Abstract
A 68-year-old Chinese man was found to have a lobular mass in the sphenoid sinus which extended to the clivus and the roof of the nasopharynx on a staging MRI scan performed for his high-grade parotid salivary duct carcinoma. Further positron emission tomography scan showed that this lesion was fluorodeoxyglucose (FDG) avid. This proved to be a diagnostic dilemma. The patient underwent a total parotidectomy, left selective neck dissection and a transphenoidal biopsy of his nasal lesion. Final histology revealed that this lesion was a synchronous ectopic sphenoid sinus pituitary adenoma (ESSPA). Initial differential diagnoses that were considered included a chordoma, metastatic carcinoma and nasopharyngeal carcinoma. However, an important differential with a neoplastic appearance and a tendency for positive FDG uptake is an ESSPA. It requires dedicated immunohistochemical staining to diagnose, and its mainstay of treatment is surgical excision.
Keywords: ear, nose and throat/otolaryngology, neuroendocrinology, head and neck cancer, radiology
Background
Sphenoid sinus tumours are rare, of which ectopic sphenoid sinus pituitary adenoma (ESSPA) is one of the most uncommon. When a hypermetabolic sphenoid lesion was identified during a positron emission tomography (PET) scan of a patient with an aggressive parotid carcinoma, it proved to be a diagnostic dilemma to rule out metastatic cancer versus a second primary tumour. We present a case report of an ESSPA and a review of the literature. This publication aims to highlight another facade of this rare entity.
Case presentation
A 68-year-old Chinese man presented with a gradually enlarging left parotid swelling of 1-year duration. He had no nasal, neurologic or systemic symptoms. On examination, he had a 3×3 cm left parotid tumour which was attached to the overlying skin. His flexible nasoendoscopy was normal apart from a subtle smooth mucosal bulge at the roof of his nasopharynx.
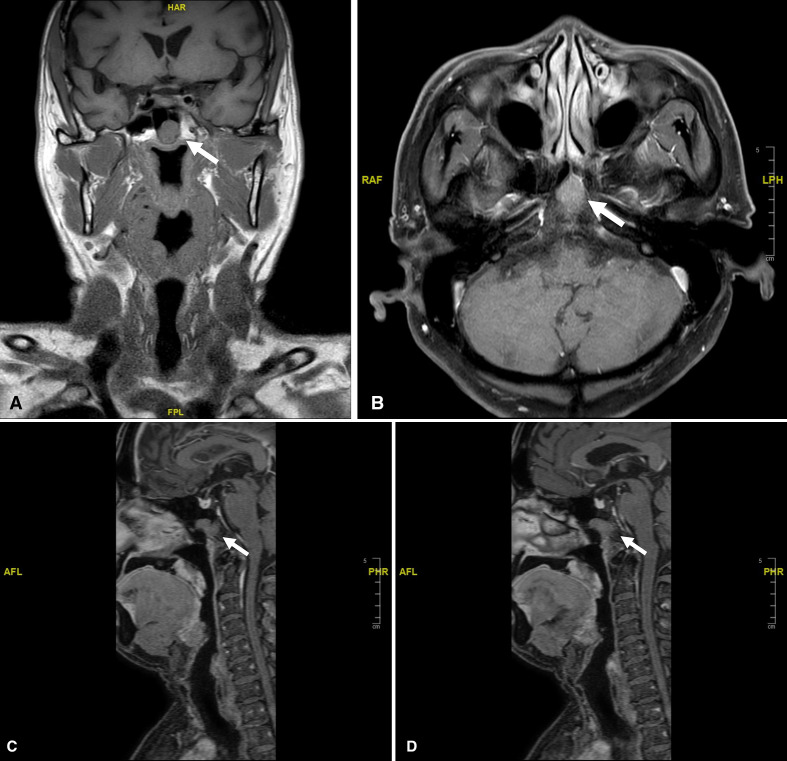
The malignant tumour was worked up preoperatively with an MRI neck and posterior nasal space. There was a 1.7×1.5×1.8 cm lobulated mass in the superficial lobe of the left parotid gland. The margins appeared ill defined and the lesion is seen to breach the parotid capsule with extension into the subcutaneous fat. There were slightly enlarged bilateral level II cervical nodes, measuring 1.5×0.9 cm on the right and 1.3×1.0 cm on the left, these were clinically indeterminate. The scan also showed an incidental finding of a lobular mass in the sphenoid sinus which extended to the clivus posteriorly and the roof of the nasopharynx inferiorly (figure 1A–D). The sphenoid sinus lesion measured 1.6×2.3 cm, appearing hypointense and isointense on T1-weighted and T2-weighted images, respectively, with moderate enhancement. The sagittal cuts show that there is no connection between this lesion and the normal sella pituitary. This incidental finding of a small circumscribed midline sphenoid lesion was initially regarded as an insignificant notochord remnant. His parotid fine needle aspiration cytology showed an oncocytic lesion (Milan category 4B).1
Figure 1.
MRI showing sphenoid lesion (white arrow). (A) T1-weighted MRI (coronal). (B) T1-weighted MRI (axial). (C) T1-weighted MRI (sagittal midline) showing a normal sella pituitary and separate sphenoid lesion. (D) T1-weighted MRI (sagittal left side) showing sphenoid lesion extends to the clivus.
Our patient was counselled for a total parotidectomy. The option for frozen section and selective neck dissection was discussed, but our patient chose to wait for the final histology before further surgery. He underwent a total parotidectomy which showed a 3 cm hard tumour in the superficial lobe of the left parotid gland. A cuff of skin was excised enbloc as the tumour was adherent to it. There were no enlarged nodes noted intraoperatively.
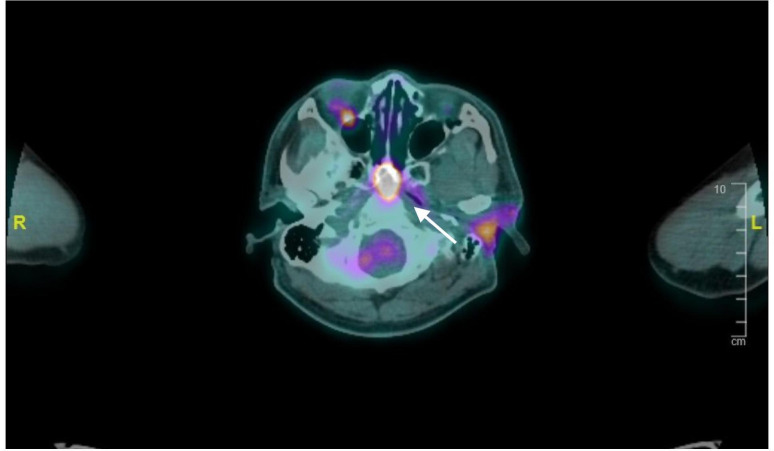
His postoperative histology showed high-grade salivary duct carcinoma with macroscopic tumour extension into subcutaneous fat. In view of this, a PET scan was arranged to assess for distant metastases and plan for adjuvant therapy. Unexpectedly, his PET scan revealed that the sphenoid sinus lesion was fluorodeoxyglucose (FDG) avid with a standardised uptake value (SUV) of 17.9 and appeared to be eroding into the sphenoid sinus and the anterior margin of the clivus (figure 2). This finding was especially worrisome in a clinical setting of aggressive parotid salivary duct carcinoma and warranted urgent further evaluation. The PET scan also showed mildly FDG avid left level II cervical nodes (SUVmax 3.9).
Figure 2.
Positron emission tomography scan showing flurodeoxyglucose–avid sphenoid sinus lesion (white arrow).
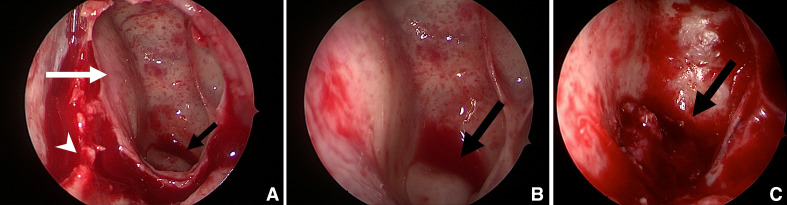
He underwent a left selective neck dissection of levels I, II, III and Va and a transphenoidal biopsy of his nasal lesion in the same setting. Endoscopically, in order to access this sphenoid sinus lesion, a limited posterior septectomy was performed and the anterior wall of the sphenoid sinus was taken down. A submucosal soft fleshy tumour was found on the central floor of the sphenoid sinus with a bony defect (figure 3A–C).
Figure 3.
Intraoperative photos of ectopic sphenoid sinus pituitary adenoma (black arrow). (A) Posterior septectomy (white arrowhead), opened left sphenoid sinus (white arrow). (B) Bulge on floor of sphenoid sinus with normal overlying mucosa. (C) Ectopic sphenoid sinus pituitary adenoma with surrounding bony defect seen after removal of left sphenoid sinus mucosa.
An intraoperative frozen section showed a small round blue cell tumour raising possibility of neuroendocrine tumour. Paraffin sections displayed monomorphic cells arranged in expanded nests highlighted with reticulin stain with intervening thin walled capillary vasculature. There were no signs of infiltration into surrounding tissue. Individual cells show round nuclei featuring salt and pepper chromatin and abundant granular eosinophilic cytoplasm. No high-grade features such as increased mitosis or necrosis or overt cytologic atypia were seen. Immunohistochemical staining showed dot like positive pancytokeratin Cam5.2 staining and diffuse positive staining for neuroendocrine markers such as chromogranin and synaptophysin. Further immunostaining for pituitary hormones revealed patchy positive staining for thyroid-stimulating hormone and follicle-stimulating hormone as well as rare cells staining for luteinising hormone and growth hormone. He was diagnosed to have an ESSPA in correlation with imaging details confirming intact sella turcica without a concurrent pituitary adenoma. Subsequent hormonal serology evaluation found that his prolactin was mildly elevated at 318 miU/L and sex hormone binding globulin was elevated at 76 nmol/L. However, these were not clinically significant and the rest of his pituitary hormonal work-up (ACTH, ILGF 1, TSH, T4, cortisol, FSH LH) was unremarkable. The neck dissection was negative for metastasis, making this a pT3 pN0 M0, stage III left parotid salivary duct carcinoma; with an incidental ESSPA.
Outcome and follow-up
He has undergone adjuvant radiotherapy for his high-grade salivary duct carcinoma, and is on close follow-up with regards to his ESSPA. We will monitor the growth of this ESSPA. If it continues to grow or result in complications, we will consider endoscopic excision with margins which is treatment of choice of ESSPAs.2 He remains asymptomatic from this lesion at the 6-month mark.
Discussion
Isolated sphenoid sinus lesions have been increasingly reported in medical literature, and this has helped clinicians formulate differential diagnoses when dealing with such cases. A recent meta-analysis of 1133 cases of isolated sphenoid sinus opacifications showed that while majority of cases are inflammatory in nature, a considerable proportion turn out to be neoplastic disease. Inflammatory causes include chronic rhinosinusitis (31.7%), mucoceles (20.3%) and fungal sinusitis (12.5%). Benign neoplasms such as inverted papillomas (5.7%), intracranial lesions such as pituitary adenomas (7.0%) and malignant neoplasms (7.7%) make up majority of the remaining cases.3 Metastatic disease is uncommon, with metastases from the prostate, breast, lung, kidney and bone marrow being reported.4 Primary malignancies are extremely rare and include adenocarcinomas and squamous cell carcinomas. On the other hand, it is far commoner for tumours to extend into the sphenoid cavity: pituitary tumours from the sella superiorly, chordomas from the clivus posteriorly and nasopharyngeal carcinomas from the nasopharynx inferiorly.5 6
Peculiar to our case, our patient’s FDG-avid sphenoid sinus lesion in a background of aggressive parotid cancer brought about concern of a metastatic salivary duct carcinoma or a synchronous sinonasal cancer.7 8
On one hand, medical literature shows that incidental sinonasal FDG uptake on PET-CT can indicate either inflammatory disease or a neoplastic lesion, and is unfortunately unable to differentiate the two based on SUV. A retrospective cohort study by Tzelnick et al found that 60% of these lesions turned out to be chronic inflammation and 40% were neoplastic (these lesions include inverted papillomas, squamous cell carcinoma, sinonasal sarcoma, lymphoma, adenoid cystic carcinoma, carcinoid and lung metastasis).9 On the other hand, the lesion in our case did not appear like chronic or fungal sinusitis on MRI. Most of his sphenoid sinus remained well aerated, instead this was mass lesion with erosion of the bony sphenoid floor and clivus. Differentials specific to our case included metastatic carcinoma, nasopharyngeal carcinoma, chordoma, rathke’s cleft cyst, neuroendocrine tumours such as olfactory neuroblastoma and an ectopic pituitary adenoma.
ESSPA is a rare entity that arises from a remnant of the Rathke’s pouch. The overall incidence rate of pituitary adenomas is 2.7 cases per 100 000,10 and among pituitary adenomas, the literature suggests that only 0.48% are ectopic. A comprehensive literature review on ESSPAs published in 2012 found a total 75 cases.11 It commonly affects middle aged patients and they present in a myriad of different ways. These include nasal symptoms (chronic sinusitis and obstruction), neurological symptoms (headache and visual disturbances), endocrine symptoms (hirsuitism) and asymptomatic incidental findings.11 12 On MRI, ectopic pituitary adenomas have well defined margins and show no relationship to the sella pituitary gland.
In a retrospective review of the CT and MRI findings of 8 sphenoid sinus ectopic pituitary adenomas, these lesions appear isointense in 6 (75%) patients and hypointense in 2 (25%) on T1-weighted images. On T2-weighted images, the lesions appeared hyperintense in 2 (25%) patients and isointense in 6 (75%). Ectopic pituitary adenomas show mild-to-moderate heterogeneous contrast enhancement and exhibited a cribriform-like appearance.13–15 They can sometimes display expansion and erosion into surrounding structures, however are not as aggressive and destructive as compared with chordomas and metastatic lesions. There is a high incidence of nasopharyngeal carcinoma in South-East Asian Chinese; however, these lesions are centred around the fossa of rosenmuller and typically also have characteristically aggressive appearance when it extends from the nasopharynx into surrounding structures such as the sphenoid sinus.
As an ectopic pituitary adenoma is such a rare entity, to our knowledge, no study to date has analysed its tendency for FDG uptake. The FDG-PET scan measures increased glucose uptake that occurs in malignant cells. Brain tissue also typically shows intense uptake of FDG because it metabolises glucose exclusively. In our patient, the ESSPA showed a markedly raised SUV of 17.9, consistent with a neoplasm of cerebral origin. In a related study of FDG PET findings in patients with pituitary lesions, about 80% of pituitary adenomas showed positivity (SUVmax >2.4) on PET scans. SUVmax ranged from 1.9 to 20.5 and correlated to the size of adenomas (r=0.559, p<0.01).16 Included in this study, 6 out of 7 rathke’s cleft cyst did not show FDG uptake on PET scans. This incidental finding of an ESSPA in a patient with head and neck cancer has enabled us to discover the tendency for ESSPAs to be FDG avid. This knowledge can help a clinician to have a high index of suspicion for ectopic pituitary adenomas when dealing with FDG avid lesions in the sphenoid sinus or midline of the nasal cavity.
Histologically, the transphenoidal biopsy of this lesion showed monomorphic cells with nuclei featuring salt and pepper chromatin and abundant granular eosinophilic cytoplasm. Along with diffuse positive staining of chromogranin and synaptophysin, the neuroendocrine nature of the tumour was confirmed. The differentials of a paraganglioma and olfactory neuroblastoma were excluded by testing for cytokeratin and S100. Ectopic pituitary adenomas are diffusely positive for cytokeratin and negative for S100, paragangliomas are negative for cytokeratin while olfactory neuroblastomas show focal or negative cytokeratin staining. Both paragangliomas and olfactory neuroblastomas show positive staining for S100 in peripheral sustentacular pattern, Olfactory neuroblastomas also tend to occur more anteriorly in the region of the cribiform plate and ethmoid sinuses as compared with ESSPA. Dedicated immunohistochemical staining of anterior pituitary hormones confirms the diagnosis of ESSPA in correlation with clinical and imaging findings. PET scan excludes the differential of a metastatic neuroendocrine tumour in this patient.
Pertaining to its surgical appearance, this ESSPA was in the midline, its overlying mucosa appeared healthy and normal. Even though there was a bony defect in the surrounding intersinus septum, sphenoid sinus floor and clivus, its boundaries were well defined and smooth, distinct in appearance from infiltrative carcinomas. This is in keeping with a pathologic study of 32 cases of ESSPAs which found frequent bone involvement and an overlying intact respiratory epithelium.11 We postulate that this is a result of the Rathke’s pouch remnant’s natural location and the subsequent mass effect of the adenoma rather than frank invasion of surrounding bone.
ESSPAs are benign pituitary gland neoplasms without a metastatic potential. The aim of treatment is to alleviate symptoms which result from mass effect (eg, nasal, visual or neurological) and treat hormone excess. Surgical removal is the mainstay of treatment. This can be done endoscopically by optimising surgical access, raising the surrounding mucosa, removing the tumour in a piecemeal fashion, paying attention to the tumour boundaries and adjacent bone to ensure complete excision. Surgical excision is often successful with low recurrence rates.11 Other forms of treatment include medical therapies such as bromocriptine which is shown to reduce the size of prolactin secreting tumours, but these patients will require lifelong treatment.17 Radiotherapy can be used in a selected cases of large or incompletely excised tumours, but its efficacy is contentious and radiation related risks need to be considered. As our patient’s ESSPA was non-functional, and not affecting surrounding neurovascular structures, we opted to observe this incidental lesion, and focus our care on his parotid cancer.
In conclusion, when encountering a sphenoid sinus lesion, ESSPA, though rare, should be considered as a differential diagnosis. This is especially pertinent when evaluating an FDG-avid lesion that can be incorrectly diagnosed as metastatic disease. Only with a high index of suspicion can the appropriate immunostains be performed to confirm the diagnosis. This will enable an accurate diagnosis and appropriate management.
Patient’s perspective.
I had no symptoms from the nose and went to see the doctor for a neck lump. When I was told that I had a nose lesion despite a normal flexible nasoendoscopy, I was quite surprised and taken aback. I was dealing with a newly diagnosed cancer as well. I had some concerns that it could be a spread of the cancer. But overall, I am glad that the surgeries went well.
Learning points.
Ectopic sphenoid sinus pituitary adenoma is a rare but important differential for sphenoid sinus lesions.
Ectopic pituitary adenoma has a tendency for fluorodeoxyglucose avidity on positron emission tomography scan, and can masquerade as invasive or metastatic carcinoma.
When investigating suspicious sinonasal lesions, biopsy and dedicated immunohistochemical staining is important for accurate diagnosis and appropriate management.
Footnotes
Contributors: AC contributed substantially to the conception and design of the case report as well as the literature review and discussion. AYQS provided the scan images which includes the MRI sagittal reconstruction image and important intellectual content regarding scan interpretation. BM was critical in interpreting the histological findings of the lesion and providing important intellectual content with regards to the immunohistochemical stains required to diagnose such a lesion. JLT was the clinician in direct care of the patient. He made substantial contributions to the conception and design of the case report and revised it critically. All authors approve of the current version. All authors agree to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rossi ED, Baloch Z, Pusztaszeri M, et al. The Milan system for reporting salivary gland cytopathology (MSRSGC): an ASC-IAC-sponsored system for reporting salivary gland fine-needle aspiration. J Am Soc Cytopathol 2018;7:111–8. 10.1016/j.jasc.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 2.Oruçkaptan HH, Senmevsim O, Ozcan OE, et al. Pituitary adenomas: results of 684 surgically treated patients and review of the literature. Surg Neurol 2000;53:211–9. 10.1016/S0090-3019(00)00171-3 [DOI] [PubMed] [Google Scholar]
- 3.Moss WJ, Finegersh A, Jafari A, et al. Isolated sphenoid sinus opacifications: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2017;7:1201–6. 10.1002/alr.22023 [DOI] [PubMed] [Google Scholar]
- 4.Barrs DM, McDonald TJ, Whisnant JP. Metastatic tumors to the sphenoid sinus. Laryngoscope 1979;89:1239–43. 10.1002/lary.1979.89.8.1239 [DOI] [PubMed] [Google Scholar]
- 5.Ng YH, Sethi DS. Isolated sphenoid sinus disease: differential diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2011;19:16–20. 10.1097/MOO.0b013e32834251d6 [DOI] [PubMed] [Google Scholar]
- 6.Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer 2004;101:613–9. 10.1002/cncr.20412 [DOI] [PubMed] [Google Scholar]
- 7.Ali S, Bryant R, Palmer FL, et al. Distant metastases in patients with carcinoma of the major salivary glands. Ann Surg Oncol 2015;22:4014–9. 10.1245/s10434-015-4454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwentner I, Obrist P, Thumfart W, et al. Distant metastasis of parotid gland tumors. Acta Otolaryngol 2006;126:340–5. 10.1080/00016480500401035 [DOI] [PubMed] [Google Scholar]
- 9.Tzelnick S, Bernstine H, Domachevsky L, et al. Clinical implications of incidental sinonasal positive FDG uptake on PET-CT. Otolaryngol Head Neck Surg 2019;160:729–33. 10.1177/0194599818821862 [DOI] [PubMed] [Google Scholar]
- 10.McDowell BD, Wallace RB, Carnahan RM, et al. Demographic differences in incidence for pituitary adenoma. Pituitary 2011;14:23–30. 10.1007/s11102-010-0253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson LDR, Seethala RR, Müller S. Ectopic sphenoid sinus pituitary adenoma (ESSPA) with normal anterior pituitary gland: a clinicopathologic and immunophenotypic study of 32 cases with a comprehensive review of the English literature. Head Neck Pathol 2012;6:75–100. 10.1007/s12105-012-0336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenig BM, Heffess CS, Adair CF. Ectopic pituitary adenomas (EPA): a clinicopathologic study of 15 cases. Mod Pathol 1995;8. [Google Scholar]
- 13.Yang BT, Chong VFH, Wang ZC, et al. Sphenoid sinus ectopic pituitary adenomas: CT and MRI findings. Br J Radiol 2010;83:218–24. 10.1259/bjr/76663418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FitzPatrick M, Tartaglino LM, Hollander MD, et al. Imaging of sellar and parasellar pathology. Radiol Clin North Am 1999;37:101–21. 10.1016/S0033-8389(05)70081-6 [DOI] [PubMed] [Google Scholar]
- 15.Slonim SM, Haykal HA, Cushing GW, et al. MRI appearances of an ectopic pituitary adenoma: case report and review of the literature. Neuroradiology 1993;35:546–8. 10.1007/BF00588720 [DOI] [PubMed] [Google Scholar]
- 16.Seok H, Lee EY, Choe EY, et al. Analysis of 18F-fluorodeoxyglucose positron emission tomography findings in patients with pituitary lesions. Korean J Intern Med 2013;28:81–8. 10.3904/kjim.2013.28.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langford L, Batsakis JG, Lauren L. Pituitary gland involvement of the sinonasal tract. Ann Otol Rhinol Laryngol 1995;104:167–9. 10.1177/000348949510400217 [DOI] [PubMed] [Google Scholar]