Abstract
Geriatric population is increasing rapidly worldwide, and fragility fracture and complication following orthopaedic surgery in elderly people have now become major challenges for surgeons. Further studies are required to identify potentially modifiable factors associated with surgical site infection (SSI) in geriatric patients. This retrospective, multicenter study was conducted at four level I hospitals in China. During the 31‐month study period, a total of 2341 patients (65 years or older) underwent orthopaedic surgery and complete data were recorded from September 2015 to April 2018. Demographics information, medications and additional comorbidities, surgery‐related variables, and laboratory indexes were extracted and analysed. Receiver‐operating characteristic analysis was performed to detect the optimum threshold of continuous variables. Independent risk factors of SSI were identified by univariate and multivariate analyses. Finally, 63 patients suffered from wound infection within the follow‐up period, indicating a 2.7% incidence rate of SSI. Statistical results showed that open injury (odds ratio [OR], 9.5; 95% confidence interval [CI], 5.4‐16.7), American Society of Anesthesiologists classified III‐IV score (OR, 2.2; 95% CI, 1.3‐3.8), surgical duration of >132 minutes (OR, 2.9; 95% CI, 1.1‐5.0), serum albumin (ALB) of <36.4 mg/L (OR, 2.0; 95% CI, 1.6‐3.4), and blood glucose (GLU) of >118 mg/dL (OR, 3.1; 95% CI, 1.1‐5.3) were independent risk factors of postoperative SSI. With the application of sensitive and modifiable variables such as surgical duration and the levels of ALB and GLU, more geriatric patients with sub‐high risk of postoperative SSI could be identified.
Keywords: geriatric patients, infection, multivariate analysis, risk factors, traumatic surgery
1. INTRODUCTION
Although there is no clear definition, people older than 65 years are considered to be elderly,1 and the geriatric population is increasing rapidly worldwide.2, 3 In China, individuals aged 65 years or older accounted for 10.4% of the domestic population in 2015, and this proportion has increased to 11.9% by 2018, reaching approximately 166 million. Meanwhile, with the improvement of medical and health conditions and expansion of advanced medical service systems, the average life expectancy of the population increased substantially. The life expectancy at birth for Chinese people has increased more than 5 years in the recent 10 years, and a newborn citizen could expect to live up to the age of 76.3 years in China 2015. The growing elderly population has become the focus of attention in all areas of modern society.
Osteoporosis and fragility fracture, which are frequently encountered in the elderly, should be carefully considered.4, 5 It is reported, the medical expenses for hip fractures in China will reach $60 billion in 2020, and nearly $240 billion will be needed by 2040. Complications following orthopaedic surgery in elderly people have now become major challenges for orthopaedic surgeons. Surgical site infection (SSI), which has been found more prevalent in geriatric surgical patients,6 represents a significant portion of ward infection and is associated with an increased morbidity,7 prolonged hospital stay, and higher health care bills. From an economic perspective, SSI accounted for 17% of nosocomial infections and cost between $1 billion and $10 billion annually.8 However, geriatric people may accompany with some adverse medical comorbidities, and the aged skin and soft tissues are usually fragile and less tolerant of surgical trauma. It has been demonstrated that approximately half of the SSIs can be prevented by application of evidence‐based prevention strategies,9 of which the identification of sensitive risk factors is a cost‐effective method. Prognostic risk factors associated with postoperative SSI included operating time, body mass index (BMI), gender, diabetes mellitus, length of hospital stay, delay in surgery, and so on,10, 11, 12, 13 and were confirmed in published studies. However, most of these predictors were unmodifiable, and there were no clear‐cut values for some continuous variables, such as operative duration, level of serum albumin (ALB), blood glucose (GLU), and intraoperative blood loss. Few studies identified easily remediable factors to reduce infection rates following orthopaedic surgery in geriatric patients, and the reliability for some of those studies may be compromised by the limited including variables for adjustment in a multivariate logistical analysis model.
Given that we have designed this multicenter study, we follow with the aim (a) to describe the characteristics of SSI in elderly patients over a 12‐month follow‐up period at four tertiary hospitals; (b) to detect the optimal cut‐off value for variables, which is associated with postoperative SSI; (c) to identify potentially modifiable factors associated with SSI following orthopaedic surgery in geriatric patients.
2. PATIENTS AND METHODS
2.1. Study design
This retrospective and multicenter study was conducted at four level I hospitals from September 2015 to April 2018. Detailed information extracted from the electronic medical records (EMRs) on each patient (65 years or older) who underwent orthopaedic surgery was collected by investigators. During hospitalisation of patients, we reviewed their electronic EMRs and results of bacterial culture for the signs of wound infection. After being discharged from hospital, all the patients were followed up for any evidence of SSI occurrence via telephone assessment and interview. The exclusion criteria were as follows: (a) periprosthetic fractures; (b) old fractures (>21 days from trauma); (c) osteoarticular tuberculosis; (d) pathological fracture (primary or metastatic tumour); (e) traumatic fracture treatment by conservative methods (skin or skeletal traction, external fixation apparatus, plaster immobilisation, or thermoplastic plate brace); (f) patients admitted only for treatment of SSI but without initial surgery in the four selected hospitals; and (g) patients with incomplete data of medical record.
2.2. Data collection and definition of variables
In the present study, we included more than 40 variables to identify the risk factors associated with SSI. Demographic variables included age, gender, occupation, residential status (rural, urban), BMI (<18.5, underweight; 18.5‐23.9, normal; 24‐27.9, overweight; 28‐31.9, obesity; and ≥32, morbid obesity), and cigarette and alcohol consumption. Additional comorbidities included hypertension, rheumatoid disease, diabetes mellitus, liver and kidney diseases, anaemia, tumours (benign or malignant), respiratory disorders, immune system disease, cardiac and cerebrovascular disease, stroke, peripheral vascular disease, previous surgery for any organ and system. Surgical‐related variables including surgery type (emergent or elective), surgical duration, anaesthesia time, anaesthetic type (intrathecal, general, combination), perioperative intravenous usage of antibiotics, intraoperative blood loss and transfusion, intraoperative body temperature, experience of surgeon (chief, associate chief, attending), time of surgery (from the hospital admission to the start of surgery), surgical site, and the American Society of Anesthesiologists (ASA, I‐V) classification system were analysed to evaluate the patients' physiological homeostasis and physical status,14 length of hospital stay, application of drainage system, frequency of intraoperative fluoroscopy, and injury type (close or open fracture). Preoperative laboratory indexes (24 hours within hospitalisation) included electrolyte, serum total protein (TP), ALB, globulins, white blood cells (WBCs), neutrophil granulocytes, lymphocytes (LYMs), monocytes, eosinophil granulocyte (EOSs), basophil granulocytes, red blood cells, haemoglobin (HGB), blood platelet (PLT), and GLU.
Definition of surgical site infection was based on the criteria of the United States Center for Disease Control and Prevention.15 This classification system categorises SSI into three types: (a) superficial incisional, (b) deep soft tissue of the incision, and (c) organ/space infection. Superficial SSI, defined as infection occurring within 30 days postoperation, involves the skin and subcutaneous tissue of the surgical site only; one or more symptoms are observed: redness, swelling, and pain of the incision; purulent discharge; spontaneous wound dehiscence; and positive results of bacterial culture. Deep wound infection is defined as infection that occurs within 90 days postoperation, and it involves the fascial and muscular layers.
This study was approved by the ethics committee of all the participant hospitals and received written consent from all the study participants.
The homogeneity of the data could be compromised if the time span of patients' recruitment was too long. Taking this condition into consideration, the time window was set from September 2015 to April 2018. Finally, all enrolled patients were divided into two groups according to the occurrence of SSI. The case group was defined as patients with SSI, and the control group included patients who were not suffered from infection. All the data mentioned above were extracted and collected by investigators who were specifically trained in epidemiology.
2.3. Statistical analysis
Whitney U test was used for non‐normally distributed continuous variables and t test for normally distributed variables, and the significance was set at P < .05 (*P < .05; **P < .01; ***P < .001; ****P < .0001). Receiver‐operating characteristic (ROC) analysis was performed to detect the optimum cut‐off value for continuous variables (such as surgical duration, BMI, ALB, GLU intraoperative blood loss, and body temperature and other laboratory indexes). Prognostic risk factors that demonstrated to have statistical significance (P < .10) in the univariate analysis were entered into the multivariable logistic analysis to identify independent predictors of wound infection. A stepwise backward elimination approach was used to exclude confounding covariates from the final multivariate model, and a P value of <.05 was considered to be statistically significant. The Hosmer‐Lemeshow test was used to evaluate goodness of fit of the final model, and an acceptable fitness was enacted as P > .05. All statistical procedures were performed by using the SPSS 19.0 software package (SPSS Inc., Chicago, Illinois).
3. RESULTS
3.1. Features of study sample
During the 31‐month study period, a total of 2341 patients with traumatic geriatric fracture were recruited based on the inclusion and exclusion criteria, and the mean age of the study sample was 72.5 ± 6.4 years. Of them, 930 (39.7%) were male and 1411 (60.3%) were female, with a male‐to‐female ratio of 1:1.5. Of which 189 (8.1%) patients suffered from open fracture and 2152 (91.9%) patients diagnosed with closed injury. According to the trauma site, 287 (12.3%) cases of upper extremity, 1540 (65.8%) cases of lower extremity, 430 (18.4%) cases of spine fracture, 30 (1.3%) cases of pelvic and acetabular fracture, and 54 (2.3%) patients with multiple fractures were hospitalised. The length of hospital stay ranges from 2 to 139 days, with a mean time of 17.3 ± 10.7 days, and the mean surgical duration was 110.6 ± 57.4 minutes.
3.2. Characteristics of SSI
Among the 2341 patients, a total of 63 SSIs occurred within the follow‐up period, indicating a 2.7% incidence rate of SSI. Superficial SSI was observed in 53 cases and deep infection in 10 cases, with the incidence of 2.3% and 0.4%, respectively. The mean length of hospitalisation for SSIs and no‐infected patients was 30.6 ± 19.2 days and 16.9 ± 10.1 days, respectively, and the difference was statistically significant (P, .000). The earliest diagnosis of SSI was at 3 days postoperative and the latest was at the 127th day, and the longest duration of infection was up to 43 days. Microbiological cultures were conducted in 47 infected patients, and 83.0% (39/47) of the patients had a positive culture. Staphylococcus aureus was the most common causative pathogen (23), followed by Pseudomonas aeruginosa (15), Enterococcus faecalis (12), methicillin‐resistant S. aureus (MRSA) (10), and Staphylococcus epidermidis (7), and 13 patients suffered from polymicrobial infection.
3.3. Risk factors for SSI
ROC analysis was performed to detect the optimum cut‐off value for some continuous variables, which could affect the incidence of SSI (Figure 1). Area under the ROC curves (AUCs) and their corresponding optimum cut‐off values, 95% CI for these quantitative data, were summarised in Table 1. In the univariate analysis, open injury (OR, 13.8; P, .000), ASA class III‐IV (OR, 2.7; P, .000), surgical duration >132 minutes (OR, 1.7; P, .033), intraoperative blood loss >285 mL (OR, 2.2; P, .006), hypertension (OR, 1.7; P, .038), LYM <1.79 × 109/L (OR, 1.9; P, .006), HGB <134.6 g/L (OR, 1.9; P, .040), ALB <36.4 mg/L (OR, 3.3; P, .001), emergency operation (OR, 2.9; P, .002), drainage use (OR, 1.9; P, .011), intraoperative body temperature <36.2°C (OR, 1.5; P, .032), diabetes mellitus (OR, 3.5; P, .000), GLU >118 mg/dL (OR, 3.3; P, .000), BMI >24.9 kg/m2 (OR, 3.7; P, .012), and hospital stay >21 day (OR, 1.9; P, .016) were demonstrated to be associated with SSI (Table 2). In the multivariate logistic regression analysis model, all of the 15 abovementioned factors were entered for adjustment. Final statistical results showed open injury (OR, 9.5; 95% CI, 5.4‐16.7), ASA classified III‐IV score (OR, 2.2; 95% CI, 1.3‐3.8), surgical duration >132 minutes (OR, 2.9; 95% CI, 1.1‐5.0), ALB<36.4 mg/L (OR, 2.0; 95% CI, 1.6‐3.4), and GLU >118 mg/dL (OR, 3.1; 95% CI, 1.1‐5.3) were independent risk factors of postoperative SSI (Table 3). The result of Hosmer‐Lemeshow test demonstrated a preferable fitness (X 2 = 4.645, P = .795).
Figure 1.
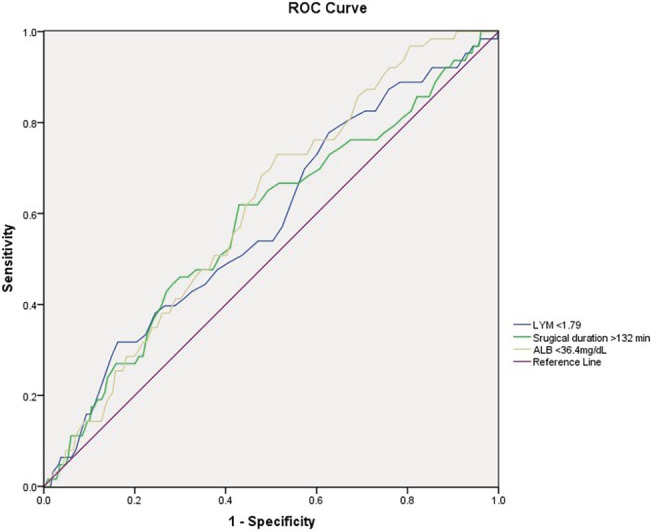
The optimum cut‐off values of continuous variables tested by receiver‐operating characteristic analysis
Table 1.
Optimum cut‐off value of continuous variables identified by the ROC analysis
| Variables | Cut‐off value | Area under the ROC curve (AUC) | 95% CI |
|---|---|---|---|
| Surgical duration (minute) | 132 | 0.582 | 0.511‐0.653 |
| Intraoperative body temperature (°C) | 36.2 | 0.545 | 0.518‐0.592 |
| Lymphocyte (LYM, 109/L) | 1.79 | 0.580 | 0.506‐0.654 |
| Body mass index (BMI, kg/m2) | 24.9 | 0.539 | 0.512‐0.595 |
| Blood glucose (GLU, mg/dL) | 118 | 0.553 | 0.507‐0.852 |
| Serum albumin (ALB, g/L) | 36.4 | 0.613 | 0.551‐0.675 |
| Intraoperative blood loss (mL) | 285 | 0.568 | 0.514‐0.614 |
Abbreviations: CI, confidence interval; ROC, receiver‐operating characteristic.
Table 2.
The association between single‐risk indicators and postoperative surgical site infection
| Variables | SSI (n = 63, 2.7%) | No SSI (n = 2278, 97.3%) | P value |
|---|---|---|---|
| Open injury | 32 (50.8) | 157 (6.9) | .000*** |
| Surgery type | .002** | ||
| Emergency operation | 53 (84.1) | 1466 (64.4) | |
| Selective surgery | 10 (15.9) | 812 (35.6) | |
| Cigarette consumption | 10 (15.9) | 297 (13.0) | .462 |
| Alcohol abuse | 21 (33.3) | 475 (20.9) | .083 |
| Gender (female) | 31 (49.2) | 1380 (60.6) | .076 |
| Residential status (urban area) | 20 (31.7) | 619 (27.2) | .504 |
| BMI > 24.9 kg/m2 | 31 (49.2) | 1035 (45.4) | .012* |
| Diabetes mellitus | 6 (9.5) | 313 (13.7) | .000*** |
| Hypertension | 19 (30.2) | 924 (40.6) | .038* |
| Anaemia | 1 (1.6) | 26 (1.1) | .787 |
| HGB (<134.6 g/L) | 39 (61.9) | 1624 (71.3) | .040* |
| Liver and kidney disease | 1 (1.6) | 57 (2.5) | .915 |
| Surgical duration (>132 min) | 18 (28.6) | 604 (26.5) | .033* |
| Intraoperative blood loss (285 mL) | 17 (27.0) | 1002 (44.0) | .006** |
| ASA score (III‐IV) | 40 (63.5) | 880 (38.6) | .000*** |
| Intraoperative body temperature (<36.2°C) | 8 (122.7) | 287 (12.6) | .032* |
| Anaesthetic type (general) | 11 (17.5) | 697 (30.6) | .090 |
| Surgical site | .936 | ||
| Spine | 10 (15.9) | 382 (16.8) | |
| Pelvic and acetabulum | 2 (3.2) | 39 (1.7) | |
| Lower extremity | 40 (63.5) | 1537 (67.5) | |
| Preoperative antibiotics use | 12 (19.0) | 336 (14.7) | .390 |
| Length of hospital stay (>21 days) | 39 (61.9) | 423 (18.6) | .016* |
| Drainage use | 24 (38.1) | 1228 (53.9) | .011* |
| TP (<55 g/L) | 7 (11.1) | 119 (5.2) | .232 |
| ALB (<36.4 g/L) | 10 (15.9) | 365 (16.0) | .001** |
| WBC# (reference 4–10 × 109/L) | .787 | ||
| <4 | 1 (1.6) | 69 (3.0) | |
| >10 | 7 (11.1) | 327 (14.4) | |
| LYM (<1.79 × 109/L) | 32 (50.8) | 1426 (62.6) | .006** |
| GLU (>118 mg/dL) | 24 (38.1) | 698 (30.6) | .000*** |
| EOS (references 0.02‐0.52 × 109/L) | .185 | ||
| <0.02 | 8 (12.7) | 394 (17.3) | |
| >0.52 | 0 (0.0) | 47 (2.1) | |
| PLT (references 100‐300 × 109/L) | .854 | ||
| <100 | 2 (3.2) | 31 (1.4) | |
| >300 | 10 (15.9) | 245 (10.8) |
Abbreviations: ALB, albumin; ASA, American Society of Anesthesiologists; BMI, body mass index; EOS, eosinophils; GLU, blood glucose; HGB, haemoglobin; LYMs, lymphocytes; PLT, blood platelets; TP, total protein; WBCs, white blood cells.
P < .05; **P < .01; ***P < .0001.
Table 3.
OR, 95% CI, and P value for independent risk factors in the multivariable logistic regression analysis of surgical site infection
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Open injury | 9.5 | 5.4‐16.7 | .000*** |
| ASA (III‐IV) | 2.2 | 1.3‐3.8 | .006** |
| Surgical duration > 132 min | 2.9 | 1.1‐5.0 | .028* |
| GLU > 118 mg/dL | 3.1 | 1.1‐5.3 | .000*** |
| ALB < 36.4 g/L | 2.0 | 1.6‐3.4 | .004** |
Abbreviations: ALB, serum albumin; ASA, American Society of Anesthesiologists; CI, confidence interval; GLU, blood glucose, OR, odds ratio.
P < .05; **P < .01; ***P < .0001.
4. DISCUSSION
From a cohort of 2341 geriatric patients, during more than 12 months follow‐up after traumatic orthopaedic surgeries, 63 encountered wound infection for a gross incidence rate of 2.7%; of them, 53 had superficial SSI and 10 had deep infection. These results are comparable with those in previous literature studies.16, 17, 18 In the multivariate analysis of this present study, open injury, ASA‐graded III‐IV score, surgical duration >132 minutes, ALB <36.4 mg/L, and GLU >118 mg/dL were independent risk factors of SSI.
SSI is one of the most common and adverse complications of open fracture, Ojo et al19 have confirmed that the incidence rate of SSI after open injury of Gustilo I was 0%‐2%, and following Gustilo II and III types, it was 2%‐15% and 5%‐50%, respectively. Recent evidence in our study has reaffirmed that geriatric patients with open fracture had an almost 9.5‐fold increased risk of SSI in traumatic orthopaedic surgeries and the outcomes were statistically significant (P = 0.000) and robust. There were 170 open fractures in the study sample of our study and 32 SSIs were observed, indicating an 18.8% incidence of wound infection after open geriatric fracture. Meanwhile, open fracture is a well‐accepted risk factor for deep infection because of more extensive soft‐tissue injury and free contamination of the wound with skin and ambient flora. Consistent with previous articles,20, 21 2.4% patients suffered from deep SSI in this study. Multiple operations were needed in the treatment of open fracture with contaminated or dirty wound, and some scholars even advocated that it is beneficial to the postoperative wound infection control to reduce the time between initial trauma to surgery to 6 hours; however, prolonged debridement time and increased surgical frequency will inevitably aggrandise the exposure opportunity between wound and pathogenic bacterium, at the same time, physiological homeostasis of the traumatic patients would be interfered substantially. These abovementioned conditions could reduce coping strategies and resilience of the body, especially when the patients are of advanced age. Unlike the other potential factors for SSI, open injury cannot be modified or avoided by patients and orthopaedist. For these high‐risk patients, early administration of antibiotics and debridement have been demonstrated as an imperative of the successful management of open fractures.
Surgery in elderly patients represents an at‐risk procedure in terms of both anaesthetic and surgical plan, and the ASA classification system was a practicable and excellent assessment tool for estimation of the inpatients' physical status and endurance capability to surgery and anaesthesia. The ASA score has been identified as an independent risk factor of postoperative SSI in orthopaedics and other disciplines.13, 14, 15, 16, 17, 18, 19, 20, 21, 22 In the present study, the ASA classified III‐IV score was associated with quite a high risk of SSI (OR = 2.2). Reese et al23 conducted a retrospective cohort study, which identified 1735 adult orthopaedic patients who underwent open reduction and internal fixation of the long bone fractures, they found that every extra grade of ASA increased the risk of SSI approximately by 2.1 times. Similar evidence was also reported by Ren et al,24 wherein they recruited 3378 elderly patients (≥60) in their study who admitted to the department of orthopaedics due to traumatic injury, deformity, degenerative and osteopathy disease, and the statistical results of univariate and multivariable analyses confirmed that ASA ≥ III was associated with more than 2‐fold increased risk of infection.
Compared with the ASA score, surgical duration was also considered as a better marker of adverse physiological conditions, injury severity, technical difficulty, more extensive soft stripping, and extensive exposure of the wound. The difference in absolute surgical duration between the SSI and no‐infected group for all traumatic geriatric patients in our study was 28.3 minutes (102.4 vs 130.7). It has been reported that the risk of a postoperative SSI would increase approximately 78% with every extra hour of surgical duration in tibial plateau fracture patients.25 The relationship between prolonged operating time and SSI was also verified in spinal injury. Veeravagu et al,26 in a prospective analysis of 24 774 surgical cases of spinal decompression and fusion surgery, demonstrated surgical duration up to 3 hours as a significant predictor of postoperative wound infection. Although most operating room of level I hospitals were equipped with air conditioning systems, most of them were found to contain airborne fungi, albeit at lower concentrations.27 With the prolongation of operating time, exposure time of surgical incision and deep tissues of orthopaedic patients to the airborne pathogenic bacteria will increase correspondingly and the immunity of human body to microorganism will compromise under anesthetisation. All the published literature studies identified 90 minutes, 3 hours, or 5 hours as independent risk factors of SSI.26, 28, 29 In our study, a surgical duration of 132 minutes was demonstrated as the optimum cut‐off value in the prediction of postoperative SSI. In the application of the new accurate value in multivariable analysis, elderly patients who had undergone orthopaedic surgeries with an operating time longer than 132 minutes were at 2.9 times risk of SSI compared with the normal patients. Based on our finding, more sub‐high‐risk patients could be identified in the clinical practices. However, there are many modifiable and no‐manipulated variables that affect surgical duration, and surgeons should be aware of the effect of operating time on SSI and optimise their workflow accordingly.25
The relationship between hyperglycaemia and adverse orthopaedic surgical outcomes, such as infectious complications, has been described.30 Consistent with published studies, diabetes mellitus was associated with an increased risk of SSI in univariate analysis (OR, 3.5; P, .000), but the multivariable analysis showed that GLU of >118 mg/dL rather than diabetes was an independent risk factors of postoperative wound infection (OR, 3.14; 95% CI, 1.07–5.29). However, many of previous investigations have been focused on the influence of diabetes mellitus on the postoperative implications following orthopaedic surgery.31, 32 However, 37.5% of medical patients and 33% surgical patients who were admitted to the hospital without a history of diabetes have hyperglycaemia.33 Elderly patients with hyperglycaemia are prone to urinary tract infection and the risk of SSI following pelvic acetabular and hip fractures will increase significantly. Elevated preoperative serum GLU level in geriatric patients increases the risk of SSI, independent of diabetic status. When the GLU is higher than 252 mg/dL, the phagocytic function of WBCs will be compromised, which will inevitably weaken the body's anti‐infective ability. Colonisation and reproduction of certain bacteria, such as anaerobic and microaerobic microorganism, are accelerated in high‐concentration glucose tissue.34, 35 As a modifiable variable, hyperglycaemia is a sensitive factor in preventing postoperative wound infection.
Nutritional deficiency has been identified to be an independent predictor of postoperative morbidities, including infection, prolonged length of stay, haematoma, and complications of the renal, neurovascular, and cardiovascular system, and adequate attention should be given to patients' nutritional intake. In the present study, a lower level of ALB level (<36.4 g/L) instead of malnutrition (ALB <35 g/L) was associated with increased prevalence of SSI. Charles et al36 retrospectively analysed 77 785 patients who underwent total knee arthroplasty, and they found that patients with low ALB were more likely to have a superficial (OR, 1.27) and deep (OR, 3.64) SSI compared with that with normal ALB level. More importantly, ALB level was modifiable, and Myint et al37 reported that the number of infection episodes was reduced significantly after hip fracture patients accepted oral nutritional supplementation. Similar results were reported by the authors in different disciplines; however, the prognostic value and significance of a more sensitive ALB value in the geriatric orthopaedic surgeries remain ambiguous. ROC curve identified 36.4 g/L as the optimal threshold for ALB in our study. In the process of univariate and multivariable analyses, ALB <36.4 g/L would increase the wound infection risk by 3.3‐fold and 2.0‐fold, respectively, and these outcomes were statistically significant and robust. With the clear threshold of ALB, wound infection in elderly patients could be more effectively predicted and prevented by orthopaedists.
The potential limitations of this study should to be taken into account. Firstly, the sample size of overall infected patients was small, which did not permit us to detect the relationship between other comorbidities, laboratory indexes, and SSI. Secondly, a retrospective study inevitably inherits the selective bias. In addition, some infected patients were identified via telephone review after being discharged from hospital and the incidence rate of SSI may be underestimated. Despite these limitations, the present study shows apparent strengths. ROC analysis was performed to define a highly sensitive cut‐off value for ALB level, serum GLU, and surgical duration in the prediction of postoperative wound infection.
5. CONCLUSION
In the present study, we reported a 2.7% incidence rate of SSI in geriatric patient following orthopaedic surgery. Consistent with other studies, open injury and ASA graded III‐IV score were still well‐accepted prognostic risk factors of SSI. Meanwhile, modifiable variables, such as surgical duration and levels of ALB and GLU were confirmed to be associated with an increased risk of postoperative wound infection. With the application of these sensitive and modifiable factors, individualised treatment strategy can be formulated to assist patients with high risk of SSI to achieve a better outcome.
Le J, Dong Z, Liang J, et al. Surgical site infection following traumatic orthopaedic surgeries in geriatric patients: Incidence and prognostic risk factors. Int Wound J. 2020;17:206–213. 10.1111/iwj.13258
Jinbo Le, Zhijie Dong, and Zhenshuan Zhao contributed equally to this study.
REFERENCES
- 1. Baek GH. Are we prepared for geriatric orthopedics? Clin Orthop Surg. 2010;2(3):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Douglass CW, Jiménez MC. Our current geriatric population: demographic and oral health care utilization. Dent Clin N Am. 2014;58(4):717‐728. [DOI] [PubMed] [Google Scholar]
- 3. Labib N, Nouh T, Winocour S, et al. Severely injured geriatric population: morbidity, mortality, and risk factors. J Trauma. 2011;71(6):1908‐1914. [DOI] [PubMed] [Google Scholar]
- 4. Nieves JW, Bilezikian JP, Lane JM, et al. Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int. 2010. Mar;21(3):399‐408. [DOI] [PubMed] [Google Scholar]
- 5. Karlsson MK, Hasserius R, Obrant KJ. Individuals who sustain nonosteoporotic fractures continue to also sustain fragility fractures. Calcif Tissue Int. 1993;53(4):229‐331. [DOI] [PubMed] [Google Scholar]
- 6. Tengve B, Kjellander J. Antibiotic prophylaxis in operations on trochanteric femoral fractures. J Bone Joint Surg Am. 1978;60(1):97‐99. [PubMed] [Google Scholar]
- 7. Partanen J, Syrjälä H, Vähänikkilä H, Jalovaara P. Impact of deep infection after hip fracture surgery on function and mortality. J Hosp Infect. 2006;62(1):44‐49. [DOI] [PubMed] [Google Scholar]
- 8. Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare‐associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101‐114. [DOI] [PubMed] [Google Scholar]
- 10. Acklin YP, Widmer AF, Renner RM, Frei R, Gross T. Unexpectedly increased rate of surgical site infections following implant surgery for hip fractures: problem solution with the bundle approach. Injury. 2011;42(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 11. Akinleye SD, Garofolo G, Culbertson MD, Homel P, Erez O. The role of BMI in hip fracture surgery. Geriatr Orthop Surg Rehabil. 2018;9:2151458517747414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skaggs DL, Friend L, Alman B, et al. The effect of surgical delay on acute infection following 554 open fractures in children. J Bone Joint Surg Am. 2005;87(1):8‐12. [DOI] [PubMed] [Google Scholar]
- 13. Ma T, Lu K, Song L, et al. Modifiable factors as current smoking, hypoalbumin, and elevated fasting blood glucose level increased the SSI risk following elderly hip fracture surgery. J Invest Surg. 2019;19:1‐9. [DOI] [PubMed] [Google Scholar]
- 14. Dripps R. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 15. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606‐608. [PubMed] [Google Scholar]
- 16. Liang Z, Rong K, Gu W, et al. Surgical site infection following elective orthopaedic surgeries in geriatric patients: incidence and associated risk factors. Int Wound J. 2019;16(3):773‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li GQ, Guo FF, Ou Y, Dong GW, Zhou W. Epidemiology and outcomes of surgical site infections following orthopedic surgery. Am J Infect Control. 2013;41(12):1268‐1271. [DOI] [PubMed] [Google Scholar]
- 18. Carneiro M, Krummenauer EC, Alves Machado JA, Adam MS, de Souza JG, Gonçalves RA. Incidence of infection in orthopedic surgeries: do we actually know it? Am J Infect Control. 2014;42(4):461‐462. [DOI] [PubMed] [Google Scholar]
- 19. Ojo OD, Oluwadiya KS, Ikem IC, Oginni LM, Ako‐Nai AK, Daniel FV. Superficial swab cultures in open fracture management: insights from a resource‐poor setting. J Wound Care. 2010;19(10):432‐438. [DOI] [PubMed] [Google Scholar]
- 20. Yokoyama K, Itoman M, Shindo M, Kai H, Ueta S, Kobayashi A. Deep infection and fracture healing in immediate and delayed locked intramedullary nailing for open femoral fractures. Orthopedics. 1999;22(5):485‐490. [PubMed] [Google Scholar]
- 21. Dellinger EP, Miller SD, Wertz MJ, Grypma M, Droppert B, Anderson PA. Risk of infection after open fracture of the arm or leg. Arch Surg. 1988;123(11):1320‐1327. [DOI] [PubMed] [Google Scholar]
- 22. Shao J, Zhang H, Yin B, Li J, Zhu Y, Zhang Y. Risk factors for surgical site infection following operative treatment of ankle fractures: a systematic review and meta‐analysis. Int J Surg. 2018;56:124‐132. [DOI] [PubMed] [Google Scholar]
- 23. Reese SM, Knepper B, Young HL, Mauffrey C. Development of a surgical site infection prediction model in orthopaedic trauma: the Denver health model. Injury. 2017;48(12):2699‐2704. [DOI] [PubMed] [Google Scholar]
- 24. Ren M, Liang W, Wu Z, Zhao H, Wang J. Risk factors of surgical site infection in geriatric orthopedic surgery: a retrospective multicenter cohort study. Geriatr Gerontol Int. 2019;19(3):213‐217. [DOI] [PubMed] [Google Scholar]
- 25. Colman M, Wright A, Gruen G, Siska P, Pape HC, Tarkin I. Prolonged operative time increases infection rate in tibial plateau fractures. Injury. 2013;44(2):249‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine. 2009;34(17):1869‐1872. [DOI] [PubMed] [Google Scholar]
- 27. Alfonso‐Sanchez JL, Martinez IM, Martín‐Moreno JM, González RS, Botía F. Analyzing the risk factors influencing surgical site infections: the site of environmental factors. Can J Surg. 2017;60(3):155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim BD, Hsu WK, De Oliveira GS Jr, Saha S, Kim JY. Operative duration as an independent risk factor for postoperative complications in single‐level lumbar fusion: an analysis of 4588 surgical cases. Spine. 2014;39(6):510‐520. [DOI] [PubMed] [Google Scholar]
- 29. Dubory A, Giorgi H, Walter A, et al. Surgical‐site infection in spinal injury: incidence and risk factors in a prospective cohort of 518 patients. Eur Spine J. 2015;24(3):543‐554. [DOI] [PubMed] [Google Scholar]
- 30. Rizvi AA, Chillag SA, Chillag KJ. Perioperative management of diabetes and hyperglycemia in patients undergoing orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(7):426‐435. [DOI] [PubMed] [Google Scholar]
- 31. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621‐1629. [DOI] [PubMed] [Google Scholar]
- 32. Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207(3):326‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levetan CS, Passaro M, Jablonski K, Kass M, Ratner RE. Unrecognized diabetes among hospitalized patients. Diabetes Care. 1998;21(2):246‐249. [DOI] [PubMed] [Google Scholar]
- 34. Glynn MK, Sheehan JM. The significance of asymptomatic bacteriuria in patients undergoing hip/knee arthroplasty. Clin Orthop Relat Res. 1984;185:151‐154. [PubMed] [Google Scholar]
- 35. Fitzgerald RH Jr, Nolan DR, Ilstrup DM, Van Scoy RE, Washington JA 2nd, Coventry MB. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59(7):847‐855. [PubMed] [Google Scholar]
- 36. Nelson CL, Elkassabany NM, Kamath AF, Liu J. Low albumin levels, more than morbid obesity, are associated with complications after TKA. Clin Orthop Relat Res. 2015;473(10):3163‐3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myint MW, Wu J, Wong E, et al. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: a single blind randomised controlled trial. Age Ageing. 2013;42(1):39‐45. [DOI] [PubMed] [Google Scholar]


