Abstract
To evaluate the efficacy and safety of human amniotic membrane (HAM) allograft in treating chronic diabetic foot ulcers (DFUs), a comprehensive search of randomised controlled trials in MEDLINE, EMBASE, PubMed, CENTRAL and Web of Science was conducted to December 7, 2019. Two reviewers independently screened the studies, extracted data, and evaluated the quality of studies. The primary outcome was the proportion of complete healing. The secondary outcomes were mean time to complete healing and adverse events. Statistical analyses were performed using RevMan 5.3. We identified 257 articles, of which 7 articles (465 participants) were included in the meta‐analysis. The proportion of complete wound healing in HAM plus standard of care (SOC) group was 3.88 times as high as that in SOC alone (RR: 3.88 [95% CI: 2.34, 6.44]) at 6 weeks, and 2.01 times at 12 weeks (RR: 2.01 [95%CI: 1.45, 2.77]). The intervention group had a significantly shorter time to complete healing (MD: −30.33 days, [95% CI: −37.95, −22.72]). The number needed to treat within 6 weeks was 2.3 ([95% CI: 1.8, 3.1]). No significant difference was shown in adverse events. Results were consistent in a sensitivity analysis. Hence, HAM plus SOC is effective and safe in treating chronic DFUs.
Keywords: diabetic foot ulcer, human amniotic membrane, meta‐analysis, systematic review, wound closure
Abbreviations
- AHAM
acellular human amniotic membrane
- DAMA
dehydrated amniotic membrane allograft
- DFUs
diabetic foot ulcers
- dHACA
dehydrated human amnion and chorion allograft
- dHACM
dehydrated human amnion/chorion membrane allograft
- dHAM
dehydrated human amniotic membrane
- HAA
human amniotic allograft
- HAM
human amniotic membrane
- HSAM
hypothermically stored amniotic membrane
- hVWM
human viable wound matrix
- NNT
number needed to treat
- RCT
randomised controlled trials
- RDs
risk differences
- RRs
risk ratios
- SOC
standard of care
- TCC
total contact casts
- UTN
Universal Trial Number
1. INTRODUCTION
Diabetes mellitus is one of the most serious chronic non‐communicable diseases in the world, and is currently the seventh leading cause of death worldwide.1 According to the international diabetes federation, there were 451 million diabetes patients over the age of 18 years in the world in 2017.2 It was predicted that the number would increase to 693 million by 2045,2 which means a heavy financial burden for both governments and patients.3 Long‐term hyperglycaemia can cause a variety of complications, among which diabetic foot ulcer (DFU) is the main cause of amputation and disability.4 It is estimated that at least one limb lost due to DFUs every 30 seconds around the world.5
Patients with diabetic feet often experience problems such as dysesthesia, amyotrophy, foot malformation, cracked skin and feet ischemia, for long‐term hyperglycaemia toxicity often leads to neuropathy and peripheral vascular disease. The dullness or disappearance of sensation makes it difficult to detect and treat timely when foot trauma occurs. Meanwhile, changes in the wound microenvironment, including persistent inflammation, growth factor deficiencies, cell senescence, bioburden, and increased levels of destructive proteases, make it even harder to heal.6 As a result, many DFUs are extremely difficult to cure. Some of them tend to be infected, even gangrened. In that situation, amputation is sometimes the only way to save lives. Even worse, not only is the quality of life with DFUs significantly low but also the disease burden is tremendously high.7, 8, 9 In England only, the annual health care cost for diabetic ulceration and amputation was between 1.3 billion and 1.5 billion dollars, more than the combined cost of lung, prostate, and breast cancers.7 Therefore, finding effective treatments for DFUs is of vital importance.
At present, the standard treatments for DFUs include strict glycaemic control, thorough debridement, moist dressings, offloading of weight‐bearing ulcers, evaluating the circulation in the legs, and antibiotics if the ulcer is infected.4 Nevertheless, even with aggressive treatment, many DFUs remain notoriously difficult to cure.10 Clinicians have been looking for more effective treatments for DFUs. Several advanced modalities have been proven to have encouraging results, such as negative pressure wound therapy, oxygen therapies, human growth factors, acellular bioproducts, and nonsurgical debridement agents.10 However, a more promising wound‐healing product has been introduced, namely human amniotic membrane (HAM).11, 12 HAM is a reproductive tissue representing the innermost layer of the human placenta. It is composed of a single epithelial layer, a thick basement membrane, and a connective tissue matrix.13 HAM has been used to treat eye and skin injuries for more than 20 years. Recently, some clinicians tried to apply HAM to refractory DFUs.11, 14 Then, Zelen and colleagues conducted a randomised controlled trial comparing healing characteristics of DFUs treated with HAM versus standard of care (SOC).12 Subsequently, some similar studies began to emerge. However, it is not easy to obtain a large enough sample size in a study, and there is still no definite conclusion whether it is effective and safe. Thus, we conducted a systematic review and meta‐analysis to evaluate the efficacy and safety of HAM plus SOC and SOC alone in the treatment of DFUs.
2. MATERIALS AND METHODS
This systematic review and meta‐analysis was developed and followed a standard protocol that was registered on PROSPERO (2019: CRD42019147511) and can be found online.15 The review includes two of three research questions in the protocol. The last question examining the cost‐effectiveness of HAM has not been conducted because only few original studies provided this information.
The meta‐analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) 2009 guidelines.16 This article was based on previously conducted studies and did not involve any experiments on human participants or animals.
2.1. Eligibility criteria and exclusion criteria
Eligible trials had to fulfil the following criteria using the PICOS strategy: (a) population (P): type 1 or type 2 diabetic patients with foot ulcers for more than 4 weeks; (b) intervention (I): HAM plus SOC; (c) comparison (C): SOC alone; (d) outcomes (O): not limited; (e) study design (S): randomised controlled trial.
Non‐human studies, non‐randomised controlled trials, reviews, and protocols with no outcome report were excluded. Moreover, previous reports of the same study were also excluded.
2.2. Primary and secondary outcomes
The primary outcome in this review was the proportion of complete wound healing. The secondary outcomes were mean time to complete healing, proportion of patients with ≥50% ulcer area reduction, and adverse events. Complete healing is defined as complete (100%) re‐epithelialisation of the ulcers that occurred without any drainage or need for dressing.
2.3. Literature search
To identify relevant trials, the electronic databases Ovid Medline, Embase, PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science were searched. The database of ongoing trails (www.clinicaltrials.gov) was also searched to identify additional eligible studies. Medical Subject Headings (MeSH) and free terms were combined as the retrieval strategy. The search strategies were adapted to each database. For example, the search details in Ovid Medline are shown in Appendix A. Published time was restricted from database inception to 16 August 2019, with updation on 7 December 2019. Language restrictions were not imposed.
2.4. Data extraction
Two investigators independently screened the titles and abstracts and excluded obviously irrelevant manuscripts. The full texts of possible eligible studies were obtained and double screened for eligibility. Meanwhile, we scrutinised the Universal Trial Number (UTN) of each study to exclude earlier publication of the same number. If some UTNs were unavailable, we compared the authors, intervention settings, study design, and enrolment period to determine the differences between studies. Disagreements were resolved through discussion.
The following information was collected from each eligible randomised controlled trial: (a) publication data (authors, title of the study, year of publication, study design, intervention setting, UTN, period of enrollment); (b) Patient data (age, BMI, HbA1c); (c) ulcer baseline condition (location, ulcer size); (d) intervention (the brand and manufacturer of allograft in each study, sample size); (e) control (sample size); (f) outcomes (proportion of complete healing, mean time to complete healing, proportion of patients with ≥50% ulcer area reduction, and adverse events). If both intent‐to‐treat and per‐protocol analyses were reported in the study, we only extracted data from intent‐to‐treat analysis.
2.5. Assessment of risk of bias
Two reviewers independently evaluated the risks of bias using the following six domains of Cochrane Risk of Bias Tool for randomised trials: random number generation, allocation concealment, blinding of patients and personnel, blinding of outcome estimators, incomplete outcome data, and selective reporting. If there were any disagreements, all the authors would discuss it.
The risk of bias of each study was assessed in the Review Manager (RevMan version 5.3) according to the Cochrane risk‐of‐bias tool for randomised trials.17
2.6. Statistical analyses
Clinical and methodological heterogeneities were evaluated based on study characteristics. If the study characteristics had good homogeneity, data were combined quantitatively using RevMan. Statistical heterogeneity was identified using the Cochran Q test and the I 2 statistic. If I 2 statistic was lower than 50%, it was considered that the heterogeneity was acceptable, and a fixed‐effects model was applied. If not, it was regarded that the heterogeneity was substantial, and a random‐effects model with Mantel–Haenszel weighting was chosen. All analyses used the intent‐to‐treat approach. For binary outcomes, the results were reported as risk differences (RDs) and relative risks (RRs). For continuous outcomes, mean differences (MDs) with corresponding 95% CIs were presented. All statistical tests were two sided and conducted at a significance level of 0.05. The number needed to treat (NNT) was also calculated to inform the effectiveness of the treatment. Furthermore, the Kaplan‐Meier curves of time to complete healing were pooled in R software (version 3.6.1) to illustrate the time needed to complete healing by using Tierney's method.18 Finally, a sensitivity analysis was performed to assess whether the result is robust by adding two studies19, 20 in which both intervention and control groups use total contact casts (TCC), an extra method of offloading plantar ulcers.
3. RESULTS
3.1. Study selection and characteristics
Of 257 citations, nine studies12, 19, 20, 21, 22, 23, 24, 25, 26 met eligibility criteria and were included in the qualitative systematic review. Seven studies with 465 participants were included in the final meta‐analysis (Figure 1). Two studies19, 20 were excluded from the quantitative analyses because both intervention and control groups were using TCC.
Figure 1.
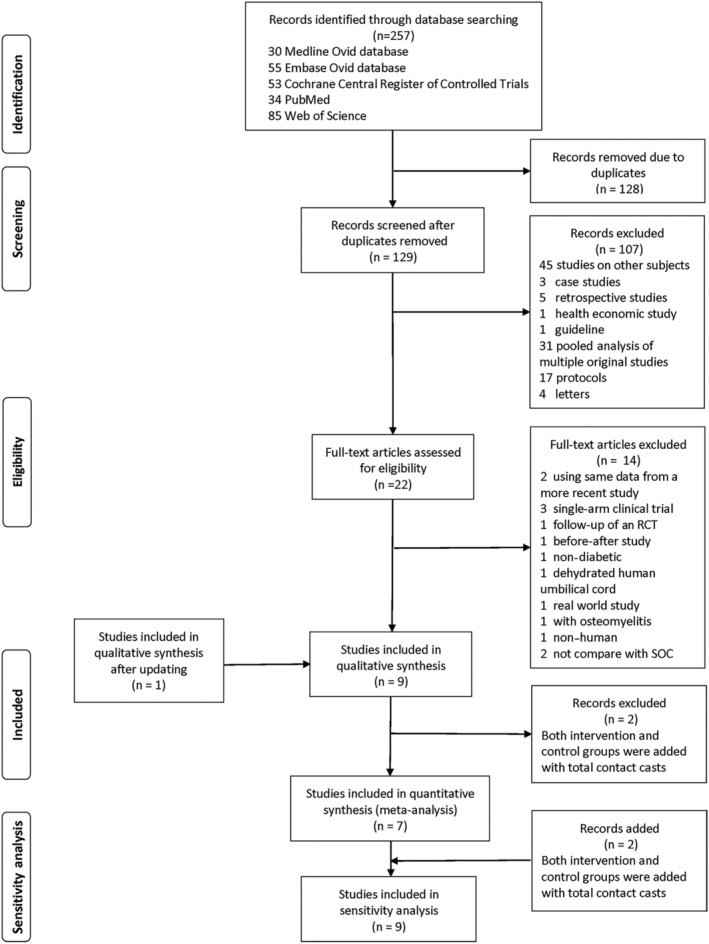
PRISMA flow diagram of the study selection for the systematic review and meta‐analysis
The baseline characteristics of participants in these studies were very similar (Table 1) and heterogeneities in trial design procedures were quite low. Patients included in all the studies were Wagner grades 1 and 2, except one study22 Wagner grade 2‐4. Most studies20, 21, 23, 24, 25, 26 had 1 to 2 weeks screening or run‐in period before randomisation. At that stage, all potentially eligible patients obtained a history collection, a thorough physical examination, and a careful wound assessment. Meanwhile, the wound was treated with standard wound care such as thorough surgical debridement, moist wound healing, and off‐loading. Only if their ulcers lasted more than 4 weeks and were unresponsive to standard wound care could they be involved to randomisation. All included studies were followed up weekly. Most of the studies were followed up for 12 weeks, while two studies22, 23 for 6 weeks and one study20 for 16 weeks.
Table 1.
General characteristics of included studies
| Author/year | Universal trial number | Mean age (years) /(SD) | BMI (kg/m2)/(SD) | HbA1c (%) | Ulcer size (cm2)/(SD) | Ulcer location | Intervention (brand and case number) | Type of HAM (processing method) | Control (case number) | Duration of follow‐up |
|---|---|---|---|---|---|---|---|---|---|---|
| Zelen et al (2013) | NCT01552499 | I = 56.4 (14.7) C = 61.7 (10.3) | I = 30.4 (5.7) C = 35.4 (6.6) | Not mentioned | I = 2.6 (1.9) C = 3.4 (2.9) | Forefoot, digital, heel and midfoot | dHACM (EpiFix®, MiMedx, Kennesaw, GA) + SOC (n = 13) | Amnion and chorion (Dehydration) | SOC (n = 12) | 12 weeks or until complete healing |
| Lavery et al (2014) | Not mentioned | I = 55.5 (11·5) C = 55.1 (12·0) | I = 33.5 (7.7) C = 32.2 (7.9) | I = 8.0 (1.6) C = 7.8 (1.5) | I = 3.41 (3.23) C = 3.93 (3.22) | Dorsal and plantar | hVWM (Grafix® Osiris Therapeutics, Inc., Columbia, MD) + SOC3(n = 50) | Amnion and chorion (cryopreservation) | SOC (n = 47) | 12 weeks |
| Mohajeri‐Tehrani et al (2016) | Not mentioned | I = 55.44 (11.27) C = 60.00 (9.30) | I = 29.30 (7.06) C = 27.4 (5.21) | I = 7.4 (1·1) C = 7.5 (1·1) | I = 9.48 (9.35) C = 8.61 (10.05) | Toe, heel, plantar, forefoot, lateral maleol | AHAM (Life Patch, International Bioimplant Company, Tehran, Iran) + SOC (n = 27) | Amnion (Cryopreservation) | SOC (n = 30) | 6 weeks |
| Snyder et al (2016) | NCT02209051 | I = 57.9 (12.49) C = 58.6 (6.97) | I = 34.9 (5.9) C = 35.1 (8.1) | Not mentioned | I = 4.7 (5.43) C = 6.9 (6.75) | Forefoot, hindfoot, metatarsals, midfoot, Phalanges | DAMA (AMNIOEXCEL, Derma Sciences Inc, Princeton, NJ) + SOC (n = 15) | Amnion (Dehydration) | SOC (n = 14) | 6 weeks |
| Zelen et al (2016) | NCT01921491 | I = 63.3 (12.25); C = 60.6 (11.55) | I = 33.9 (6.99); C = 34.7 (9.35) | I = 7.5 (1.51); C = 8.2 (1.78) |
I = 2.6 (2.97); C = 3.1 (3.17) |
Toe, forefoot, midfoot, hindfoot, ankle | dHACM 2 (EpiFix®, MiMedx Group Inc., Marietta, GA) + SOC (n = 32) | Amnion and chorion (dehydration) | SOC3 (n = 35) | 12 weeks or until 1 week after complete healing |
| DiDomenico et al (2018) | NCT02399826 | I = 60.1 (11.77); C = 61.0 (10.66) | I = 34.0 (9.30); C = 34.5 (9.42) | I = 7.6 (1.47); C = 7.9 (1.48) | I = 2.1 (1.46); C = 3.1 (3.58); | Toe, forefoot, midfoot, heel, ankle, hindfoot | dHACA (AmnioBand, Musculoskeletal Transplant Foundation, Edison, NJ) + SOC (n = 40) | Amnion and chorion (dehydration) | SOC (n = 40) | 12 weeks |
| Tettelbach et al (2019) | NCT01693133 | I = 57.4 (10.6); C = 57.1 (10.5) | I = 35.8 (8.9); C = 34.6 (8.5) | I = 7.8 (1.4); C = 8.8 (1.8) | I = 3.2 (2.8); C = 3.9 (3.8) | Toe, forefoot, midfoot, ankle, hindfoot | dHACM (EpiFix, MiMedx Group Inc., Marietta, GA) + SOC (n = 54) | Amnion and chorion (dehydration) | SOC (n = 56) | 12 weeks |
| Thompson et al (2019) | NCT02344329 | I = 58.50 (12.96); C = 55.17 (18.32) | Not mentioned | I = 9.63 (2.77); C = 8.47 (2.44) | I = 1.54 (1.74); C = 2.78 (3.04) | Not mentioned | HAA (AmnioExcel; Integra Lifesciences, Plainsboro, New Jersey) + SOC + TCC (TCC‐EZ; Derma Sciences Inc, Plainsboro, New Jersey) (n = 7) | Amnion (Dehydration) | SOC + TCC (n = 6) | 12 weeks or until complete healing |
| Serena et al. 2019 | Not mentioned | I = 59.2 (7.61); C = 59.6 (10.72) | Not mentioned | Not mentioned | I = 3.12 (3.86); C = 3.33 (4.62) | Not mentioned | HSAM + SOC + TCC (n = 38) | Amnion (Cryopreservation) | SOC + TCC (n = 38) | 16 weeks |
Abbreviations: AHAM, acellular human amniotic membrane; DAMA, dehydrated amniotic membrane allograft; dHACA, dehydrated human amnion and chorion allograft; dHACM, dehydrated human amnion/chorion membrane allograft; HAA, human amniotic allograft; HAM, human amniotic membrane; hVWM, human viable wound matrix; HSAM, hypothermically stored amniotic membrane; TCC, total contact casts; SOC, standard of care.
3.2. Risk of bias
The risk of biases is shown in Figure 2. The primary bias comes from the implementation of the blind method. Eight studies (88.9%) were unable to blind patients. Fortunately, in two studies,24, 26 study adjudicators and validators were both blinded to group assignment when examining photographic images and confirming healing. Four studies (44.4%) reported how they generated randomisation sequences and four (44.4%) reported using an opaque envelope to conceal allocation. Seven studies (77.8%) reported adverse events, while two studies did not, which might produce reporting bias.
Figure 2.
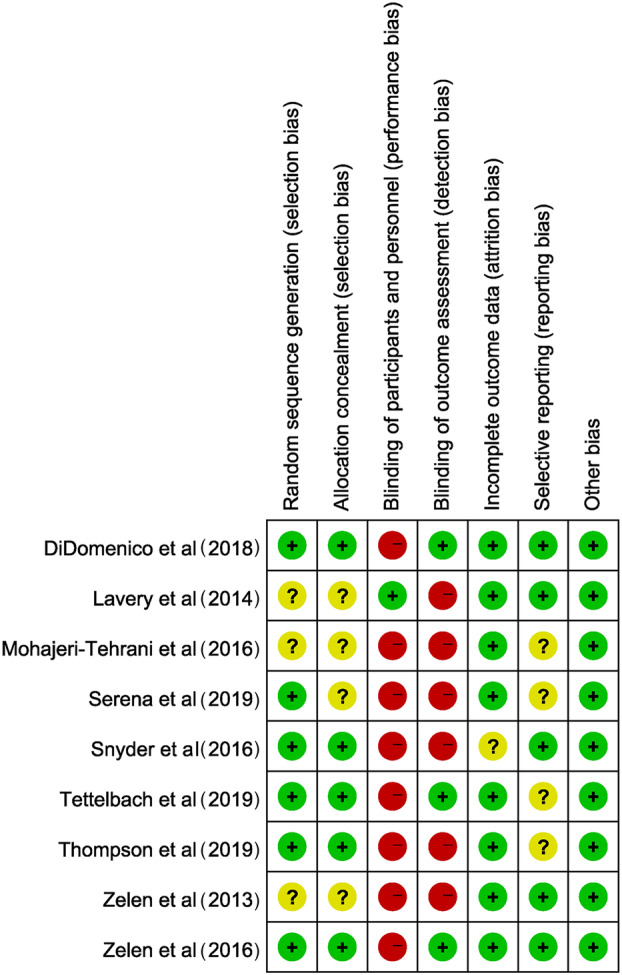
Risk of bias quality summary
3.3. Primary outcomes
The proportion of complete wound healing with HAM plus SOC was significantly higher than that of SOC alone at 6 weeks (RD: 0.48, [95% CI: 0.21, 0.74]) and 12 weeks (RD: 0.40, [95% CI: 0.26, 0.53]). When using relative risk (RR) as an indicator, the proportion of complete wound healing with HAM plus SOC was 3.88 times as high as that of SOC alone (RR: 3.88, [95% CI, 2.34, 6.44]) at 6 weeks and 2.01 times at 12 weeks (RR: 2.01, [95% CI, 1.45, 2.77]) (Figure 3).
Figure 3.
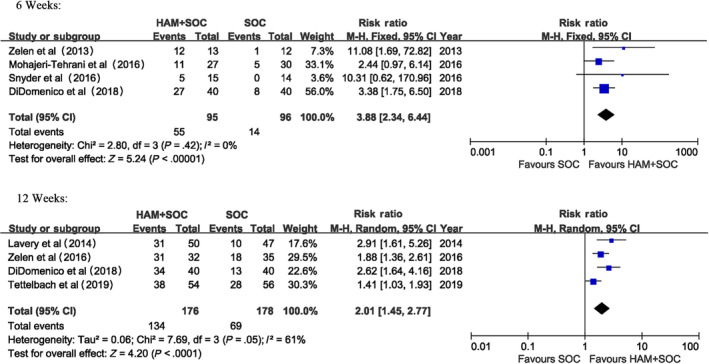
Proportion of complete healing (6 weeks and 12 weeks)
3.4. Secondary outcomes
The HAM plus SOC group had a significantly shorter time to complete wound closure compared with SOC alone (MD: −30.33 days, [95% CI: −37.95, −22.72]). The Kaplan‐Meier curves pooled for patients with complete healing also illustrated the prominent superiority of HAM (Figure 4 and Figure 5). The NNT within 6 weeks is 2.3 ([95% CI, 1.8, 3.1]) The rate of more than 50% area reduction is 1.64 times as much as using SOC (RR: 1.64, [95% CI: 1.23, 2.17]).
Figure 4.
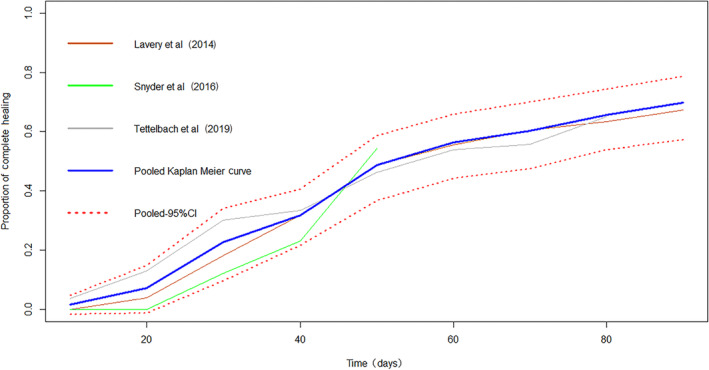
Kaplan‐Meier curves of time to complete healing (HAM + SOC group)
Figure 5.
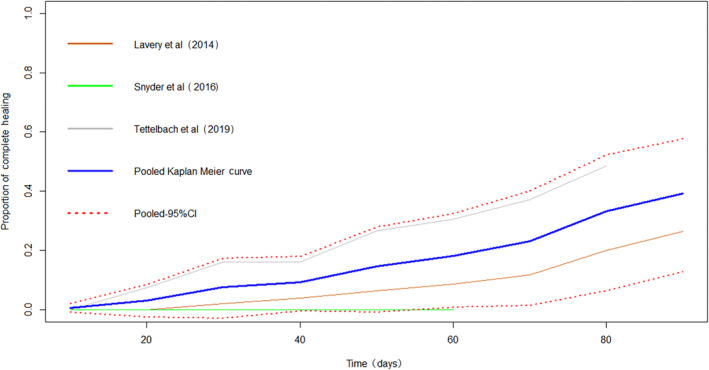
Kaplan‐Meier curves of time to complete healing (SOC group)
The occurrence of adverse events varies across different studies (Appendix B), which may be due to different scopes the researchers focused on. However, most studies emphasised wound‐related infections and possible product‐related adverse events. The wound‐related infections including cellulitis and osteomyelitis occurred in both groups with no significant difference (RD: ‐0.06, [95% CI: −0.13, 0.01]) (Figure 6). Possible product‐related adverse events including wound maceration and positive wound cultures were only seen in one study26 with a proportion of 5.6%.
Figure 6.
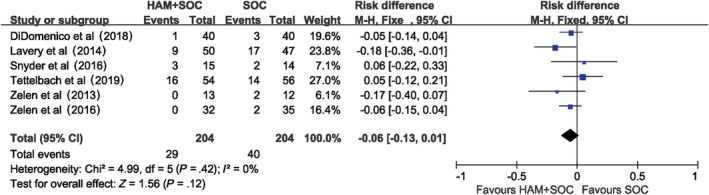
Wound‐related infections
3.5. Sensitivity analysis
By adding two studies with TCC in both intervention and control groups, we found the proportion of complete wound healing with HAM plus SOC was 35.0% higher than that of SOC alone at 12 weeks, which is quite similar to that without it. In addition, RR for the proportion of complete wound healing with HAM plus SOC versus SOC alone reduced just a little at 12 weeks (RR: 1.8, [95% CI, 1.33, 2.43]), but still much higher than 1 (Figure 7). Furthermore, the intervention group can save more than 20 days to complete wound closure (MD for mean time to complete healing: −22.18, [95%CI, −28.81, −15.55]), which is similar to our previous result.
Figure 7.
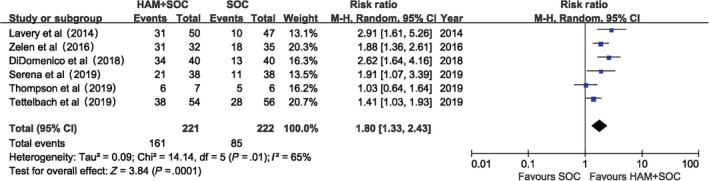
Proportion of complete healing (12 weeks) sensitivity analysis. notes: by adding 2 studies that both intervention and control groups were with total contact casts in addition
4. DISCUSSION
This systematic review of RCTs evaluated the efficacy and safety of HAM plus SOC in patients with chronic DFUs. A total of 465 diabetic patients with foot ulcers (231 randomised to the invention group and 234 to the control group) were finally included in the meta‐analysis. The meta‐analysis demonstrated that HAM plus SOC could significantly accelerate wound healing. Our results are consistent with previous meta‐analyses.27, 28, 29 However, previous meta‐analyses27, 28 pooled the same study24 twice, which would enhance the weight of that study and make the result not valid enough. On the contrary, we scrutinised the UTN, authors, intervention settings, study design, and enrolment period of all included studies carefully to ensure that each study was included only once. Moreover, we included three new studies19, 20, 26 published in recent years.
The most obvious finding from the analysis was that HAM plus SOC did accelerate the process of wound healing. HAM plus SOC achieved a much higher probability of wound recovery than SOC alone, about four times at 6 weeks and two times at 12 weeks. It also had a significantly shorter time to complete wound closure, about 30 days earlier. In addition, every two to three patients treated with HAM plus SOC will would get one additional patient successfully cured within 6 weeks. Hence, HAM plus SOC might decrease the chance of amputation and improve the quality of patients' life. The sensitivity analysis showed that our result was quite robust. The main reason for the excellent curative effect may due to its special properties. It contains a large number of multiple growth factors and multiple proangiogenic factors, which can induce human dermal fibroblast proliferation and angiogenesis. Besides, it also has anti‐inflammatory and antimicrobial properties, and can be tolerated by the immune system. These properties make it an outstanding facilitator when serving as a scaffold for cell proliferation and differentiation in wound healing.11
Another interesting finding was that most participants pooled in this meta‐analysis were obese. It has been illuminated that obesity is a risk factor of pressure ulcers and can delay wound healing, because accompanied by increased body weight, insulin sensitivity reduces, blood flow shrinks and diabetic severe injury surges.30 Hence, early aggressive therapy in obese patients such as HAM plus SOC may avoid deterioration to chronic refractory ulcers.
There were two types of processing methods included in this review: dehydration or cryopreservation (Table 1). The complete healing rates of dehydrated amniotic membranes at 6 weeks and 12 weeks were slightly higher than those of cryopreserved amniotic membranes. But due to the small number of studies on cryopreserved amniotic membranes, this review was unable to demonstrate whether the difference was valid. Likewise, there were two types of HAM, with or without chorion (Table 1). The efficacy between different types of HAM cannot be compared, for without TCC, there had been only two studies22, 23 on amnion alone.
Although there were some adverse events reported in the involved studies, including osteomyelitis and cellulitis, there was no statistical difference between the intervention group and the control group (Appendix B). Most studies12, 22, 24, 25 showed no product‐related events, except one study26 reported one case of wound maceration and two cases of positive wound bacterial cultures. Other adverse events were sparse.
Our review has some strengths. First, to our knowledge, this is the most comprehensive and systematic meta‐analysis conducted by searching in all the major databases. Second, we carefully verified trial information, including the UTN, authors, intervention settings, study design, and enrolment period, so that duplicate publication would not be included and multiple publication biases were avoided. Third, we included new studies published the recent years. Fourth, we used intent‐to‐treat analysis, so that the comparability of baseline between intervention group and control group could not be destroyed. Fifth, this is the first review that pooled Kaplan‐Meier curves for patients with complete healing. Clinicians can get a pretty good idea of how many people were cured at different periods of treatment. Finally, we conducted a sensitivity analysis to evaluate how robust our result was, which was not seen in previous meta‐analyses.27, 28, 29
This review has several limitations. First, all included studies were performed in the United States, except for one in Iran.22 Whether it is as effective in other ethnic groups is still unclear. Second, there are still some potential biases, especially from the implementation of blinding patients that due to the special feature of surgical trials. Third, although some studies have shown that HAM has a cost‐effectiveness advantage in treating DFUs in the United States, economic data from other countries are still lacking. Further studies are needed to determine whether it is cost‐effective worldwide. Fourth, change in the quality of life is important for patients with DFUs, but our meta‐analysis failed to pool them together, because no original study investigated it.
5. CONCLUSIONS
This meta‐analysis of randomised controlled trials showed that HAM plus SOC is effective and safe in treating patients with chronic DFUs, which can significantly increase wound healing rate and reduce complete healing time. Early use of HAM plus SOC in obese patients may avoid deterioration to chronic refractory ulcers. However, more studies are needed to assess the quality of life and cost‐effectiveness in different populations.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
THE SEARCH STRATEGY DETAILS IN OVID MEDLINE
1. exp Extraembryonic Membranes/
2. exp Placenta/
3. exp Allografts/
4. exp Biological Dressings/
5. amnio*.mp.
6. chorion*.mp.
7. serosa.mp.
8. extraembryonic membranes.mp.
9. placenta.mp.
10. maza.mp.
11. allograft.mp.
12. allogeneic.mp.
13. biological dressing.mp.
14. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
15. exp Diabetic Foot/
16. diabetic foot.mp.
17. diabetic feet.mp.
18. 15 or 16 or 17
19. exp Randomized Controlled Trial/
20. random*.mp.
21. 19 or 20
22. 14 and 18 and 21
ADVERSE EVENTS
| Author/year | Proportion of adverse events | Wound‐related infections | Possible product‐related adverse events |
|---|---|---|---|
| Zelen et al (2013) | I = 8% (1/13); C = 33% (4/12) | I = 0% (0/13); C:16.7% (2/12) (2 cellulitis) | 0 |
| Lavery et al (2014) | I = 44% (22/50); C = 66% (31/47) | I = 18% (9/50); C = 36.2% (17/47) | Not mentioned |
| Mohajeri‐Tehrani et al (2016) | Not mentioned | Not mentioned | 0 |
| Snyder et al (2016) | I = 27% (4/15); C = 21% (3/14) | I = 20% (3/15): (1 ulcer infection, 1 osteomyelitis, 1 cellulitis); C = 14% (2/14): (1 ulcer infection, 1 cellulitis) | Not mentioned |
| Zelen et al (2016) | Not mentioned by groups | I = 0% (0/32); C = 6% (2/35): (2 ulcer infections, 1 ulcer infection and osteomyelitis) | 0 |
| DiDomenico et al (2018) | I = 8% (3/40); C = 20% (8/40) | I = 3% (1/40); C = 8% (3/40) | 0 |
| Tettelbach et al (2019) | Not calculated by patients, but by events | I = 30% (16/54): (6 ulcer infections, 7 cellulitis, 3 osteomyelitis); C = 25% (14/56): (5 ulcer infections, 8 cellulitis, 1 osteomyelitis) | 3 |
| Thompson et al (2019) | Not mentioned | Not mentioned | Not mentioned |
| Serena et al (2019) | Not mentioned | Not mentioned | Not mentioned |
Su Y‐N, Zhao D‐Y, Li Y‐H, et al. Human amniotic membrane allograft, a novel treatment for chronic diabetic foot ulcers: A systematic review and meta‐analysis of randomised controlled trials. Int Wound J. 2020;17:753–764. 10.1111/iwj.13318
Contributor Information
Ya‐Na Su, Email: anna.ya@qq.com.
Jing Li, Email: lijing68@hotmail.com.
REFERENCES
- 1. WHO . Top 10 causes of death: Global Health Observatory (GHO) data, 2016. Geneva, Switzerland. https://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/.
- 2. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271‐281. [DOI] [PubMed] [Google Scholar]
- 3. James SLG, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grennan D. Diabetic foot ulcers. JAMA. 2019;321(1):114. [DOI] [PubMed] [Google Scholar]
- 5. IDF . International Diabetes Federation. Diabetes Atlas 8th Edition, 2017. https://diabetesatlas.org/.
- 6. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiat Med Surg. 2015;32(1):135. [DOI] [PubMed] [Google Scholar]
- 7. Kerr M, Barron E, Chadwick P, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019;36(8):995‐1002. [DOI] [PubMed] [Google Scholar]
- 8. Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Burden of diabetic foot ulcer in Nigeria: current evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria. World J Diabetes. 2019;10(3):200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tchero H, Kangambega P, Lin L, et al. Cost of diabetic foot in France, Spain, Italy, Germany and United Kingdom: a systematic review. Annales D Endocrinol. 2018;79(2):67‐74. [DOI] [PubMed] [Google Scholar]
- 10. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci 2018;1411(1):153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah AP. Using amniotic membrane allografts in the treatment of neuropathic foot ulcers. J Am Podiatr Med Assoc. 2014;104(2):198‐202. [DOI] [PubMed] [Google Scholar]
- 12. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008;15:88‐99. [DOI] [PubMed] [Google Scholar]
- 14. Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 2014;11(6):711‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su YN, Zhao DY, Li YH, Li J. Human placental membrane allograft in treatment of diabetic foot ulcer: a systematic review and meta‐analysis 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=147511.
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. British Med J. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collaboration: London, United Kingdom; 2011. [Google Scholar]
- 18. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson P, Hanson DS, Langemo D, Anderson J. Comparing human amniotic allograft and standard wound care when using Total contact casting in the treatment of patients with diabetic foot ulcers. Adv Skin Wound Care. 2019;32(6):272‐277. [DOI] [PubMed] [Google Scholar]
- 20. Serena TE, Yaakov R, Moore S, et al. A randomized controlled clinical trial of a hypothermically stored amniotic membrane for use in diabetic foot ulcers. J Comp Eff Res. 2019;9:23‐34. [DOI] [PubMed] [Google Scholar]
- 21. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix() for the treatment of chronic diabetic foot ulcers: results of a multi‐centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohajeri‐Tehrani MR, Variji Z, Mohseni S, et al. Comparison of a bioimplant dressing with a wet dressing for the treatment of diabetic foot ulcers: a randomized, controlled clinical trial. Wounds. 2016;28(7):248‐254. [PubMed] [Google Scholar]
- 23. Snyder RJ, Shimozaki K, Tallis A, et al. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcers. Wounds. 2016;28(3):70‐77. [PubMed] [Google Scholar]
- 24. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi‐centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DiDomenico LA, Orgill DP, Galiano RD, et al. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: a prospective, randomised, multi‐centre clinical trial in 80 patients. Int Wound J. 2018;15(6):950‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haugh AM, Witt JG, Hauch A, et al. Amnion membrane in diabetic foot wounds: a meta‐analysis. Plast Reconstr Surg Glob Open. 2017;5(4):e1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laurent I, Astere M, Wang KR, Cheng Q‐F, Li QF. Efficacy and time sensitivity of amniotic membrane treatment in patients with diabetic foot ulcers: a systematic review and meta‐analysis. Diabetes Ther. 2017;8(5):967‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paggiaro AO, Menezes AG, Ferrassi AD, De Carvalho VF, Gemperli R. Biological effects of amniotic membrane on diabetic foot wounds: a systematic review. J Wound Care. 2018;27(2):S19‐S25. [DOI] [PubMed] [Google Scholar]
- 30. Tapking C, Houschyar KS, Rontoyanni VG, et al. The influence of obesity on treatment and outcome of severely burned patients. J Burn Care Res. 2019;40(6):996‐1008. [DOI] [PubMed] [Google Scholar]


