Abstract
Robotic-assisted technology has shown to be promising in coronary and peripheral vascular interventions. Early case reports have also demonstrated its efficacy in neuro-interventions. However, there is no prior report demonstrating use of the robotic-assisted platform for spinal angiography. We report the feasibility of the robotic-assisted thoracic and lumbar spinal angiography.
Keywords: angiography, spine, technology, technique
Case presentation
A patient with a history of follicular lymphoma and renal cell carcinoma was found to have multiple bony metastases including a large disabling lesion centred at the T4 vertebral body. Spinal angiography and preoperative tumour embolisation were planned using the CorPath GRX Robotic System (Corindus, a Siemens Healthineers Company, Waltham, Massachusetts, USA).1 Segmental artery selection from aorta is a part of the current CorPath indications, and the primary operator had completed the phase I training on the robotic system with the fulfilment of five successful clinical cases. The robotic console is positioned outside of the angio-suite. It is equipped with a 26-inch monitor to view biplane live fluoroscopic views, and three joysticks and touchscreen controls. The robotic system is capable of advancing, retracting and rotating the catheter and guidewire separately. It is also capable of deploying an additional rapid-exchange device or a guidewire.
The spinal angiography procedure was performed under general anaesthesia using femoral artery approach. For the manual portion of the procedure, a 5-French groin sheath was inserted in the groin and a 5-French Mickelson catheter was advanced to the descending aorta. The catheter was then connected to a rotating haemostatic valve and a micro guidewire (V-18 ControlWire, Boston Scientific, Marlborough, Massachusetts, USA) was inserted for the subsequent robotic manipulation. The catheter was continuously flushed with heparinised saline and another side port was connected to an extended connection tubing that allowed for contrast injections manually or using a contrast media injector. The robotic arm was then brought into position and the catheter-guidewire combination was loaded into the single-use cassette (figure 1).
Figure 1.
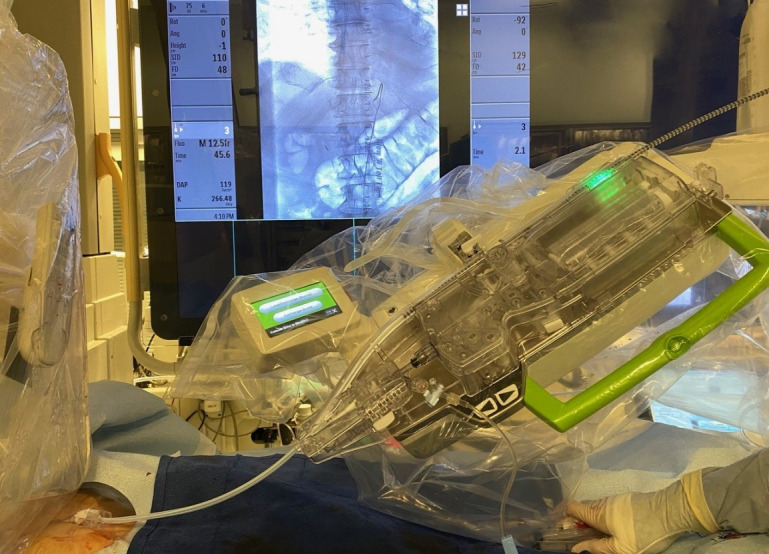
Robotic arm loaded with the catheter-guidewire combination into the single-use cassette.
The primary operator sat behind the control console to manipulate the catheter. At the bedside, the supporting operator monitored flush lines and connection tubings as well as manual contrast injections from the side port as needed. Using the robotic controls, the operator steered the catheter in the aorta followed by selective catheterisation of each segmental artery of the thoracic and lumbar spines. From time to time, the soft micro guidewire was advanced into the segmental artery to confirm the successful engagement instead of a manual puff of contrast media. Anterior spinal arteries were visualised from injections in the left T6, T8 and T9 segmental arteries, and the Artery of Adamkiewicz from the left L1 branch (figure 2). A total of 22 segmental arteries were selected. Once tumour stain was identified from bilateral T3, T4 and T5 segmental arteries, an Echelon 10 microcatheter (Medtronic, Irvine, California, USA) was manually catheterised into those feeders via the 5-French catheter followed by particle and coil embolisations. The total fluoroscopy time of the entire procedure was 59 min. The patient experienced no complications and was unchanged on the day following the procedure.
Figure 2.
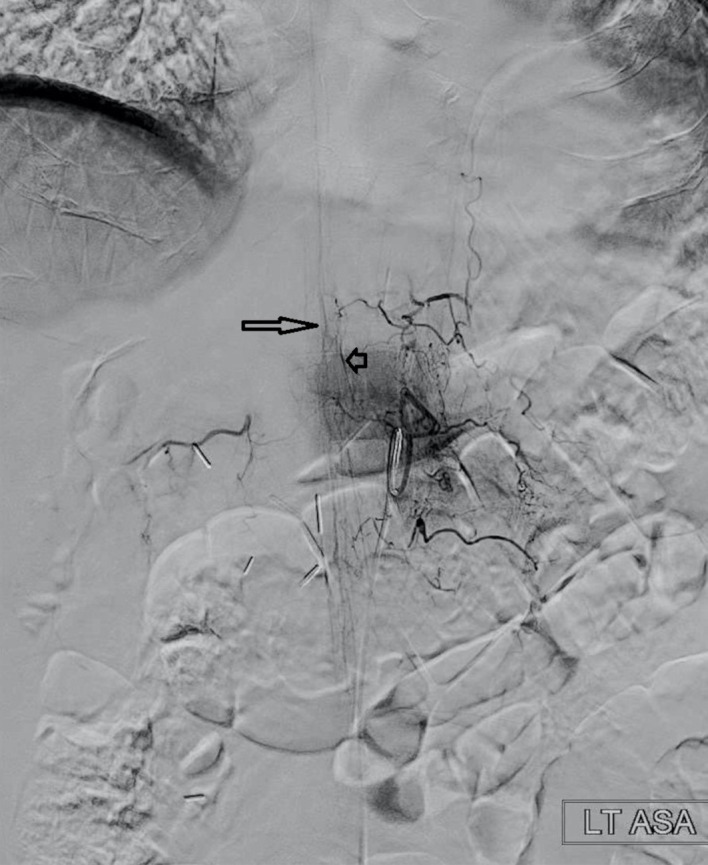
Artery of Adamkiewicz (short arrow) arising from the left L1 branch identified using robotic spinal angiography. The anterior spinal artery is identified with the long arrow.
Discussion
To our knowledge, this is the first report of in-human clinical use of robotic assistance for spinal angiography. While the Corindus robot is designed for coronary and peripheral vascular procedures,2–4 we summarised our initial experience including the technical advantages and challenges to perform robotic spinal angiography. The catheter was primarily manipulated using a push–pull and rotation joystick control solely based on the visual information (figure 3). Particular attention was given to the deflection of the catheter tip in lieu of haptic feedback. Also, we developed the wire-aid guidance (WAG) technique instead of using a short puff of contrast media to confirm the successful catheterisation to a segment artery. The WAG technique uses a soft micro guidewire to be pushed out of the catheter when the interventionalist thinks the catheter tip has engaged a segmental artery. If the catheter tip successfully engages a segmental artery, the guidewire travels straight into it. If not, the guidewire wags downwards or upwards and runs along the wall of the aorta (figure 4A, B). Given the current workflow using the robotic system, the WAG technique makes the overall procedure easier and faster than the established contrast-injection method. It can also further reduce overall contrast load given to the patient. The challenges include lack of haptic feedback, the learning curve and time required to adjust to new system, and the short working length or short range of the robotic arm motion which currently is 20 cm. (Ideally it should be around 40 cm.)
Figure 3.
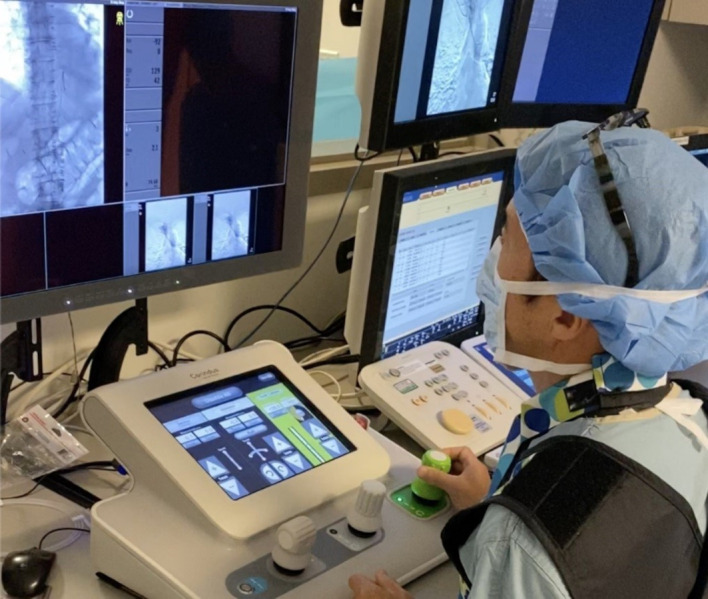
Catheter manipulation using a push–pull and rotation joystick control solely based on the visual information.
Figure 4.
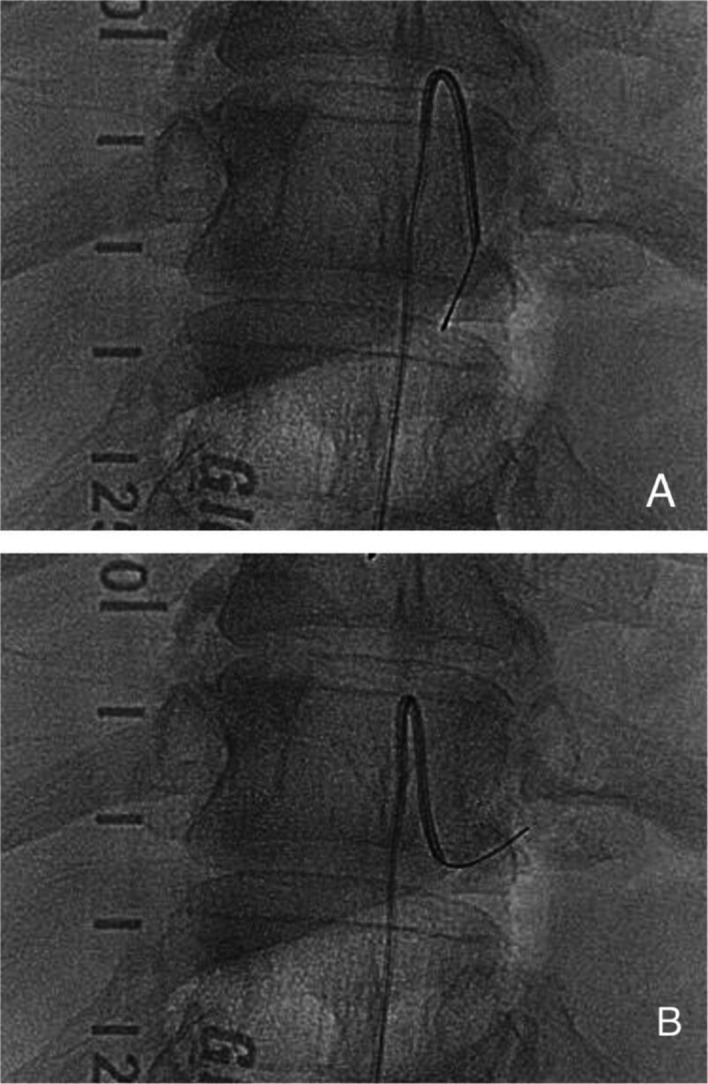
(A) Guidewire wags downwards or upwards and runs along the wall of the aorta, suggesting unsuccessful engagement of the segmental artery. (B) Catheter tip successfully engages a segmental artery with the guidewire travelling straight into it.
Reports on the Corindus robotic system for coronary and peripheral interventions have indicated reduced radiation dose to the operator. Importantly, spinal angiography is a longer procedure in duration over the large body part and close to the operator. Therefore, the use of robotic system is advantageous in reducing the cumulative radiation exposure to the operator. Recent studies have reported feasibility of the robotic system for performing neurointerventional procedures including carotid artery stenting.5–7 Spinal angiography is technically demanding since multiple small arteries have to be catheterised using visual and haptic feedback. Nonetheless, by using the afore-mentioned technique and devices, robotic spinal angiography is feasible. Moreover, the operator is seated more conveniently with easy access to all screens and for better visualisation and better characterisation and documentation of anatomical and radiographic findings without the need to break sterility.8
There are some limitations to consider. First, presence of notable atherosclerotic changes or anatomic variances may add to the complexity of the procedure using robotic approach. Second, the lack of ability to move the angiography table from the control console of the robotic system disrupts the workflow in spinal angiography. Future integration of angio-suite, angiography machine and the robotic system will be a solution.
Conclusion
Our experience supports the efficacy of the current robotic system for precise and fine movements of the catheter for optimal navigation and selection of small segmental arteries for spinal angiography.
Learning points.
Robotic spinal angiography is feasible without haptic feedback.
Visual information such as catheter deflection could compensate the lack of haptic feedback.
Wire-aid guidance technique is useful for segmental artery catheterisation using the robotic system.
Footnotes
Contributors: ST and HS developed the concept and design of the study. ST, HS, GPC, DE and GD contributed to preparation of the manuscript and scientific content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ST: Corindus Vascular Robotics: advisory board, shareholder.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Britz GW, Panesar SS, Falb P, et al. Neuroendovascular-specific engineering modifications to the CorPath GRX robotic system. J Neurosurg 2019:1830–6. 10.3171/2019.9.JNS192113 [DOI] [PubMed] [Google Scholar]
- 2.Weisz G, Metzger DC, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: precise (percutaneous Robotically-Enhanced coronary intervention) study. J Am Coll Cardiol 2013;61:1596–600. 10.1016/j.jacc.2012.12.045 [DOI] [PubMed] [Google Scholar]
- 3.Mahmud E, Schmid F, Kalmar P, et al. Feasibility and safety of robotic peripheral vascular interventions: results of the RAPID trial. JACC Cardiovasc Interv 2016;9:2058–64. 10.1016/j.jcin.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Smilowitz NR, Weisz G. Robotic-assisted angioplasty: current status and future possibilities. Curr Cardiol Rep 2012;14:642–6. 10.1007/s11886-012-0300-z [DOI] [PubMed] [Google Scholar]
- 5.Mendes Pereira V, Cancelliere NM, Nicholson P, et al. First-in-human, robotic-assisted neuroendovascular intervention. J Neurointerv Surg 2020;12:338–40. 10.1136/neurintsurg-2019-015671.rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajja KC, Sweid A, Al Saiegh F, et al. Endovascular robotic: feasibility and proof of principle for diagnostic cerebral angiography and carotid artery stenting. J Neurointerv Surg 2020;12:345–9. 10.1136/neurintsurg-2019-015763 [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Sachdeva R, Al-Bayati AR, et al. Robotic assisted carotid artery stenting for the treatment of symptomatic carotid disease: technical feasibility and preliminary results. J Neurointerv Surg 2020;12:341–4. 10.1136/neurintsurg-2019-015754 [DOI] [PubMed] [Google Scholar]
- 8.Gonen L, Chakravarthi SS, Monroy-Sosa A, et al. Initial experience with a robotically operated video optical telescopic-microscope in cranial neurosurgery: feasibility, safety, and clinical applications. Neurosurg Focus 2017;42:E9. 10.3171/2017.3.FOCUS1712 [DOI] [PubMed] [Google Scholar]


