspension tests, wound extracts were diluted at 1:10 ratio, thus, in the mean, the final protein content of 0.114% was comparable to the protein content of the in vitro testing conditions according to DIN EN 13727.8
Table 2.
Protein content (%) in different wound exudates derived from different collection methods compared with the DIN EN 13727:2009 standard test
| Method | Protein content (%) | |
|---|---|---|
| Mean ± SD | Median (min‐max) | |
| 24‐hour sponge method (n = 13)a , b | 1.85 ± 1.06 | 2.41 (0.32‐2.89) |
| Ultrasonic suspension (n = 11)a , b | 0.37 ± 0.55 | 0.19 (0.02‐1.96) |
| Vacuum exudate (n = 5)a | 1.00 ± 0.77 | 1.27 (0.08‐1.70) |
| Total | 1.14 ± 1.05 | 0.52 (0.02‐2.89) |
| Standard test: clean conditionsc | 0.03 (NA) | NA |
| Standard test: dirty conditionsc | 0.3 (NA) | NA |
Abbreviations: max, maximum; min, minimum; NA, not applicable.
For suspension tests, exudate was diluted at 1:10 ratio.
Two samples of wound exudate existed for patient No. 13, which were collected via 24‐hour sponge method and ultrasonic suspension.
According to DIN EN 13727:2009.
3.3. Microbial infection of chronic leg ulcers
In total, 39 different bacterial isolates were detected in the 20 chronic wounds analysed for microbial colonisation. The microbial diagnostic results are displayed in Table 3. Staphylococcus species were the most commonly detected bacterial isolates in the wounds assessed. These were found in 60% of all wounds, followed by Proteus species detected in 40% of all wounds. Isolates of the Proteus species were often detected in combination with Pseudomonas species, which were present in 35% of all analysed wounds. Further detected bacterial species include Escherichia coli, Corynebacterium striatum, Streptococcus agalactiae, Acinetobacter species, MRSA, and Enterobacter cloacae.
Table 3.
Microbial diagnostics of bacterial species colonising ulcus cruris
| Bacterial species | No. of isolates (N = 39) |
|---|---|
| Staphylococcus aureus (including MRSA) | 10 (25.7%) |
| Coagulase‐negative staphylococci | 6 (15.4%) |
| Pseudomonas aeruginosa | 5 (12.8%) |
| Proteus mirabilis | 5 (12.8%) |
| Proteus species | 3 (7.7%) |
| Escherichia coli | 3 (7.7%) |
| Pseudomonas species | 2 (5.1%) |
| Corynebacterium striatum | 2 (5.1%) |
| Streptococcus agalactiae | 1 (2.6%) |
| Acinetobacter species | 1 (2.6%) |
| Enterobacter cloacae | 1 (2.6%) |
Abbreviations: MRSA, methicillin‐resistant Staphylococcus aureus.
The total germ count found in wound exudates is summarised in Table 4. With an average germ count of 3.1 × 108 cfu/ml, the chronic wounds in general were highly contaminated with bacteria. The number of cfu varied strongly between the different wounds, ranging from 2.0 × 105 to 3.4 × 109 cfu/ml. For comparison, quantitative suspension tests according to DIN EN 13727:20098 are performed with 1.5 to 5.0 × 108 cfu/ml.
Table 4.
Total germ count (cfu) in wound exudates depending on the collection method and compared with the DIN EN 13727:2009 standard test
| Method | Total germ count (cfu/ml) | |
|---|---|---|
| Mean | Median (min‐max) | |
| 24‐hour sponge method (n = 13) | 5.5 × 108 | 7.5 × 107 (2.0 × 105 to 3.4 × 109) |
| Ultrasonic suspension (n = 11) | 2.4 × 107 | 4.6 × 106 (2.8 × 105 to 1.0 × 108) |
| Vacuum exudate (n = 5) | 2.9 × 108 | 5.8 × 107 (5.0 × 105 to 7.2 × 108) |
| Total (N = 29) | 3.1 × 108 | 2.6 × 107 (2.0 × 105 to 3.4 × 109) |
| DIN EN 13727:2009 | 1.5‐5.0 × 108 | |
Abbreviations: cfu, colony‐forming units; max, maximum; min, minimum.
3.4. Quantitative suspension tests according to DIN EN 13727:2009
The results of the quantitative suspension tests are illustrated in Figure 1. According to DIN EN 13727:2009,8 full bactericidal efficacy is achieved, when the total germ count is reduced by at least five common logarithm levels (lg RF ≥ 5.00). In general, the testing conditions using wound exudate reduced the bactericidal efficacy of all tested antiseptic agents, when compared with their efficacy under standardised testing conditions according to DIN EN 13727:2009.8 Nevertheless, the extent of this reduction strongly differed between the tested antiseptic agents.
Figure 1.
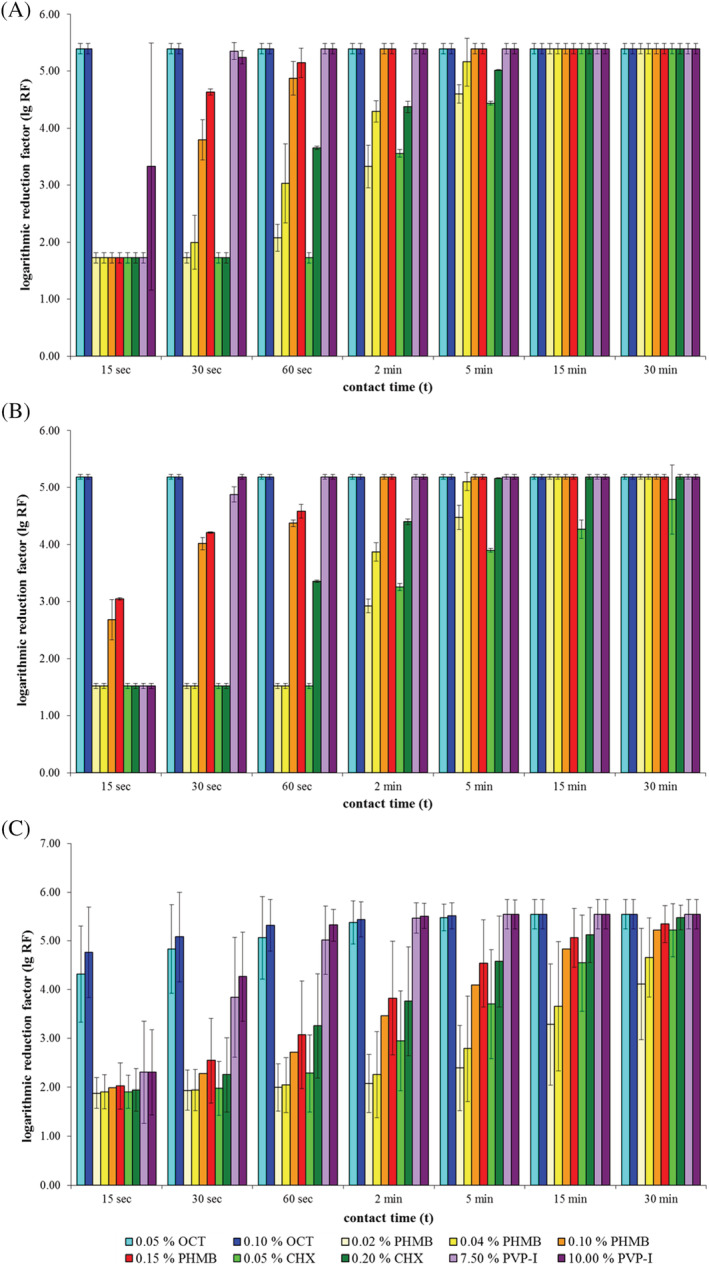
Results of quantitative suspension tests according to DIN EN 13727 under low burden conditions with 0.03% albumin (A), high burden conditions with 0.3% albumin and 0.3% sheep erythrocytes (B), and practical conditions using wound exudate (C)
Overall, OCT solutions exhibited the best bactericidal efficacy under challenge with wound exudate, reaching full efficacy after 30 seconds (OCT 0.10%) and 60 seconds (OCT 0.05%), respectively. PVP‐I solutions reached full bactericidal efficacy after 60 seconds of exposure time. CHX and PHMB solutions were the least efficient in the practical test, requiring much longer exposure times than OCT and PVP‐I. CHX solutions required an exposure time of 15 minutes (0.20%) and 30 minutes (0.05%), respectively. The highest concentration of PHMB (0.15%) required an exposure time of 15 minutes in order to reach full bactericidal efficacy according to DIN EN 13727:2009.8 The less concentrated PHMB solutions (0.02% and 0.04%) did not reach sufficient bactericidal efficacy within the maximum tested exposure time of 30 minutes.
4. DISCUSSION
Standardised in vitro tests according to DIN EN 13727 use albumin and sheep erythrocytes to simulate wound bed conditions.7, 8 However, these testing conditions are based on theoretical considerations and do not necessarily reflect the clinical reality. For the first time, the bactericidal efficacy of different antiseptics was compared under more realistic testing conditions by using human wound exudate as organic challenge in tests based on DIN EN 13727 protocol.8 In this way, in vitro test results may enable more accurate predictions on the potential performance of an antiseptic agent in clinical practice. In the present study, all antiseptic agents exhibited reduced bactericidal efficacy, when compared with their efficacy under standardised testing conditions with albumin and sheep erythrocytes. These effects were the most obvious under shorter exposure times.
As demonstrated in previous studies, the presence of organic load generally reduces the efficacy of antiseptics.7, 15, 16, 17, 18, 19 Similar results were observed in the present study, when comparing the bactericidal efficacy under low and high burden conditions, with OCT exhibiting the most efficient biocidal efficacy, followed by PVP‐I. Interestingly, despite the fact that wound exudates contained on average less protein than the high burden test solutions, the tested antiseptic agents were less efficient when challenged with human wound exudate (see Table 2). In case of OCT, the higher protein content under high burden conditions had little effect on the biocidal efficacy of the antiseptic. OCT remained the most efficient antiseptic when tested with human wound exudate; nonetheless, its efficacy was reduced and longer exposure times were necessary. Thus, the protein content in wound exudate alone does not seem to be the determining factor responsible for the reduction of the bactericidal efficacy. Perhaps, not the protein concentration alone but also the organic composition of the wound exudate may have also influenced the performance of the antiseptics. Further studies testing different wound exudate components will be necessary to address this question.
Another influencing factor may have been the accompanying bacterial flora found in wound exudates. In addition to the MRSA test strain, human wound exudate test solutions contained a highly diverse bacterial flora, which in some samples exceeded the required total germ count of standardised tests. Thus, the presence of a diverse bacterial flora and a high bioburden may have been the causal factor that made longer exposure time necessary.20 To date, standardised tests require the assessment of bactericidal efficacy using specific bacterial strains, rather than a mixture of different species. In future applications, it may be reasonable to use a mixture of different bacterial strains that are potentially found in chronic wounds.
A comparison between the standard test temperature and actual wound temperatures revealed a difference of more than 12°C. The measured wound temperatures are comparable to the mean wound temperature determined in a study by McGuiness et al.21 The factor temperature has been shown to affect both the activity of antiseptics and wound healing. Although the efficacy of antiseptics increases at higher temperatures, optimal wound healing is achieved between 36 and 38°C.21, 22 Thus, to realistically simulate wound bed conditions in standard tests, it may be reasonable to increase the test temperature to mean wound temperatures.
Furthermore, evidence exists that the pH is an important factor during the healing process of wounds.23 Different pH ranges seem to be required for different phases of wound healing.11 A reduction of wound pH has been observed in the course of the healing process, while chronic wounds seem to be in a state of alkalinity.24, 25 The mean pH level of 8.1 (± 0.5) measured in this study is in line with these observations. To date, standardised tests according to DIN EN 13727 do not require a specific pH in the test solution.8 Wiegand et al could demonstrate that the pH can influence the bactericidal efficacy of some antiseptics; furthermore, the efficacy under different pH levels is also dependent on the bacterial species.26 These observations together with the increasing evidence on the importance of pH during wound healing support the recommendation to consider the performance of antiseptics at different pH levels in future applications.
In conclusion, in vivo conditions are simulated more realistically using human wound exudates instead of standardised organic load as challenges. Next to the proteins found in wound exudate, other components such as the diverse microbial flora seem to influence the antimicrobial activity of antiseptics. However, further studies are necessary to specifically identify the responsible factors. In addition, the present study demonstrates that standardised in vitro test conditions only partly reflect actual wound bed conditions. For future applications of antiseptic agents, it is recommendable to take the environment into account, in which it will be used. In this way, the biocidal efficacy of antiseptics can be assessed in in vitro settings that resemble clinical conditions as closely as possible and enable more reliable predictions on their performance in daily routine wound management.
CONFLICT OF INTEREST
N.R. and P. G.‐B. are employees of Schülke & Mayr GmbH. The authors declare no further conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank Veronika Schneck, PhD, and Andrea Rathmann‐Schmitz, PhD (Bonn, Germany), for their support in the preparation and critical review of the manuscript. The study was funded by Schülke & Mayr GmbH.
Radischat N, Augustin M, Herberger K, Wille A, Goroncy‐Bermes P. Influence of human wound exudate on the bactericidal efficacy of antiseptic agents in quantitative suspension tests on the basis of European Standards (DIN EN 13727). Int Wound J. 2020;17:781–789. 10.1111/iwj.13336
Funding information Schülke & Mayr GmbH
REFERENCES
- 1. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4(9):560‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dissemond J, Korber A, Lehnen M, Grabbe S. Methicillin‐resistenter Staphylococcus aureus (MRSA) in chronischen Wunden: Therapeutische Optionen und Perspektiven. Methicillin‐resistant Staphylococcus aureus (MRSA) in chronic wounds: therapeutic options and perspectives. J Dtsch Dermatol Ges. 2005;3(4):256‐262. [DOI] [PubMed] [Google Scholar]
- 4. Dissemond J, Schmid EN, Esser S, Witthoff M, Goos M. Bacterial colonization of chronic wounds. Studies on outpatients in a university dermatology clinic with special consideration of ORSA. Hautarzt. 2004;55(3):280‐288. [DOI] [PubMed] [Google Scholar]
- 5. Daeschlein G. Antimicrobial and antiseptic strategies in wound management. Int Wound J. 2013;10(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DIN Deutsches Institut für Normung e.V . DIN EN 1040: Chemische Desinfektionsmittel und Antiseptika—Quantitativer Suspensionsversuch zur Bestimmung der bakteriziden Wirkung (Basistest) chemischer Desinfektionsmittel und Antiseptika—Prüfverfahren und Anforderungen (Phase 1); Deutsche Fassung EN 1040:2005. Berlin, Germany: Beuth Verlag GmbH; 2005. [Google Scholar]
- 7. Pitten FA, Werner HP, Kramer A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J Hosp Infect. 2003;55(2):108‐115. [DOI] [PubMed] [Google Scholar]
- 8. DIN Deutsches Institut für Normung e.V . DIN prEN 13727: Chemische Desinfektionsmittel und Antiseptika—Quantitativer Suspensionsversuch zur Bestimmung der bakteriziden Wirkung im humanmedizinischen Bereich—Prüfverfahren und Anforderungen (Phase 2, Stufe 1). Deutsche Fassung DIN prEN 13727:2009. Berlin, Germany: Beuth Verlag GmbH; 2009. [Google Scholar]
- 9. Cutting KF. Wound exudate: composition and functions. Br J Community Nurs. 2003;8(Suppl. 3):S4‐S9. [DOI] [PubMed] [Google Scholar]
- 10. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound‐healing: a new perspective for wound‐therapy? Arch Dermatol Res. 2007;298(9):413‐420. [DOI] [PubMed] [Google Scholar]
- 12. Koburger T, Hubner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP‐iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother. 2010;65(8):1712‐1719. [DOI] [PubMed] [Google Scholar]
- 13. Qadri SM, Ueno Y, Imambaccus H, Almodovar E. Rapid detection of methicillin‐resistant Staphylococcus aureus by crystal MRSA ID system. J Clin Microbiol. 1994;32(7):1830‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DIN Deutsches Institut für Normung e.V . DIN prEN 12353: Chemische Desinfektionsmittel und Antiseptika—Aufbewahrung von Testorganismen für die Prüfung der bakteriziden (einschließlich Legionella), mykobakteriziden, sporiziden, fungiziden und viruziden Wirkung. Deutsche Fassung DIN prEN 12353:2011. Berlin, Germany: Beuth Verlag GmbH; 2011. [Google Scholar]
- 15. Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004;17(4):863‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirsch T, Lahmer A, Kapalschinski N, Goertz O, Lehnhardt M, Seipp H‐M. Einfluss von Albumin auf die Wirksamkeit antiseptischer Substanzen. Zeitschrift für Wundheilung. 2012;2012(1):7‐10. [Google Scholar]
- 18. Kapalschinski N, Seipp H‐M, Goertz O, Mikhail B, Kolbenschlag J, Daigeler A, Lehnhardt M, Hirsch T. Antiseptische Therapie bei Brandverletzungen Wirkungsverlust von Polyhexanid gegenüber Staph. aureus und MRSA durch Albumin. Meeting Abstract. In: 31. Jahrestagung der Deutschsprachigen Arbeitsgemeinschaft für Verbrennungsbehandlung. DAV; 2013. Mayrhofen, Austria.
- 19. Kramer A, Widulle H, Assadian O. Vergleichende Charakteristik häufig in Desinfektionsmitteln und Antiseptika eingesetzter Wirkstoffe. In: Kramer A, Assadian O, eds. Wallhäußers Praxis der Sterilisation, Antiseptik und Konservierung. Qualitätssicherung der Hygiene in Industrie, Pharmazie und Medizin. Stuttgart, Germany: Thieme; 2008:624‐631. [Google Scholar]
- 20. Schwebke I. Wirksamkeitsbewertung von Desinfektionsmitteln: Methoden zur Prüfung der Wirksamkeit von Desinfektionsmitteln. In: Fachtagung Zulassung/Registrierung von Biozid‐Produkten: Schwerpunkt Desinfektionsmittel; 2009; Dresden, Germany 2009, 68–78. [Google Scholar]
- 21. McGuiness W, Vella E, Harrison D. Influence of dressing changes on wound temperature. J Wound Care. 2004;13(9):383‐385. [DOI] [PubMed] [Google Scholar]
- 22. Gelinas P, Goulet J, Tastayre GM, Picard GA. Effect of temperature and contact time on the activity of eight disinfectants—a classification. J Food Prot. 1984;47(11):841‐847. [DOI] [PubMed] [Google Scholar]
- 23. Dissemond J, Witthoff M, Brauns TC, Haberer D, Goos M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt. 2003;54(10):959‐965. [DOI] [PubMed] [Google Scholar]
- 24. Jones EM, Cochrane CA, Percival SL. The effect of pH on the extracellular matrix and biofilms. Adv Wound Care (New Rochelle). 2015;4(7):431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gethin G. The significance of surface pH in chronic wounds. Wounds UK. 2007;3(3):52‐56. [Google Scholar]
- 26. Wiegand C, Abel M, Ruth P, Elsner P, Hipler UC. pH influence on antibacterial efficacy of common antiseptic substances. Skin Pharmacol Physiol. 2015;28(3):147‐158. [DOI] [PubMed] [Google Scholar]


