Abstract
The decomposition of urea into ammonia by urease‐producing bacterium shows an elevation in the pH level, which can lead to incontinence‐associated dermatitis (IAD). This study aimed to examine the efficacy of a combination of antiseptic and urease inhibitor in inhibiting the decomposition of urea by the urease‐producing bacterium Proteus mirabilis. We performed in vitro assays to compare the effects of a combination of antiseptic and urease inhibitor, antiseptic only, urease inhibitor only, and an untreated control with the effects of a urea‐containing solution. Cultured P. mirabilis was mixed with urea‐containing solution, followed by the addition of antiseptic and/or urease inhibitor. The main outcome used to assess the efficacy of the different treatments was ammonia concentration at 4‐hours post‐treatment initiation, and multiple comparison analysis was performed using Dunnett's test to compare the results between groups. Ammonia concentrations in samples treated with either antiseptic or urease inhibitor were lower than those in the untreated control, while the combination of antiseptic and urease inhibitor resulted in decreased ammonia concentrations compared with either treatment alone. Therefore, the application of both urease inhibitor and antiseptic is more effective for the inhibition of urea decomposition by urease‐producing bacteria. Novel preventive strategies using these reagents may be effective for preventing IAD.
Keywords: ammonia, incontinence‐associated dermatitis, microbiology, urea, urease
1. INTRODUCTION
Incontinence is one of the most common health problems affecting older adults. The reported prevalence of incontinence among nursing home residents is 48% to 59.8%: 7.7% to 14% with urinary incontinence only, 8% to 12.4% with faecal incontinence only, and 24% to 39.7% with both urinary and faecal incontinence. 1 , 2 Older adults suffering from incontinence are also subject to various skin changes, 3 placing them at substantial risk of cutaneous disorders such as incontinence‐associated dermatitis (IAD). IAD is defined as inflammation characterised by redness, with or without blistering, and erosion that occurs with chronic or repetitive exposure to urine or faeces. 4 Despite the implementation of preventative strategies, 5 , 6 , 7 high IAD prevalence and incidence rates (ranging from 5.2% to 27%) have been reported worldwide, with cumulative incidence rates ranging from 3.4% to 50%. 8 IAD negatively affects patients by causing discomfort such as itching or pain, 8 which impacts quality of life. Therefore, the development of an effective preventive care strategy for IAD is a pressing nursing issue.
Previous studies examined the contributions of digestive enzymes and intestinal bacteria to the development of IAD using a rat IAD model. 9 , 10 Results showed that proteases, one of the most common digestive enzymes found in faeces, caused skin barrier impairment and induced tissue damage in the dermis and around hair follicles in the macerated skin. Bacterial inoculation of skin macerated by proteolytic solution resulted in the formation of bacteria‐rich clusters comprising abundant bacterial cells and inflammatory cells within the papillary dermis, with remarkable tissue damage observed around the clusters. These results suggested that IAD results from protease‐induced skin damage, with bacteria then invading the areas with compromised barrier function, further damaging the dermal tissue. Therefore, maintaining skin barrier function against external irritants such as enzymes and bacteria is important for preventing IAD.
In the current study, we focused on skin pH, which is one component of skin barrier function. The skin surface is normally acidic, with a pH ranging from 4 to 6. 11 , 12 This acidic pH helps prevent colonisation by pathogenic bacteria. 11 However, elevated pH was shown to impair skin barrier function by increasing the activity of serine proteases and reducing the activity of ceramide‐generating enzymes. 13 , 14 Therefore, the acidic pH contributes to the maintenance of skin barrier function. When skin is exposed to urine and/or faeces, urease, a major bacterial virulence factor, converts urea into ammonia. Ammonia helps elevate the skin surface pH, resulting in impaired pathogen defence and skin barrier function. As a result, the pathogenic bacterial load increases, with protease activity also increasing in response to the increased skin pH. Therefore, an increased skin pH puts incontinence patients at high risk of developing IAD.
Urease activity is high among bacterial species belonging to the genus Proteus, 15 , 16 which is one of the important intestinal bacteria that have also been associated with the development of urinary infection. 17 Therefore, both urease‐ and protease‐producing bacteria are commonly found in patients with double incontinence or urinary infection, 17 , 18 suggesting that the affected patients are at high risk of developing IAD.
We hypothesised that IAD could be prevented by inhibiting bacterial urease production and preventing the pH elevation. In the current study, we examined the efficacy of a combination of antiseptic and urease inhibitor for inhibiting urea decomposition into ammonia by Proteus mirabilis in vitro, thereby preventing the increase in pH. Because urease may remain around dead bacterial cells, a urease inhibitor and an antiseptic were examined for their ability to inhibit urease production.
2. METHODS
2.1. Bacterial culture
P. mirabilis Hauser 1885 was selected as a model species for this study and was purchased from the National Institute of Technology and Evaluation (Tokyo, Japan). P. mirabilis was cultured overnight in Luria‐Bertani (LB) broth at 37°C with aeration to an optical density at 600 nm (OD600) = 1.0, equivalent to a concentration of 1.2 × 109 colony‐forming units (CFU)/mL. Bacterial culture was diluted to 1:10 for use in the subsequent assays.
2.2. Preparation of urea‐containing solution and artificial urine
We used two types of urea‐containing solvent in the assays: 2% urea‐containing solution and artificial urine. A 20% (wt/vol) urea‐containing stock solution was prepared in distilled water. The stock solution was diluted to 1:10 in LB broth for use in the assays. Artificial urine (10× concentration) was diluted to 1:10 in distilled water for use in the assays. The diluted artificial urine contained 2% urea (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), 0.8% sodium chloride (FUJIFILM Wako Pure Chemical Corporation), 0.03% magnesium sulphate heptahydrate (FUJIFILM Wako Pure Chemical Corporation), and 0.08% calcium chloride dihydrate (FUJIFILM Wako Pure Chemical Corporation) in ion‐exchanged water. The concentration of each component in artificial urine is set to be similar to human urine. 19 Artificial urine was filter‐sterilised immediately prior to use using a 0.45‐μm syringe filter.
2.3. Survival assays and ammonia production in medium supplemented with urea‐containing solution
We used the assay to examine the effects of antiseptic and/or urease inhibitor by checking the bacterial survival and ammonia production (patent number: JP2019‐62806A). Briefly, cultured P. mirabilis was mixed with urea‐containing solution, followed by the addition of antiseptic and/or urease inhibitor depending on the treatment of each experimental group. Bacterial cell density and ammonia concentration were measured after a certain period. Bacterial cell density indicated the growth and survival of P. mirabilis, and ammonia concentration indicated the ammonia production because of the decomposition of urea caused by P. mirabilis.
Four experimental groups were used for the assays in the presence of 2% urea‐containing solution: bacteria and urea only (BU), antiseptic plus bacteria and urea (BU + A), urease inhibitor plus bacteria and urea (BU + I), and antiseptic and urease inhibitor plus bacteria and urea (BU + AI). A no‐urea culture (B) was used as the negative control (Figure 1A).
FIGURE 1.
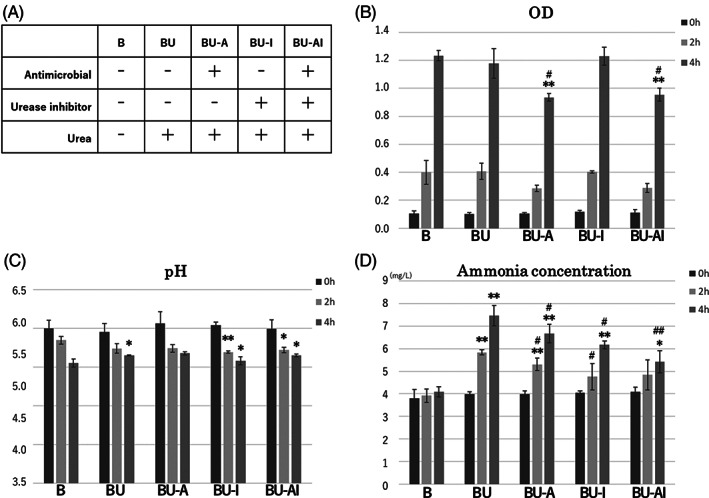
Efficacy of a combination of antiseptic and urease inhibitor for the inhibition of urea decomposition into ammonia by P. mirabilis in urea‐containing solution. A, Four treatment groups with urea (BU, BU‐A, BU‐I, and BU‐AI) and one without urea (B) were prepared. B, OD values were significantly decreased in cultures BU‐A and BU‐AI compared with culture B at 4 hours post‐inoculation. C, The culture pH was significantly increased in the BU treatment compared with that of culture B at 4 hours post‐inoculation. D, The ammonia concentration was significantly increased in the BU culture compared with that of culture B at both 2 and 4 hours post‐inoculation. Cultures BU‐A, BU‐I, and BU‐AI showed significant decreases in ammonia concentration compared with the BU culture at 4 hours post‐inoculation. *P < .05 compared with culture B at the same time point; **P < .01 compared with culture B at the same time point. # P < .05 compared with the BU culture the same time point; ## P < .01 compared with the BU culture at the same time point
For the BU, BU + A, BU + I, and BU + AI cultures, 1 mL of P. mirabilis culture (OD600 = 1.0) was mixed with 1 mL of urea‐containing stock solution and 8 mL of LB broth. The negative control culture (B) contained 1 mL of distilled water instead of the urea‐containing stock solution. Antiseptic reagent Ag‐zeolite (Zeomic, Sinanen Zeomic Co., Aichi, Japan), which is an inorganic agent comprised of silver ion and a microporous, aluminosilicate mineral named zeolite, was added at a concentration of 100 ppm to the BU + A and BU + AI cultures. Green tea extract (FS‐LO 40, Shiraimatsu Pharmaceutical Co., Tokyo, Japan), which is a natural plant extract generated by dry distillation of green tea leaf and acts as a urease inhibitor, was added at a concentration of 1000 ppm to the BU + I and BU + AI cultures.
All cultures were incubated at 37°C on a shaker, with samples collected at 0, 2, and 4 hours post‐inoculation. At each time point, OD600, pH, and ammonia concentration measurements were conducted using a spectrophotometer (DS‐11; DeNovix Inc., Wilmington, DE, USA), pH meter (LAQUAtwin B‐712; Horiba Ltd., Kyoto, Japan) and ammonia meter (Ion meter TiN‐9001i; Tokyo Chemical Laboratories, Tokyo, Japan), respectively.
2.4. Survival assays and ammonia production in artificial urine
The experiments using artificial urine as a solvent were performed to consider more similar conditions in clinical absorbent pads. For the survival and ammonia production assays in artificial urine, 1 mL of cultured P. mirabilis (OD600 = 1.0) was mixed with 9 mL of artificial urine. Several cultures contained 100 mg of water‐absorbent polymer. A water‐absorbent polymer is a cross‐linked polyacrylic acid that contains sodium atoms and can absorb water by a process of osmosis. Water‐absorbent polymer creates a similar condition in the absorbent layer of an adult diaper pad and was also prepared to reproduce the conditions found inside an incontinence diaper. Ag‐zeolite (antiseptic reagent) and green tea extract (urease inhibitor) were added at various concentrations (Ag‐zeolite: 0, 25, 50, 75, or 100 ppm; green tea extract: 0, 250, 500, 750, or 1000 ppm) to the appropriate cultures.
All cultures were incubated at 37°C on a shaker. Samples were collected at 0 and 4 hours post‐inoculation and used for OD600, pH, and ammonia concentration measurements, as described above.
2.5. Data analysis
Dunnett's test was used to carry out multiple comparison analysis of the OD600, pH, and ammonia concentration results between the control and treatment groups at each time point. P‐values <.05 were considered statistically significant. Data were reported as means ± SDs.
3. RESULTS
3.1. Effects of antiseptic and urease inhibitor on bacterial survival and ammonia production in medium with urea‐containing solution
We first examined the ability of a combination of urease inhibitor and antiseptic to inhibit the decomposition of urea into ammonia by P. mirabilis in a medium supplemented with urea‐containing solution. OD600, pH, and ammonia concentration measurements were performed at 0, 2, and 4 hours post‐inoculation of the cultures (Figure 1B–D). Bacterial cell density, as determined by OD600 values, was significantly decreased in the BU‐A and BU‐AI cultures compared with culture B at 4 hours post‐inoculation, indicating that the addition of antiseptic effectively inhibited bacterial growth. The pH was significantly higher in the BU culture compared with that of culture B at 4 hours post‐inoculation, while the ammonia concentration was significantly higher in the BU culture compared with culture B at both 2 and 4 hours post‐inoculation. These results indicated that the additional urea in the BU culture was converted into ammonia by P. mirabilis. In comparison, cultures BU‐A, BU‐I, and BU‐AI all showed significantly lower levels of ammonia compared with the BU culture at 4 hours post‐inoculation, indicating that the antiseptic and urease inhibitor effectively inhibited ammonia production. Moreover, the ammonia concentration of the BU‐AI culture was significantly lower than that of the BU‐A culture (P = .03) and lower than that of the BU‐I culture (P = .06), indicating that the combination of antiseptic and urease inhibitor more effectively inhibited urea decomposition than antiseptic or urease inhibitor alone. We also observed a time‐dependent decrease in the pH within the same experimental group of each culture (B, BU, BU‐A, BU‐I, and BU‐AI) at the same time as the time‐dependent increase in ammonia concentration.
3.2. Effects of antiseptic and urease inhibitor on bacterial survival and ammonia production in artificial urine
Next, we examined the effects of the antiseptic and urease inhibitor on urea decomposition by P. mirabilis in artificial urine, which more closely mimics the urine of clinical patients. We also added a water‐absorbent polymer to the artificial urine to reproduce the conditions found in an absorbent pad. Various concentrations of antiseptic and urease inhibitor were used in these assays, and the optical density, pH, and ammonia concentration were measured at 0 and 4 hours post‐inoculation of the cultures (Figure 2A–C). Immediately after inoculation, the OD values of the artificial urine cultures containing water‐absorbent polymer (OD600 = 0.2) (Figure 2A) were approximately twice those of the urea‐containing cultures (Figure 1B), which did not contain a water‐absorbent polymer. At 4 hours post‐inoculation, the OD values of all treatment groups were significantly decreased compared with that of the culture with no antiseptic or urease inhibitor, with the degree of difference observed to be antiseptic concentration‐dependent. While culture pH varied between treatment groups at baseline, random changes in pH were observed at 4 hours post‐inoculation, indicating that the pH was affected by both ammonia and other reagents such as antiseptic, urease inhibitor, and the bacteria themselves. In all cultures containing antiseptic and/or urease inhibitor, except for the culture containing 25 ppm antiseptic and 250 ppm urease inhibitor, the ammonia concentration was significantly lower than that of the no antiseptic and no urease inhibitor control. In all cases, the ammonia concentration decreased as the concentration of urease inhibitor and antiseptic increased, with the culture containing the highest concentrations of antiseptic (100 ppm) and urease inhibitor (1000 ppm) showing the lowest ammonia concentration among all treatments at 4 hours post‐inoculation. Moreover, measurements of ammonia concentration with 750 ppm urease inhibitor and 100 ppm antiseptic, 1000 ppm urease inhibitor and 75 ppm antiseptic, and 1000 ppm urease inhibitor and 100 ppm antiseptic were 74.2 ± 6.1, 61.8 ± 1.6, 56.7 ± 4.5 mg/L, respectively, at 4 hours post‐inoculation. These measurements were ≥ 50% lower than ammonia concentration of no antiseptic and no urease inhibitor control (153.2 ± 9.9 mg/L).
FIGURE 2.
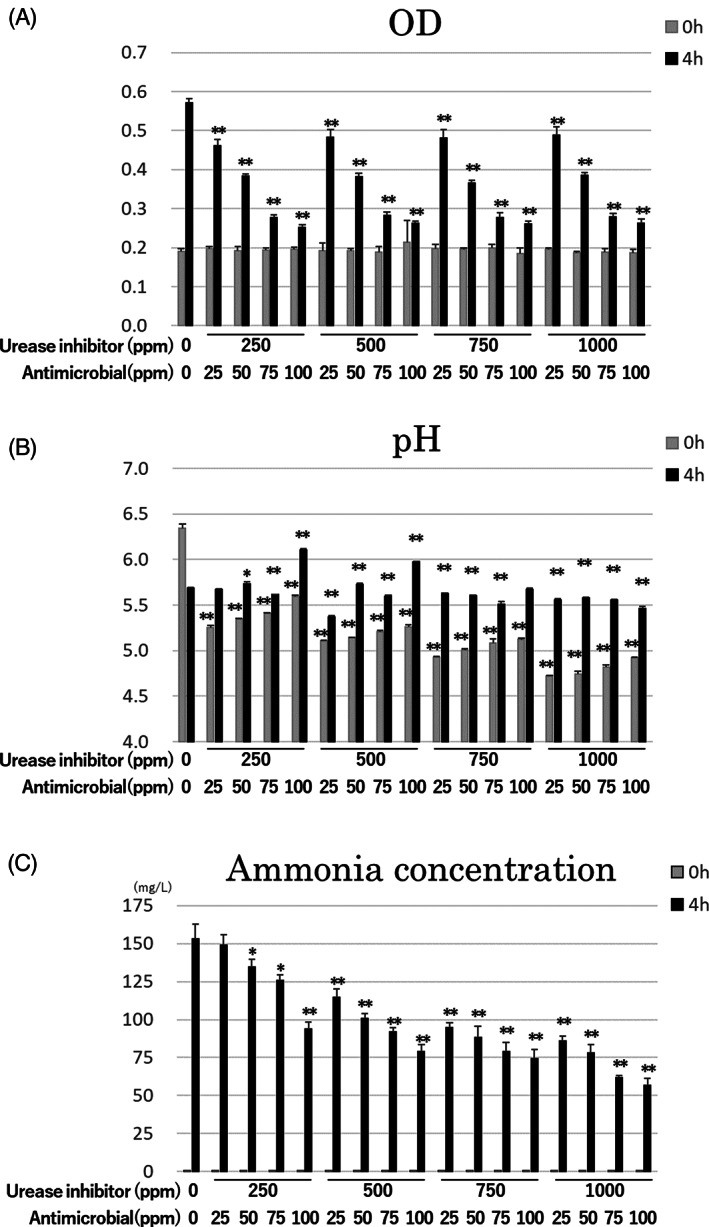
Efficacy of various concentration of antiseptic and urease inhibitor for the inhibition of urea decomposition into ammonia by P. mirabilis in artificial urine. A, OD values at 4 hours post‐inoculation for all treatment groups were significantly decreased compared with the culture without antiseptic or urease inhibitor, with decreases in OD occurring in an antiseptic concentration‐dependent manner. B, The pH values of the different cultures varied immediately after inoculation (0 hours), with random pH changes observed at 4 hours post‐inoculation. C, The ammonia concentrations of all treatment groups were significantly decreased compared with that of the culture without antiseptic or urease inhibitor, with decreases in ammonia concentration associated with increases in the concentrations of urease inhibitor and antiseptic. *P < .05 compared with the culture containing 0 ppm urease inhibitor and 0 ppm antiseptic at the same time point; **P < .01 compared with the culture containing 0 ppm urease inhibitor and 0 ppm antiseptic at the same time point
4. DISCUSSION
This is the first study to examine a new concept for the prevention of the development of IAD by preventing skin pH increases as a result of urea decomposition. Our results showed that a combination of antiseptic and urease inhibitor effectively inhibited urea decomposition into ammonia through the inhibition of bacterial urease production. We found that both urease inhibitor and antiseptic inhibited ammonia increases, although no pH increases were observed.
The assays in the current study were carried out in two different media: LB medium supplemented with urea‐containing solution and artificial urine. The urea‐containing solution only contained urea and allowed us to examine the inhibition of urea decomposition under simplified conditions. We used artificial urine in subsequent assays to examine the efficacy of the treatment under conditions more similar to those found in clinical incontinence patients. The efficacy of the antiseptic and urease inhibitor treatment was shown under both experimental conditions. The results suggested that inhibition of urea decomposition is effective for the prevention of IAD and that the use of antiseptic and urease inhibitor in clinical incontinence patients is beneficial for the prevention of adverse events caused by ammonia.
We used a water‐absorbent polymer in the artificial urine assays to better reproduce the conditions found inside absorbent pads. At 4 hours post‐inoculation, ammonia concentrations were up to 20‐fold higher (~150 mg/L) (Figure 2C) than those observed in medium without the water‐absorbent polymer (~7.5 mg/L) (Figure 1D). We hypothesize that the urea‐containing solution and bacterial culture were concentrated by the water‐absorbent polymer, resulting in the observed increase in ammonia concentration.
Next, we examined the effects of different concentrations of urease inhibitor and antiseptic ammonia concentrations in artificial urine. OD values at 4 hours post‐inoculation were significantly decreased for all treatments compared with the no antiseptics and no urease inhibitor control, with the OD observed to decrease in an antiseptic concentration‐dependent manner. These results indicate that the concentration of the antiseptic is directly related to the level of inhibition of bacterial growth. Three treatments, including 750 ppm urease inhibitor and 100 ppm antiseptic, 1000 ppm urease inhibitor and 75 ppm antiseptic, and 1000 ppm urease inhibitor and 100 ppm antiseptic, decreased the ammonia concentration by at least 50% at 4 hours post‐inoculation compared with the no antiseptic and no urease inhibitor control. These results indicate that, while both antiseptic and urease inhibitor inhibit ammonia production, a combination of high concentrations of both antiseptic and urease inhibitor results in a more effective inhibition of ammonia production. However, because there is no threshold of ammonia concentration for effective prevention of IAD, further examination is necessary to consider how much ammonia concentration should be decreased for the effective prevention of IAD in clinical settings.
We attempted to reproduce in vitro the mix of urine and bacteria that is observed in clinical situations; however, we could not reproduce the pH increase caused by urea decomposition into ammonia. Theoretically, the culture pH should increase in line with increases in ammonia concentration. However, in this study, the pH decreased in a time‐dependent manner in cultures supplemented with urea‐containing solution. It is possible that P. mirabilis affects the decreases in pH. A previous study reported that P. mirabilis differentiates into swarmer morphotypes under acidic conditions. 20 Although there are no reports of acid production by P. mirabilis, it may produce some acid as a virulence factor in optimal growth environments. Moreover, in the assays using artificial urine, the pH level at 0 hours (immediately after inoculation) varied among treatments, with the pH level appearing to increase with increases in urease inhibitor concentration and to decrease with increases in antiseptic concentration. Urease inhibitor, antiseptic, and water‐absorbent polymer also affected the variation in pH level. Therefore, we recommend that pH level should not be used as an indicator of ammonia concentration and high‐risk conditions for the development of IAD in in vitro studies because of several factors other than ammonia that affect the pH.
Although pH level could not be used as an indicator to assess the effects of the treatment in the current study, ammonia levels reflected the efficacy of the urease inhibitor and antiseptic in the inhibition of urea decomposition into ammonia. Our findings suggest that the use of both urease inhibitor and antiseptic may effectively prevent the development of IAD. Thus, preventive strategies or products containing both urease inhibitor and antiseptic, such as absorbent pads containing both treatments, will likely be effective for the prevention of IAD in clinical settings, significantly improving the quality of life of many patients suffering from IAD.
We chose 4 hours post‐inoculation for collecting the endpoint measures. A clinical study performed by Fader et al. 21 showed that the 4‐hour pad change frequency was effective compared with 8‐hour pad changes in preventing the skin from becoming wetter, which can contribute to preventing skin maceration and the development of IAD. Moreover, rat model showed that 4‐hour skin treatment with agarose gel containing a proteolytic solution resulted in skin maceration, as well as inner tissue damage in histology. 9 Based on these previous reports, we consider that our experimental design of collecting the endpoint measures at 4‐hour post‐inoculation is adequate.
This study has several limitations. First, the efficacy of antiseptic and urease inhibitor was only examined in vitro. Further research, including in vivo assays and clinical surveys, is needed to confirm the efficacy of the combined treatment for preventing IAD. Second, we only used P. mirabilis in the current study. There are several other bacterial species commonly found in the urine and/or faeces of clinical incontinence patients. Therefore, additional studies using several different bacterial species are needed to better understand the effects of urine and bacteria in the development of IAD and to assess whether the urease inhibitor/antiseptic treatment combination is also effective against these other species.
5. CONCLUSION
This study examined the efficacy of antiseptic and urease inhibitor in inhibiting urea decomposition into ammonia by P. mirabilis‐produced urease. While both the antiseptic and the urease inhibitor prevented urea decomposition, a combination of the two was more effective than either treatment alone. Novel preventive strategies or products using both a urease inhibitor and an antiseptic will be effective for the prevention of IAD in a clinical setting.
CONFLICT OF INTEREST
The reagents of artificial urine, urease inhibitor, and antiseptic were generously provided by Daio Paper Corporation, Tokyo, Japan. The company also lent the device used for the measurement of ammonia concentration. Conducting the study and writing of the article were carried out without financial support from any company. One of the authors (Takeo Minematsu) belongs to the department sponsored by Saraya Cooperation, Osaka, Japan. The company had no role on the study concept, design, data collection and analysis, and manuscript drafting.
AUTHOR CONTRIBUTIONS
The reagents of artificial urine, urease inhibitor, and antiseptic were generously provided by Daio Paper Corporation, Tokyo, Japan. The company also lent the device used for the measurement of ammonia concentration. Conducting the study and writing of the article were carried out without financial support from any company. One of the authors (Takeo Minematsu) belongs to the department sponsored by Saraya Cooperation, Osaka, Japan. The company had no role on the study concept, design, data collection and analysis, and manuscript drafting.
ACKNOWLEDGEMENTS
We thank Tamsin Sheen, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was financially supported by JSPS KAKENHI (17H06648, 19K20756).
Mugita Y, Nakagami G, Minematsu T, Kitamura A, Sanada H. Combination of urease inhibitor and antiseptic inhibits urea decomposition‐induced ammonia production by Proteus mirabilis . Int Wound J. 2020;17:1558–1565. 10.1111/iwj.13422
Funding information JSPS KAKENHI, Grant/Award Numbers: 17H06648, 19K20756
REFERENCES
- 1. Bliss DZ, Savik K, Harms S, Fan Q, Wyman JF. Prevalence and correlates of perineal dermatitis in nursing home residents. Nurs Res. 2006;55(4):243‐251. [DOI] [PubMed] [Google Scholar]
- 2. Bliss DZ, Harms S, Garrard JM, et al. Prevalence of incontinence by race and ethnicity of older people admitted to nursing homes. J Am Med Dir Assoc [Internet]. 2013;14(6):451.e1‐451.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farage MA, Miller KW, Elsner P, Maibach HI. Structural characteristics of the aging skin: a review. Cutan Ocul Toxicol. 2007;26(4):343‐357. [DOI] [PubMed] [Google Scholar]
- 4. Gray M, Bliss DZ, Doughty DB, Ermer‐Seltun J, Kennedy‐Evans KL, Palmer MH. Incontinence‐associated dermatitis: a consensus. J Wound Ostomy Cont Nurs. 2007;34(1):45‐54. quiz 55–6. [DOI] [PubMed] [Google Scholar]
- 5. Kon Y, Ichikawa‐Shigeta Y, Iuchi T, et al. Effects of a skin barrier cream on management of incontinence‐associated dermatitis in older women. J Wound Ostomy Cont Nurs. 2017;44(5):481‐486. [DOI] [PubMed] [Google Scholar]
- 6. Beeckman D, Verhaeghe S, Defloor T, Schoonhoven L, Vanderwee K. A 3‐in‐1 perineal care washcloth impregnated with dimethicone 3% versus water and pH neutral soap to prevent and treat incontinence‐associated dermatitis: a randomized, controlled clinical trial. J Wound Ostomy Cont Nurs. 2011;38(6):627‐634. [DOI] [PubMed] [Google Scholar]
- 7. Park KH. The effect of a silicone border foam dressing for prevention of pressure ulcers and incontinence‐associated dermatitis in intensive care unit patients. J Wound Ostomy Cont Nurs. 2014;41(5):424‐429. [DOI] [PubMed] [Google Scholar]
- 8. Gray M, Beeckman D, Bliss DZ, et al. Incontinence‐associated dermatitis: a comprehensive review and update. J Wound Ostomy Cont Nurs. 2012;39(1):61‐74. [DOI] [PubMed] [Google Scholar]
- 9. Mugita Y, Minematsu T, Huang L, et al. Histopathology of incontinence‐associated skin lesions: inner tissue damage due to invasion of proteolytic enzymes and bacteria in macerated rat skin. PLoS One. 2015;10(9):e0138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mugita Y, Minematsu T, Nakagami G, Sanada H. Influence of digestive enzymes on development of incontinenceassociated dermatitis: inner tissue damage and skin barrier impairment caused by lipidolytic enzymes and proteases in rat macerated skin. Int Wound J. 2018;15(4):623‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Proksch E. pH in nature, humans and skin. J Dermatol. 2018;45(9):1044‐1052. [DOI] [PubMed] [Google Scholar]
- 12. Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261‐267. [DOI] [PubMed] [Google Scholar]
- 13. Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol [Internet]. 2003;121(2):345‐353. [DOI] [PubMed] [Google Scholar]
- 14. Hachem JP, Man MQ, Crumrine D, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol [Internet]. 2005;125(3):510‐520. [DOI] [PubMed] [Google Scholar]
- 15. Armbruster CE, Mobley HLT, Pearson MM. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus. 2018;8(1). 10.1128/ecosalplus.ESP-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richmond S, Yep A. Quantification of urease activity. Methods in molecular biology. New York: Humana Press Inc.; 2019:85‐96. [DOI] [PubMed] [Google Scholar]
- 17. Drzewiecka D. Significance and roles of Proteus spp. bacteria in natural environments. Microb Ecol. 2016;72(4):741‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith JS, Ediss I, Mullinger MA, Bogoch A. Fecal chymotrypsin and trypsin determinations. Can Med Assoc J. 1971;104(8):691. [PMC free article] [PubMed] [Google Scholar]
- 19. Putnum DF. Composition and concentrative properties of human urine. NASA Contract Reports [Internet]. 1971. [cited May 15, 2020]. https://ntrs.nasa.gov/search.jsp?R=19710023044
- 20. Fujihara M, Obara H, Watanabe Y, et al. Acidic environments induce differentiation of Proteus mirabilis into swarmer morphotypes. Microbiol Immunol. 2011;55(7):489‐493. [DOI] [PubMed] [Google Scholar]
- 21. Fader M, Clarke‐O'Neill S, Cook D, et al. Management of night‐time urinary incontinence in residential settings for older people: an investigation into the effects of different pad changing regimes on skin health. J Clin Nurs. 2003;12:374‐386. [DOI] [PubMed] [Google Scholar]


