Abstract
Brucellosis is a common zoonotic disease worldwide. It has protean clinical manifestation and sometimes may has a life-threatening complication. A 4-year-old boy presented with a history of fever, myalgia and appetite loss for 3 weeks. On examination, he had hepatosplenomegaly. The initial working diagnosis was an infection, autoimmune disease and malignancy. Investigations showed positive Brucella serology, and he was started on rifampicin and cotrimoxazole. He was further investigated because of persistent fever, which revealed evidence of haemophagocytic lymphohistiocytosis (HLH). He continued treatment for brucellosis, except rifampicin which was replaced with doxycyclin due to a worsening liver function. The child showed complete clinical and biochemical improvement after 6 weeks of therapy. HLH is a life-threatening condition and should be suspected in children with brucellosis, who did not respond to appropriate antibiotics treatment. Secondary HLH does not always require specific therapy; it may improve with adequate treatment of the underlying condition.
Keywords: tropical medicine (infectious disease), paediatric prescribing, haematology (incl blood transfusion)
Background
Brucellosis is the most common zoonotic infection worldwide. The reported prevalence of brucellosis in children is 10%–30% in the endemic region. Brucella melitensis is the most common species to cause infection in humans.1 In children, infection is usually transmitted by consuming unpasteurised milk, dairy product, direct contact with the infected animals (sheep, goats, camels and buffalo) and rarely with aerosol inhalation. Its clinical manifestations varied from mild disease to severe disease complicated by a life-threatening condition. The fever is the most common presentation (>75%) of brucellosis, and it can be the sole manifestation in children.1 2
Brucellosis has an excellent prognosis with appropriate treatment; however, it can have a poor prognosis in complicated cases.1 2 Complication usually occurs with specific organ involvement, and it is more common in adult patients. Haematological manifestations include anaemia, thrombocytopenia, leucopenia or pancytopenia. Haemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening haematological complication of brucellosis.2 3
This case report aims to increase awareness among clinicians to keep brucellosis as one of the differential diagnoses for fever of unknown origin (FUO). It may get complicated with a life-threatening condition, which needs timely diagnosis and appropriate treatment.
Case presentation
A 4-year-old boy presented to us with fever, myalgia and loss of appetite for 20 days. Fever was sudden in onset, high-grade, continuous, not associated with rigour, no diurnal variation, relieved partly with oral medication. It was initially associated with 3–4 vomiting episodes per day, non-projectile, non-bilious, not blood-stained and only contained food particles. It was associated with epigastric pain, which was intermittent, non-spasmodic not associated with food intake. There was no history of cough, cold, loose motion, yellow discoloration of eye or urine, skin rash, joint pain, burning micturition/haematuria, neck stiffness, loss of consciousness, abnormal movement, headache and bleeding manifestations. There was no travel history, drinking unboiled or uncooked animal milk, or meat consumption. He consulted locally on day 2 of illness and was diagnosed with dengue fever and given supportive care. Since fever was persisting, so he received intravenous antibiotics for 2 weeks. Despite that, he continued to have high-grade fever; therefore, he was referred to our institute. There was no history of blood transfusion. His perinatal period was uneventful; development and immunisation were appropriate for age. There was no similar illness in other family members.
Examination at admission; the child was conscious and alert. In vitals, body temperature was 99.9°F, BP was 88/50 mm Hg (50th centile), HR was 116/min, respiratory rate (RR) was 24/min and SpO2 was 100% in room air. There was no pallor, icterus, cyanosis, clubbing, pedal oedema and significant lymphadenopathy. All the quadrant were moving symmetrically on the abdominal examination, and there was no abdominal distension, visible masses, dilated veins or visible peristalsis. The liver was palpable 4 cm below the right costal margin with a span of 9-cm, non-tender, firm and sharp margins. The spleen was palpable 2 cm below the left costal margin, firm, non-tender, and the notch was discernible. Chest, cardiovascular, musculoskeletal and central nervous system examination was normal. The initial working diagnosis was systemic infection (bacterial, viral, protozoal or rickettsial), and we investigated him for the same.
Investigations
Baseline haemograms are presented in table 1. The peripheral blood smear showed a normocytic, normochromic pattern with adequate cell lines, and no haemoparasite. The inflammatory markers were elevated (C reactive protein: 25.9 mg/L and erythrocyte sedimentation rate: 60 mm/hour). His liver function test (LFT) showed elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with normal serum bilirubin. The kidney function test was within the normal range. The work-up for viral infections was negative [HIV-1 and HIV-2, hepatitis B, hepatitis C and nonstructural protein-1 (NS1) antigen and dengue serology]. Work-up for enteric fever (typhoid immunoglobulin M (IgM)/IgG, Widal test and an aerobic blood culture), urinary tract infection (routine urine and culture), malaria (rapid antigen test, thin and thick smear), tuberculosis (TB) (imaging, Mantoux test, gastric lavage and induced sputum for ZN staining, GeneXpert) and rickettsial infection (Anti-Scrub Typhus IgM ELISA) were negative. Blood culture was also sterile. Ultrasound and CT scan of the chest and abdomen were normal except for hepatosplenomegaly. Two-dimensional echocardiography was done to exclude infective endocarditis, which was normal. For connective tissue work-up, antinuclear antigen was negative, and there was no evidence of uveitis on eye examination. Brucella serology was positive for IgM.
Table 1.
Investigation at baseline and follow-up
| Investigations | Normal range (unit) |
At admission | Day 17 of admission | First Follow-up (two weeks of therapy) |
Second follow-up (six weeks of therapy) |
| Haemoglobin | 120–140 (g/L) | 107 g/L | 81 g/L | 107 g/L | Not done |
| Total leucocyte counts | 4–11 (109/L) | 8.3 | 5.8 | 7.4 | |
| Differential leucocyte count | (%) | ||||
| Neutrophils (%) | 55–60 | 25.8 | 17 | 25.3 | |
| Lymphocytes (%) | 35–40 | 65.4 | 74 | 62.7 | |
| Monocytes (%) | 1–5 | 08 | 7 | 8.5 | |
| Platelets | 150–450 (109/L) | 5.7 | 3.69 | 3.48 | |
| C-reactive protein (CRP) | <0.5 (mg/L) | 25.9 mg/L | Not done | 0.32 mg/L | |
| Erythrocyte sedimentation rate (ESR) | 0–20 (mm/hour) | 60 mm/hour | 07 mm/hour | ||
| Liver function test | |||||
| Alanine aminotransferase (ALT) | 0–20 (U/L) | 168 U/L | 168 U/L | 179 U/L | 78 U/L |
| Aspartate aminotransferase (AST) | 0–20 (U/L) | 270 U/L | 402 U/L | 240 U/L | 90 U/L |
| Alkaline phosphate (ALP) | 250–450 (U/L) | 427 U/L | 707 U/L | 753 U/L | 255 U/L |
| Total bilirubin | 0–1.2 (mg/dL) | 0.41 mg/dL | 0.47 mg/dL | 0.5 mg/dL | 0.34 mg/dL |
| Triglyceride | <265 (mg/dL) | Not done | 573 mg/dL | 541 mg/dL | 196 mg/dL |
| Fibrinogen | <1.5 (g/L) | 2.58 g/L | 3.78 g/L | 1.86 g/L | |
| Ferritin | <500 (mcg/L) | 1650 mcg/L | 827.5 mcg/L | 37.0 mcg/L | |
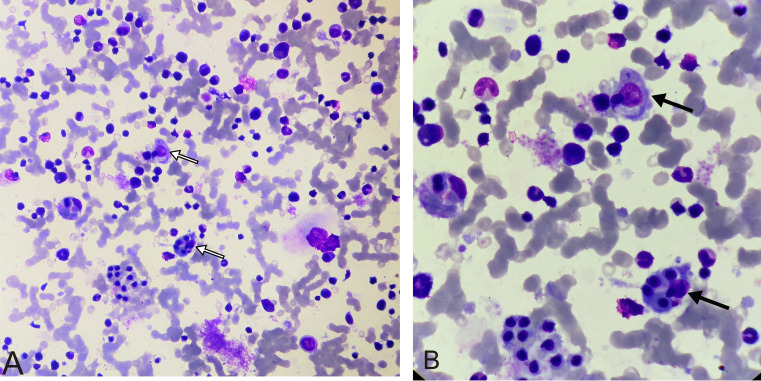
Bone marrow aspirate and biopsy smears were cellular, and all the haematopoietic components showed normal sequential maturation. There was no evidence of atypical cells, haemoparasites or granuloma. There was marked histiocytic prominence with evidence of haemophagocytosis (figure 1). Other investigations in support of HLH were also positive (table 1).
Figure 1.
Bone marrow aspirate smear. (A) Giemsa staining (40×) shows all haematopoietic components with evidence of haemophagocytosis (white arrows). (B) Giemsa staining (100×) shows erythroid and myeloid components with evidence of haemophagocytosis (black arrows).
Differential diagnosis
The initial working diagnose was infection (viral (dengue fever and HIV), bacterial (enteric fever, urinary tract infection brucellosis and infective endocarditis), mycobacterial (disseminated TB), protozoal (malaria) and rickettsia fever). The work-up for infection was negative except for positive IgM serology for Brucella. However, in view of persistent fever despite appropriate antibiotic therapy, autoimmune diseases (systemic-onset juvenile rheumatoid arthritis and other connective tissue disorder) and malignancy (lymphoma and or leukaemia) were also considered and were excluded. In echocardiography, there was no evidence of infective endocarditis. HLH secondary to brucellosis was also kept. The clinical features and investigations suggested the diagnosis of HLH.
Treatment
Initially, the child was started on injection cefotaxime considering inadequately treated enteric fever. On day 5 of admission, antibiotics were upgraded to azithromycin with a possibility of drug-resistant enteric fever. On day 8, his serology for brucellosis was turned out to be positive. As the child was <8 years old, we started him on oral rifampicin and trimethoprim+sulfamethoxazole (TMP-SMX) combination. Two days later (day 10), the child developed pain abdomen and had two vomiting episodes. Repeat LFTs showed rising AST and ALT levels. We thought of drug-induced hepatitis, and rifampicin was replaced with injection gentamycin. Despite these antibiotics, he continued to have a high spike fever. On day 15, he was started on oral doxycycline after discussing its possible adverse effects with the parents. On day 17, fever was still persisting; hence, bone marrow examination was performed to exclude malignancy and HLH. Other investigations for HLH were also sent, which were in favour of the HLH (table 1). On day 20, the report of the bone marrow confirmed the diagnosis of HLH (figure 1). However, he started to show improvement in fever by that time with decreased intensity and frequency. We discussed the possible treatment option and prognosis of HLH with the parents, and it was decided to continue same therapy for a few more days with close clinical and laboratory monitoring. He became afebrile on day 23 and he was discharged with a plan to complete antibiotics (doxycycline and TMP-SMX) for a total of 6 weeks and a follow-up in the outpatient department.
Outcome and follow-up
He was first followed up at 2 weeks of therapy to look for disease progression and adverse effects. He remained afebrile, his appetite also improved and there were no new symptoms. Liver and spleen size were also decreased. At 6 weeks of therapy, he remained asymptomatic, and laboratory parameters were also improved (table 1). There was a complete regression of the liver and spleen to normal. After 3 months of therapy, there were no new symptoms, and weight gain was also observed. After 11 months as per telemedicine consultation, there was no recurrence of symptoms.
Discussion
Brucellosis is one of the common causes of FUO in the endemic region in children. The typical presentations of brucellosis in children are fever, malaise, arthritis, hepatosplenomegaly, lymphadenopathy, abdominal pain and cytopenia.1 2 It is usually transmitted by consumption of unpasteurised dairy products; however, it can occur even in their absence, as in the index case, there was no such history.2 4 Brucellosis can be suspected based on characteristics clinical features, especially in an endemic area. The definitive diagnosis can be established by the isolation of Brucella from blood, bone marrow or other tissue. However, culture is usually insensitive, is time consuming and has poor yield. Various serological tests have been also used in the diagnosis of brucellosis.1 2
HLH is a potentially life-threatening condition characterised by marked proliferation of histiocytes and haemophagocytosis. It has two types: primary and secondary. The primary or familial HLH is due to underlying genetic defects and usually manifests in infancy. The secondary HLH (sHLH) may trigger by underlying infections, connective tissue disorder, malignancy, metabolic diseases, immunodeficiency, etc. It can manifest at any age.5 6 Systemic infection due to viruses, bacteria, protozoan, helminthic fungal and rickettsia can develop sHLH.7 8 A systemic review by Cascio et al had observed that brucellosis, rickettsial diseases and Q fever were the most common bacterial infections responsible for sHLH.3 The prevalence of all forms of HLH in children (<18 years) has been reported as 1.0/100 000; however, the exact prevalence of sHLH is unknown.9
The postulated mechanism of sHLH is an uncontrolled immunological reaction of mononuclear phagocytic system and T-cells results in cytokines (Tumor Necrosis Factor (TNF)-α and interferon-γ) storm, which can cause tissue damage.8 9 HLH 2004 protocol is the most widely used for the diagnosis of HLH; at least five out of eight criteria should be present to establish the diagnosis.3 5 9 Our case had fulfilled six out of eight criteria (fever, splenomegaly, cytopenia (haemoglobin: <90 g/L and neutrophil: <1000/dL), raised triglyceride (≥265 mg/dL), elevated serum ferritin (≥500 µg/L) and the presence of haemophagocytes on bone marrow examination).
For the treatment of brucellosis in children, antibiotics (doxycycline, rifampin, aminoglycosides, TMP-SMX, etc.) in combinations are used for 4–6 weeks.1 4 9 Deranged LFT may be due to brucellosis infection per se, or it may be the part of HLH.2 4 Such a condition poses a significant challenge in treating brucellosis as drugs (rifampicin and TMP-SMX) can further worsen liver function. It happened in the index case, which required to change in the medication. There is some concern about doxycycline’s adverse effect in children <8 years of age; however, it may be used in young children in the selected cases. Yaman et al had successfully treated a 4-year-old child with doxycycline in combination with gentamycin without any adverse effect.10
The management of sHLH includes the treatment of underlying infection along with supportive care. Although it may be sufficient in most cases, some patients may require additional therapy viz systemic steroid, IVIG, cyclosporine or etoposide.3 5 9 Yaman et al had used intravenous immunoglobulin (IVIG) in addition to antibiotics in three children with brucellosis and sHLH.10 Erduran E et al reported a case of Brucellosis and HLH in 8 years old child, and he responded well with antibiotic therapy alone and did not use any additional treatment.11 In a retrospective study of 45 children with sHLH, most of them improved with supportive care alone, and only one-third required IVIG.6 A systematic review of sHLH in zoonosis had concluded that currently the addition of immunosuppressive therapy in sHLH is not straightforward, and more evidence is required.3 Relapse may occur in 5%–15% of cases within 1 year of diagnosis.1 In the index case, the child was entirely improved with brucellosis treatment and remained asymptomatic during follow-up.
In conclusion, Brucellosis is a common cause of FUO in children, which may get complicated with sHLH, a life-threatening condition; however, timely diagnosis and institution of early treatment for underlying infection with close clinical and laboratory monitoring could be lifesaving.
Learning points.
Brucellosis should be considered in the workup of fever of unknown origin (FUO) in children even in the absence of a positive history of consumption of unpasteurised dairy products.
Persistence fever despite adequate treatment for brucellosis may point towards underlying complications.
The treatment of underlying infection in secondary haemophagocytic lymphohistiocytosis (HLH) may be sufficient in most cases, while a few cases may require additional therapy for HLH.
Close clinical and laboratory monitoring is crucial for the successful management of HLH.
Footnotes
Contributors: PK and JPG: conceptualise and design the manuscript. JM: involved in the data collection and preparation of the initial draft. AP: involved in the acquisition and formatting pathological image, and writing the initial draft. All the authors were involved in the editing and critical review of the manuscript and gave final approval for the submission. PK will act as a guarantor for this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Downes Kevin J. Brucella. In: Kliegman RM, Geme JW, Blum Nathan J, et al., eds. Nelson textbook of pediatrics. 21st edn, 2020: 1536–8. [Google Scholar]
- 2.Bosilkovski M, Krteva L, Caparoska S, et al. Childhood brucellosis: review of 317 cases. Asian Pac J Trop Med 2015;8:1027–32. 10.1016/j.apjtm.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Cascio A, Pernice LM, Barberi G, et al. Secondary hemophagocytic lymphohistiocytosis in zoonoses. A systematic review. Eur Rev Med Pharmacol Sci 2012;16:1324–37. [PubMed] [Google Scholar]
- 4.Bukhari E. Pediatric brucellosis. An update review for the new millennium. Saudi Med J 2018;39:336–41. 10.15537/smj.2018.4.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henter J-I. HLH-2004 treatment protocol of the second International HLH study 2004. Hemophagocytic Lymphohistiocytosis Study Group 2004. [Google Scholar]
- 6.Nandhakumar D, Loganatha A, Sivasankaran M, et al. Hemophagocytic lymphohistiocytosis in children. Indian J Pediatr 2020;87:526–31. 10.1007/s12098-020-03190-6 [DOI] [PubMed] [Google Scholar]
- 7.Oguz MM, Sahin G, Altinel Acoglu E, et al. Secondary hemophagocytic lymphohistiocytosis in pediatric patients: a single center experience and factors that influenced patient prognosis. Pediatr Hematol Oncol 2019;36:1–16. 10.1080/08880018.2019.1572253 [DOI] [PubMed] [Google Scholar]
- 8.Sen ES, Steward CG, Ramanan AV. Diagnosing haemophagocytic syndrome. Arch Dis Child 2017;102:279–84. 10.1136/archdischild-2016-310772 [DOI] [PubMed] [Google Scholar]
- 9.George MR. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J Blood Med 2014;5:69. 10.2147/JBM.S46255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaman Y, Gözmen S, Özkaya AK, et al. Secondary hemophagocytic lymphohistiocytosis in children with brucellosis: report of three cases. J Infect Dev Ctries 2015;9:1172–6. 10.3855/jidc.6090 [DOI] [PubMed] [Google Scholar]
- 11.Erduran E, Makuloglu M, Mutlu M. A rare hematological manifestation of brucellosis: reactive hemophagocytic syndrome. J Microbiol Immunol Infect 2010;43:159–62. 10.1016/S1684-1182(10)60025-4 [DOI] [PubMed] [Google Scholar]