Abstract
Unique properties of amniotic membrane make it a promising source for tissue engineering and a clinically useful alternative for patients suffering from chronic wounds including, for example, ulcers, burns, ocular surface damages and wounds occurring in the course of bullous diseases like stevens‐johnson syndrome and toxic epidermal necrolysis. Its use has many advantages over standard wound care, as it contains pluripotent cells, nutrients, anti‐fibrotic and anti‐inflammatory cytokines, growth factors and extracellular matrix (ECM) proteins. Placental tissues can be prepared as a medical component, an advanced therapy medicinal product or a tissue graft. In addition to basic preparation procedures such as washing, rinsing, cutting, drying and sterilisation, there are many optional steps such as perforation, crosslinking and decellularisation. Finally, transplants should be properly stored—in cryopreserved or dehydrated form. In recent years, many studies including basic science and clinical trials have proven the potential to expand the use of amniotic membrane and amnion‐derived cells to the fields of orthopaedics, dentistry, surgery, urology, vascular tissue engineering and even oncology. In this review, we discuss the role of placental tissues in skin wound healing and in the treatment of various diseases, with particular emphasis on bullous diseases. We also describe some patented procedures for placental tissue grafts preparation.
Keywords: amnion, healing, placenta, wound
1. STRUCTURE AND FUNCTION OF THE FETAL PLACENTA
Placenta is a specialised organ, it connects the mother's and foetus's tissues, and its basic function is the transfer of nutrients and various stimuli. All placental tissues, including the umbilical cord, amniotic fluid and amnion, nourish, protect the foetus and act as an immunologically privileged barrier regulating the development of the new organism. They play an important role in these processes by creating an appropriate environment for development. The newly developing foetus is supplied with nutrients through umbilical vessels and at the same time washed by amniotic fluid, which amortises and protects it. The placenta is made of a highly vascularized extracellular matrix (ECM). The matrix includes collagens I, III, IV and VI, with type I collagen being the main component of this structure.1, 2 Additionally, the placenta contains a number of glycoproteins, proteoglycans and other compounds, including fibronectin, fibrillin I, laminin, thrombospondin I, heparan sulphate and elastin.3 By allowing the distribution of various ECM components, it can affect cell differentiation, hormone and protein production, proteolytic activities as well as various repair mechanisms. It should be noted that collagen IV, laminin and heparan sulphate, which are found in the basal membrane of many organs in adults, can facilitate the basement membrane remodelling and increase the morphogenetic functional flexibility of different villus cell populations.1, 2
Amniotic fluid is made from mother's plasma. Crossing amniotic membranes, it uses osmotic and hydrostatic pressure. During foetal development, the amniotic fluid has a similar composition to the foetal plasma and is absorbed by the skin, foetal membranes, the placenta surface and the umbilical cord.4 Amniotic fluid plays an important role in foetal breathing, water‐electrolyte exchange, urinary excretion and in secretory functions of foetal organism. It mainly consists of not only water but also contains carbohydrates, proteins, lipids, electrolytes, foetal waste products (such as urea and meconium) and a low number of heterogeneous populations of foetal‐derived cells. Amniotic fluid volume increases during pregnancy up to the largest volume of 800 mL at 28th week of pregnancy.4 The amount and composition of amniotic fluid are highly controlled by various mechanisms. One of the basic components of amniotic fluid is hyaluronic acid. It increases the viscosity of this fluid simultaneously supporting the transport of various components. Hyaluronic acid may also play an important role in foetal health, for example, by inhibiting scarring, because it is associated with the first step of healing process in adult tissues and influences the activity of various growth factors and signalling molecules during wound healing.5 Amniotic fluid also contains a number of signalling molecules that play a key role in the foetus development as well as many carbohydrates, proteins, lipids, electrolytes and other nutrients. Among growth factors commonly present in amniotic fluid are, for example: epidermal growth factor (EGF), transforming growth factors α and β (TGF‐α and TGF‐β), insulin‐like growth factor I (IGF‐I), erythropoietin (EPO), granulocyte colony‐stimulating factor (GCSF) and macrophage colony‐stimulating factor (MCSF).4 Moreover, this fluid is the main regulator of the innate immune system, contains various enzymes, antibacterial peptides and immunomodulatory mediators, which protect against foetal infection.4, 5
The amniotic sac consists of two separate, but connected membranes—amnion and chorion. Amniotic membrane is most commonly used for transplantation. It is a membrane surrounding the embryo of birds, reptiles and mammals, including humans. It is made of thin, semi‐permeable tissue and constitutes the inner layer of the amnionic sac. It consists of several layers: epithelium, basal membrane, compact layer and fibroblast layer. The epithelium forms the inner lining of the amniotic sac and consists of a single layer of epithelial cells, evenly distributed on the basal membrane. This membrane is a thin layer made of collagens III and IV and glycoproteins, laminin, fibronectin and nidogen (entactin). The compact layer is almost completely devoid of cells and forms the main fibrous structure of the amnion. Thanks to collagens, the amnion provides the appropriate environment for the foetus and a barrier against its mechanical damage. Interstitial type I and III collagens form a compact structure, maintaining the mechanical integrity of the amnion, while V and VI collagens are connected filamentously with the basal membrane.6 The fibroblast layer adheres to a loose collagen network with glycoprotein islands.7
The placenta also includes the umbilical cord, which consists of Wharton jelly, which surrounds the umbilical vein and umbilical arteries. The umbilical vein transports oxygen‐rich, nutrient‐rich blood from the placenta to the foetus, whereas two arteries transport deoxygenated blood and metabolic products from the foetus to the placenta. Wharton jelly is mainly made of ECM rich in collagen and hyaluronic acid and of a small number of fibroblasts. Collagen types I, III, V and VI form an insoluble fibrous network, which co‐occurs with a glycoprotein microfibrillar network rich in fibrillin.8, 9 Hyaluronic acid (which is the main glycosaminoglycan in Wharton jelly) creates a hydrated gel that maintains umbilical cord tissue structure and protects umbilical cord vessels against extension and constriction.10, 11 The umbilical Wharton jelly also contains chondroitin sulphate, dermatan sulphate, keratin sulphate, heparan sulphate as well as a population of cells similar to stromal fibroblasts, population of myofibroblasts and mesenchymal cells (MSCs).8, 11 A relatively small number of cells in the umbilical cord, surrounded by a large volume of ECM, suggest that these cells are responsible for secretion of large amounts of ECM, in order to maintain the tissue structure.10 Wharton jelly also acts as a reservoir of high molecular weight growth factors. It contains acidic fibroblast growth factor (aFGF), basic fibroblast growth factor (bFGF), EGF, IGF‐I, insulin‐like growth factor‐binding proteins (IGFBPs), platelet‐derived growth factor (PDGF), TGF‐α and TGF‐β.10, 11 These growth factors and cytokines are responsible for cell proliferation and differentiation controlling, as well as for protein synthesis and ECM remodelling.
2. PLACENTAL TISSUES AS MATERIALS FOR USE IN MEDICINE
The placenta, which is excreted in the third stage of delivery, is classified as a biological waste in most medical units. Therefore, it is an available and a very good tissue material from which transplants, medical components or medicinal products can be prepared. Both amnion grafts and those prepared from other placental tissues originate from immunologically inert tissue, which allows them to be prepared and transplanted as living grafts. The widespread use of placental tissues (especially amniotic membrane) in medicine results from their unique properties.
2.1. Placental tissue properties supporting wound heling processes
A very important feature of foetal tissues is their low immunogenicity12 —they contain small amounts of human leukocyte antigen (HLA) and do not elicit an immune response. Therefore, it is enough to gently clean them to remove blood and unnecessary tissue residues, while maintaining the natural cell viability and biological activity of the transplant. Because the amnion is rich in nutrients and has a low immunogenicity, it is often used as a graft or dressing for damaged skin. These grafts/dressings are of natural origin, contain stem cells, ECM and a number of regulatory cytokines, which provides natural functions of tissue growth supporting and inflammation modulation.
Placental tissues have also the ability to promote cell proliferation and migration and to modulate cytokine secretion through various cell types involved in wound healing, including dermal fibroblasts, microvascular endothelial cells and stem cells.13, 14, 15, 16 Some scientists even claim that the usefulness of amnion in wound healing may be due to the fact that inflammatory processes (mediated mainly by mast cells) during healing are similar to gestational processes. That is why so‐called amnion‐derived cellular cytokine solution provides most of the necessary healing mediators.17, 18, 19, 20 This fluid is not only capable of promoting macrophage activity and accelerating epithelialisation but also improves the healing of acute and chronic wounds.21, 22, 23
Thanks to its unique properties, the amnion protects the organism against bacterial infections; reduces the loss of proteins, fluids and electrolytes; reduces the pain in burned areas and accelerates healing.14, 24 Moreover, thanks to mesenchymal cells and their ability to secrete substances such as interleukin 6 (IL‐6), interleukin 8 (IL‐8), growth‐related oncogene (GRO), monocyte chemoattractant protein‐1 (MCP‐1) and intercellular adhesion molecule (ICAM), amniotic membrane accelerates angiogenesis.16 In addition, inhibition of neutrophils by amniotic cells contributes to inflammation reduction and to collagen degradation limitation, which translates into faster wound healing.1, 15, 25
The ability of placental cells to transdifferentiate into cells of various organs (among others to cardiomyocytes, osteoblasts, fibroblasts and hepatocyte‐like cells) has been also demonstrated. Main three placental cell types displaying stem cell‐like features are amnion‐derived epithelial cells (AECs), amnion‐derived mesenchymal stromal cells (AMSCs) and chorion‐derived mesenchymal stromal cells (CMSCs). AECs and AMSCs can be obtained from the amnion in large amounts, which is possible due to the fact that the amnion is avascular and can be easily detached from the chorionic plate.22, 26 It was shown that AMSCs have the potential to induce AECs differentiation into keratinocytes, which can be used in tissue engineering to create skin substitutes.27, 28
Other important features of amniotic stem cells are their immunomodulatory properties—they can suppress several types of immune cells, including T‐ and B‐lymphocytes, natural killer (NK) cells and dendritic cells.29, 30, 31, 32, 33, 34 The most common anti‐inflammatory and anti‐fibrotic factors secreted by the placenta include hyaluronic acid, tissue inhibitors of metalloproteinases (TIMPs), interleukins, prostaglandins and migration‐inhibitory factor (MIF).22, 35, 36, 37, 38, 39 The amniotic membrane not only contains anti‐inflammatory mediators but also induces apoptosis of mononuclear cells, including lymphocytes and macrophages. The aqueous extract of the amniotic membrane decreases the expression of the major histocompatibility complex (MCH) class II, inhibits the viability of the immune system cells and enhances their apoptosis.
Mentioned anti‐inflammatory effect may indirectly contribute to the reduction of scarring. Indeed, research into the use of amniotic membrane in ophthalmology indicates that this membrane can indirectly reduce scar formation by inhibiting TGF‐β signalling at the transcription level (as a result, it leads to expression inhibition of genes responsible for scar formation).40, 41 What is more, amniotic membrane stromal matrix has the ability to maintain (in the culture of human keratinocytes) the expression of keratocan (KTN)—a protein involved in the cornea development. Therefore, the impact on TGF‐β signalling is important not only because of the inhibition of scar formation but also due to the preservation of the normal phenotype of keratinocytes. The amniotic membrane also contains PDGF, which is a highly angiogenic factor.
Finally, it exhibits analgesic activity (by acting on the fibroblasts) that has been observed in the treatment of chemical burns and severe bacterial keratitis, as well as in therapies of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and epidermolysis bullosa (EB)—genetic bullous skin diseases with severe course.24, 32
The summary of amniotic membrane unique properties and its impact on different aspects of wound healing process is shown in Figure 1.
Figure 1.
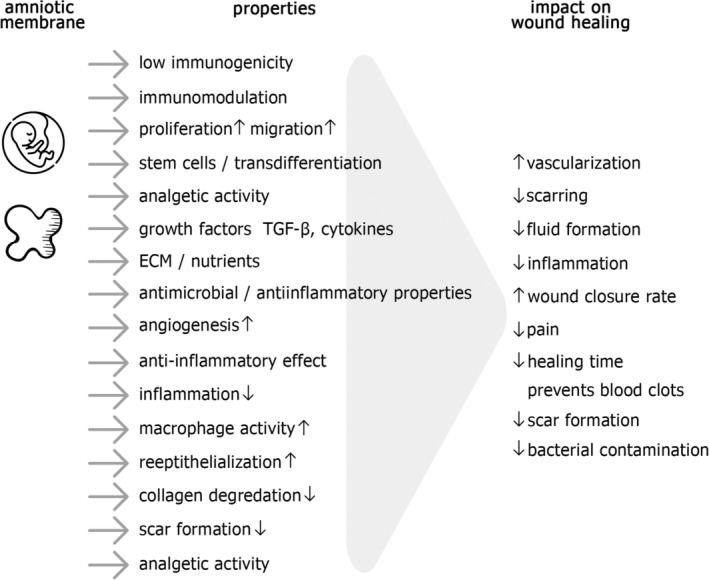
Properties of the amniotic membrane and its influence on skin wound healing process
2.2. Classical applications of placental tissues in wound healing
Due to the role played by the placenta in the development of the foetus, as well as the content of nutrients, it has been examined for its clinical use in the treatment of wounds. Because the placenta is removed from the body and utilised after the childbirth, it is an available source of tissues for transplantation. Tissues such as amniotic membrane, umbilical cord, amniotic fluid and chorion are commonly used to prepare wound healing supporting grafts for tissue regeneration or to replace damaged tissues. They are used simultaneously on the wound periphery, which accelerates its healing and prevents blood clots and scars formation.
The effectiveness of such transplants in the treatment of chronic wounds has been demonstrated, including wounds occurring in the course of diabetes, venous leg ulcers (VLUs) and burns.24, 42, 43
Diabetic foot ulcers (DFUs) are one of the most common complications of diabetes and increase the risk of premature death, fatal stroke and myocardial infarction among patients. The first randomised trial, which compared human amnion/chorion membrane products with the standard of care (SOC) in the treatment of DFUs, was performed in 2013. After 4 weeks of treatment, average ulcers surface area reduction rate was over three times higher after dehydrated amnion/chorion membrane administration, than after SOC. Then, the same research group continued the study with additional patients and the third treatment method—bioengineered skin substitute (BSS). It was shown that at week 12, amnion/chorion membrane is more effective than both SOC and BSS in terms of proportion of wounds achieving complete closure as well as number of grafts required to achieve healing.43, 44, 45 In subsequent years, other authors also reported (in their prospective randomised control trials) the high efficacy of variously prepared amnion‐derived products in the treatment of chronic DFUs.44, 46, 47, 48 To sum up, a broad meta‐analysis revealed that DFUs healing using SOC together with human amnion/chorion membrane is significantly faster than healing with SOC alone.49
Similar comparisons (amniotic membrane vs SOC) were performed in patients suffering from VLUs—the most common type of lower extremity wound. After 4 weeks, half more patients achieved 40% wound closure in amnion/chorion membrane treatment group than in control group receiving SOC alone. Moreover, follow‐up study assessing healing at 24 weeks demonstrated correlation of healing rate between the 4‐week and 24‐week trials in most patients.42, 44, 50
The use of amnion in burn treatment has a long history. It was used for the first time on burned and ulcerated skin surfaces at the beginning of the XXth century.24, 51 It is mostly used on partial‐thickness burns, because in case of full‐thickness ones, there are some problems with wound infections, amniotic membrane adherence and its dissolution.24, 52 Human amniotic membrane has been compared with other treatment methods such as using sulphadiazine cream, Biobrane, Omiderm and Water Gel in several randomised controlled trials with patients suffering from burns. It turned out, that amniotic membrane application (in comparison with alternative methods) significantly reduced oozing, bacterial contamination, hypertrophic scarring and healing time.24, 52, 53, 54 It was also shown, that irradiated human amniotic membrane is easier to applicate, provokes less pain and limits fluid formation compared with glycerol‐stored membrane.55 Moreover, the efficacy of amnion treatment was also confirmed in paediatric patients with second‐degree burns. They received either topical antibiotic cream combined with human amniotic membrane or antibiotic cream alone. In follow‐up study, amnion‐treated patients showed faster total healing time and needed fewer dressing changes, than children from control group.56
It is worth mentioning that the use of amniotic membrane in ophthalmic treatment is common and has been practiced for many years.57, 58 It has been shown that amnion effectively prevents the occurrence of inflammation and scarring as well as promotes healing in patients suffering from various ocular surface diseases.7, 59, 60, 61 Amniotic membrane is commonly used with very good clinical effects in eye ulcerations, eye burns or corneal epithelial defects.62, 63, 64, 65 Moreover, its high efficacy was shown in patients after pterygium extended removal. This surgical removal was followed by fibrin glue‐assisted amniotic membrane transplantation, which resulted in low complication rate, low recurrence rate and high cosmetic satisfaction found during postoperative follow up.66 Amnion was also used as an adjunct tool to improve glaucoma surgical outcomes by reducing adverse post‐operative phenomena such as inflammation, fibrosis, scarring, bleb leakage and hypotony.67 Another important application of amnion in ophthalmology is preparation of amniotic membrane extract (AME) and amniotic membrane extract eye drops (AMEED) in order to heal different ocular surface disorders, for example, dry eye, chemical burns, corneal ulcers, neurotrophic keratitis and epithelial defects of the cornea. Their effectiveness was confirmed both in experimental studies and clinical trials. Finally, some commercial amnion‐derived products have been developed for ophthalmic use, for example, Amniotic Cytokine Extract (ACE), Regener‐Eyes, Optiserum and PDA‐AF.58, 68, 69, 70, 71, 72, 73
Amnion transplants are also administrated (among others) on cut skin wounds, post‐excision wounds, bullous skin diseases, pressure ulcers, wounds arising near or around the nervous tissue, on damages to the mouth and eye surface.
2.3. Various types of products of amniotic origin
Tissue obtained from the placenta can be defined as a medical component, an advanced therapy medicinal product or a tissue graft. Depending on the definition and classification, there are different methods for its preparation. The human‐derived amnion in most European Union countries is prepared at Tissue Banks. Even if an amnion is prepared as a medical component or a medicinal product, only the Tissue Bank or transplant centre has the right to collect tissues. Such an institution can deliver tissues to the manufacturer either as a part of its own structure or as an external supplier. Many methods of tissue grafts preparation have been developed as part of the activities of these units. The basic classification distinguishes allografts (obtained from tissue donors) and xenografts (obtained from animal tissues).
Tissues of animal origin according to the definition are medial components, which enforce their proper legislation, specific for each country of the European Union. As medical components, tissues of animal origin must be completely purified from cells. The decellularisation process aims to remove immunoreactive cellular components and proteins, leaving the structurally intact (biologically inert) skeleton made of ECM. Such a procedure leads to a reduction of the risk of xenograft rejection and ensures an adequate safety of medical components.
Tissues of human origin are mostly prepared as transplants from placental tissues in Tissue Banks. In these banks, transplants of the amnion itself or in combination with chorion can be prepared. Depending on the method of preparation, properties and final predestination, biostatic grafts (subjected to final sterilisation) or biovital ones (maintaining their viability) can be distinguished. Tissues for biostatic grafts are subjected to final sterilisation in order to kill and remove living cells. They are only biological dressings used as dead bioprosthesis. In contrast, biovital grafts are transplants containing living cells, and their preparation and storage must be carried out so as to preserve their viability.74, 75
3. THE BASIC PROCEDURE FOR PLACENTAL TRANSPLANTS PREPARATION
A number of methods have been developed and patented for the preparation of foetal tissue grafts, but the basic procedure (showed in the Figure 2) is usually the same and starts with obtaining and purification of the material and ends with its optional sterilisation and proper storage procedure.
Figure 2.
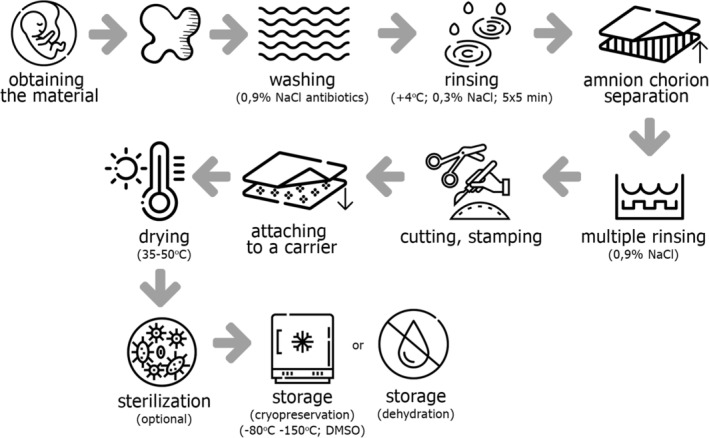
The scheme of the basic procedure of amniotic membrane transplants preparation
3.1. Obtaining the material from the donor
If it does not threaten her or the child's health, the mother may decide to donate her placenta to the Tissue Bank. Placentas are usually obtained during caesarean sections, but there is a possibility to use a naturally born placenta after carrying out all the necessary tests and qualification procedures. However, caesarean section allows to obtain tissue material in aseptic conditions, without going through the birth canal. Regardless of the delivery type, donors must be tested for infectious diseases, including human immunodeficiency virus (HIV), hepatitis B and C and syphilis, in accordance with applicable legal requirements.
The basic procedure for amniotic transplants preparation involves (in the first stage) washing of obtained placenta in a physiological saline solution and then placing it in a precisely described, liquid‐filled container for the time of its transport. The liquid is a solution of physiological saline and antibiotics. The composition of the antibiotics mixture is properly selected in accordance with the current microbiological situation of the hospital. The transport container with tissue material must be labelled in a way that allows the unequivocal identification of the donor. During its transport, the tissue material should be stored in a special refrigerator at +4°C.
3.2. The placenta and the amnion purification
The next stage of placental transplants preparation is carried out under sterile conditions, for example, under the laminar chamber at the Tissue Bank. The placenta is subjected to five rinsing cycles in physiological saline solution, on a shaker at +4°C. Each rinsing lasts 5 minutes. Then, the amnion is manually separated from the chorionic layer, and it should be rinsed again in a physiological saline solution at +4°C. The rinsing is repeated until a clean solution is obtained. After that, the amnion is laid down on a glass plate, and the excess of blood and mucus is removed. To purify placental tissues, mechanical methods allowing to remove blood clots and other contaminations can be used.
3.3. Creating a ready biological dressing
The next stage of preparation involves cutting off irregular fragments with a scalpel and then measuring the surface of obtained transplants. Products made of at least one layer of amnion, chorion or their combination are dried using a device with grooves and convex edges, which give the right shape to the graft. Such grafts are used to treat wounds after surgeries, especially within the mouth, nose, throat, vagina and anus [US20080046095 A1, February 21, 2008, Placental tissue grafts, MIMEDX].
Very important in the transplantation of amniotic or amniotic‐chorion transplants is their appropriate orientation in order to preserve their anatomical position. For this purpose, the grafts are marked by making a kind of stamp on their upper side, in the form of, for example, embossing, staining, colouring, stamp impression or incision. Natural hypoallergenic vegetable dyes are most often used as marking pigments. [WO201749210 A1, March 23, 2017, Compositions derived from placenta and methods of producing the same, STIMLABS].
When preparing a dressing from the amniotic membrane, it is necessary to pay attention that the amnion epithelial side lays directly on the net or other carrier. After application on the wound, the epithelial side with the basal membrane should be directed outwards because it promotes migration, adhesion and proliferation of the patient cells, thereby stimulating epithelialisation.76 The dressing from at least one amniotic or chorionic layer should be prepared in a way that ensures full fitting to the size of the wound, and additionally it is worth to attach it to the skin with a layer of glue. Thanks to this construction, the outer layer of the dressing adheres only to the skin surrounding the wound. This type of dressing accelerates wound healing, reduces scarring, eliminates inflammation and reduces the risk of infection. [US20130289724 A1, October 31, 2013, Amnion and chorion wound dressing and uses thereof, LIVENTA BIOSCIENCE].
Transplants of the amniotic membrane can be fitted to the wound by cutting them to the required size and shape. Next, they are applied to a carrier (eg, tissue paper), which additionally facilitate their adjustment to the size and shape of the wound. Such a transplant can be additionally enriched with a therapeutic agent. [EP861839 A1, September 2, 1998, Grafts made from amniotic membrane; methods of separating, preserving, and using such grafts in surgeries, TISSUETECH].
Further stage is the membrane drying using the drying device, during which the membrane adheres to the drying device surface and heating is carried out at 35°C to 50°C. [WO200933160 A1, March 12, 2009, Placental tissue grafts and improved methods of preparing and using the same, MIMEDX]. The last stage of transplant preparation is their packing to several sterile sacs. A label with a tissue marking code is affixed to one of these packing sacs. Transplants can be additionally subjected to radiative sterilisation (biostatic grafts) or can be stored in a freezing medium as biovital transplants.
3.4. Optional sterilisation of (biostatic) grafts
Amniotic transplants can be additionally sterilised to reduce the risk of infectious diseases originating from the donor tissue. Although the preparation of transplants is carried out under aseptic conditions, it does not provide a hundred percent microbiological safety. Therefore, in order to reduce the risk of bacterial or viral infection, sterilisation (eg, by gamma or electron beam irradiation) is performed. High levels of radiation can potentially cross‐link the tissue and induce protein denaturation within it. However, the basic advantage of this process is the preservation of biological activity of sterilised tissues, both clinically and in in vitro studies.14, 43 These data suggest that sterilisation does not significantly reduce the biological activity of amniotic transplants and additionally ensures maximal safety of patients.77
3.5. Transplants storage
After preparation, the transplants should be properly stored to preserve their properties. The most common way of tissue grafts preservation in order to prevent their degradation is cryopreservation (freezing at low temperatures). Such a freezing can prevent tissue damage by reducing their enzymatic and chemical activity, while inhibiting the microorganisms growth. However, cryopreserved grafts require specialised transport and storage as well as temperature control. For this purpose, it is necessary to use liquid nitrogen, dry ice or low temperature freezers often providing temperature of −80°C or −150°C. In cryopreservation process it is necessary to use cryoprotectants, such as dimethyl sulfoxide (DMSO) or glycerol, in order to reduce the effect of ice crystal forming inside cells. The ice can destroy their membranes and damage ECM. Nevertheless, the use of cryoprotectants may be cytotoxic in high concentrations or with longer exposure times, so it is required to carefully rinse them from the tissues before transplantation.
An increasingly popular alternative to tissues cryopreservation is their dehydration (after previous purification). Dehydration protects tissues without the necessity to freeze them in dry ice or liquid nitrogen. However, there is a risk that this process can change the microstructure of tissues and their ECM properties. Chemical dehydration involves soaking the placental tissues in a polar organic solvent until the water is completely removed. The polar organic solvent may be among others ethanol, ether, aldehyde or any combination of them. Other substances, for example, DMSO, acetone, tetrahydrofuran, ethanol and isopropanol can also be used. The dehydrated tissues are lyophilised (freeze‐dried) at temperatures of −50°C to −80°C [WO201382412 A1, June 6, 2013, Placental tissue grafts produced by chemical dehydration/freeze‐drying and methods for making and using same, MIMEDX].
4. ADDITIONAL VARIANTS IN THE PREPARATION OF PLACENTAL GRAFTS
4.1. Perforation
From the placenta not only amniotic transplants, but also chorionic transplants or combined ones (amniotic‐chorionic) can be obtained. Transplants of the amnion itself, the chorion itself or the combination of both membranes are dedicated, among others, to the treatment of chronic wounds with exudate, supporting the process of their healing. Therefore, these grafts can be additionally perforated—they can contain gaps created by the tissue perforation, in order to allow the free flow of secretions from the wound.[WO201749210 A1, March 23, 2017, Compositions derived from placenta and methods of producing the same, STIMLABS].
Perforation can lead to obtaining one or more gaps that partially pass through the amniotic and chorionic layers. Placental tissues can be perforated in different ways, so that the final grafts contain many gaps, often covering over 50% of the graft area. Such a perforation allows a significant tissue stretching, thereby increasing its length and width from 20% to 600%. The gaps may be arranged in the membrane in a cross pattern, and they may have the shape of a cross, the letter X, L or H. To facilitate the storage, the placental tissues are sticked to the rolled up bed and placed in a vial containing solution of amniotic fluid cells. [US20130084314 A1, April 4, 2013, Expandable placental membrane and methods of making and storing same, NUTECH MEDICAL].
The perforation can be performed using needles, a roller with one or more pins, a stamp with one or more insets, a hole punch, a blade, a scalpel or other sharp instrument. Obtained sheet is rinsed at least once with a washing agent in a vacuum (under pressure) with simultaneous shaking. Different washing agents are used, for example, water, sodium chloride, sodium hypochlorite and hydrogen peroxide solutions, Ringer's solution or phosphate‐buffered saline (PBS) solution. The amniotic‐chorionic graft prepared in this way, can be used for wound healing and tissues regeneration. [WO201749210 A1, March 23, 2017, Compositions derived from placenta and methods of producing the same, STIMLABS].
4.2. Collagen membrane
From the placental tissues, the amniotic and chorionic membranes can be obtained in order to prepare a collagen membrane from them. For this purpose, the amniotic membrane is separated from the chorionic membrane and then the living cells are removed. It is preferred to stick together at least two amniotic layers in the form of a sheet, tube or microsphere. As an adhesive, any synthetic glue or any biological (biocompatible, natural) glue (eg, fibronectin and fibrin) can be used. The amniotic sheets can be made by chemical, radiative, UV or hydrogel crosslinking or by hot stamping. Decellularisation does not affect the flexibility of the membrane and is performed, among others, with a weak detergent solution (pH 6‐8), a solution containing 0.01% to 1.0% of deoxycholic acid sodium salt monohydrate, nonionic and anionic detergents, Triton X‐100, sodium dodecyl sulphate or with their combination. The membrane prepared in this way exhibits very good mechanical properties (tensile strength) and preserves tertiary and quaternary protein structures. It has many applications, both as a transplant or as a dressing directly applied to wounds. Additionally, the membrane may be impregnated with antibiotics, hormones, growth factors, anti‐cancer agents, antifungal agents, molecular silver and wound healing agents. It can be also enriched with hydrogel compositions made of polyvinyl alcohol polymers, polyethylene glycols, hyaluronic acids, dextrans and their derivatives or analogs. Such membranes have a thickness of at least 10 to 40 μm and can be used in various configurations or in combination with other materials—either as a single sheet or in combination with, for example, a flexible plastic film, gauze or bandages. [EP2702871 A1, March 5, 2014, Collagen biofabric and use thereof, ANTHROGENESIS].
4.3. Crosslinking
Crosslinking of placental grafts contributes to their good adhesion to the wound. Such grafts are prepared in several possible forms: laminate containing two layers of amnion, at least one of which is crosslinked, a crosslinked chorionic layer or amnion and chorion layers, at least one of which is crosslinked. The cross‐linking agent may contain sugars and dialdehyde, epoxide, hydrazide or carbodiimide. There may be additional layers between the amnion and the chorion. Crosslinked transplants are prepared by cleansing the placenta, separating the layers of chorion and amnion and then the epithelial cells are removed and the crosslinking agent is applied. Next, the grafts are dried on the drying device, in a way ensuring that the membrane adheres to the surface of the drying device. Drying is carried out at 35°C to 50°C for 30 minutes to several days. During dehydration, there is the possibility of embossing marks on a graft by a drying device. In the final stage, the graft is cut, packaged and sterilised. [CA2826359 A1, August 23, 2012, Tissue grafts modified with a cross‐linking agent and method of making and using the same, MIMEDX) (US20080046095 A1, February 21, 2008, Placental tissue grafts, MIMEDX].
4.4. Acellular amniotic membrane
The acellular amniotic membrane can be used to treat humans (when it is prepared from human tissues), but it can also be used in veterinary when dressings are prepared from animal placenta. There is a possibility to store such a dressing at room temperature for a common use, in particular for the treatment of burns or eye injuries. It can be used not only as a single dressing layer on wounds or burns in order to support the healing process but also several amniotic membranes can be joined under pressure (in a laminate) or a three‐dimensional structure can be created and seeded by cells, for example, stem cells. After amnion and chorion membranes separating, a mechanical decellularisation is carried out by scraping the tissue on both sides and washing it in a water or 0.9%, physiological saline solution. Then, its drying under pressure at 45°C to 50°C for about 60 minutes is performed. [US20030187515 A1, October 2, 2003, Collagen biofabric and methods of preparation and use therefor, ANTHROGENESIS, CELULARITY]. Cell‐depleted, vacuum‐sealed and sterilised (by electron beam for 3‐5 hours, at a dose of 3‐5 kGy) amniotic membrane can be enriched by stem cells seeding [CN105688287 A, June 22, 2016 Amniotic membrane patch for treating skin wound and preparation method thereof, ISTEM REGENERATIVE MEDICINE SCIENTIFIC TECHNOLOGY].
4.5. Various unusual forms of the amniotic membrane
Placental tissues (in particular the amniotic membrane) can be used to prepare grafts in various forms. One of these forms is homogenised amnion powder. It can be embedded on the skeleton, which contains among others a hydrogel or a biopolymer which may be, for example, hyaluronic acid, chitosan, alginates, collagen, dextran, pectin or gelatin. The skeleton may also include a synthetic polymer consisting of oligolactide methacrylate, polyethylene glycol, polypropylene glycol and others. [CA2963273 A1, April 7, 2016, Amniotic membrane powder and its use in wound healing and tissue engineering constructs, WAKE FOREST UNIVERSITY HEALTH SCIENCES].
Moreover, the amniotic membrane can be formed as a contact lens‐shaped slice. It consists of an amniotic membrane extract applied on a collagen‐based carrier or filler. [US9295753 B1, March 29, 2016, Amniotic Membrane Preparation and Device for use as a lens or as a dressing for promoting healing, TELLO CELSO]. Amniotic fluid in a liquid form containing fragmented amniotic membrane is also used in the treatment of wounds. [WO2015134936 A1, September 11, 2015, Amnion derived therapeutic compositions and methods of use, AMNIO TECHNOLOGY]. Treatment may in particular consist of injecting amniotic fluid along the periphery of the wound and applying amniotic membrane sheet to the wound, which constitutes a scaffold with many longitudinal slots giving the amniotic membrane a shape of a mesh. In addition, other factors necessary for wound healing are provided to the wound, for example, growth factors, carbohydrates, lipids, proteins, amino acids, enzymes, hormones and so on. [US2015216910 A1 August 6, 2015, Expandable Amnion Membrane for Treating Non‐Healing Wounds, NUTECH MEDICAL].
In the treatment of wounds and in tissue regeneration, other forms of amniotic transplants are also used, among others solutions, ointments, sponges, nets, aerosols or foams obtained from the amniotic membrane. [US20140342015 A1, November 20, 2014 „Amniotic membrane hydrogel and methods of making”, WAKE FOREST UNIVERSITY HEALTH SCIENCES]. Two‐component preparations containing an amniotic membrane sheet and demineralised bone powder (DBP) are also used in the treatment. The preparation may consist of many layers, not integrated with each other. [US2014212390 A1, July 31, 2014, Placental membrane preparation and methods of making and using same, NUTECH MEDICAL].
4.6. Other (besides amnion) placental tissues as transplants
Tissue grafts obtained from the placenta may contain one amniotic layer without epithelium, they may be prepared from two layers of amnion sticked together, but they can additionally contain compounds such as chorion, Wharton jelly, natural or synthetic polymers and others. Amnion can be also softened by antibiotic application, and then separated from the chorion. Therefore, not only amnion but also chorion and Wharton jelly (being an excellent source of stem cells) are used in medicine. Another example is a crosslinked tissue graft containing dehydrated placental components, such as an amniotic membrane, chorion, Wharton jelly (or their combination) and a crosslinking agent. It has been shown that the use of such grafts prevents scars formation. They may have the form of a laminate containing at least two layers of chorion, two layers of amnion or at least one layer of each membrane together with crosslinking agent [CA2857636 A1, June 27, 2013, Cross‐linked dehydrated placental tissue grafts and methods for making and using the same, MIMEDX]. [CA2826377 A1, August 23, 2012, Laminated tissue grafts composed of Wharton's jelly and methods of making and using the same, SNOASIS MEDICAL], [WO200933160 A1, March 12, 2009, Placental tissue grafts and improved methods of preparing and using the same, MIMEDX].
5. AMNION IN THE TREATMENT OF TEN AND SJS
TEN and SJS are rare diseases considered as two variants of the same disorder, manifested by severe states histologically characterised by apoptosis of epidermal keratinocytes and by separation of the upper layers of the skin. About 75% of patients develop ocular complications in the course of these diseases.78 TEN occurs annually in 0.4 to 1.2 people per million and SJS occurs in 1 to 6 people per million. Amniotic membrane transplantation turned out to be effective both in the treatment of skin lesions and in the ocular complications therapy in patients suffering from TEN/SJS.
5.1. Characteristics of TEN and SJS
In 1922, Stevens and Johnson described for the first time two clinical cases of this disease, hence its name (SJS).79 Then, Lyell described TEN—a condition characterised by extensive changes resembling epidermal burn, that is, why TEN is now also interchangeably referred to as Lyell's Syndrome.80 Although described separately, both diseases are currently classified as bullous diseases of the epidermis, in which epidermal exfoliation can be caused by slight skin rubbing (a positive Nikolsky's sign).81 In case of both diseases, the lesions affect both skin and mucous membranes, and their classification depends on the percentage of the body surface area (BSA) affected by the disease process. In SJS lesions cover less than 10% BSA, in TEN—over 30% BSA and in case of coexisting diseases (SJS and TEN) 10% to 30% BSA is affected.81
The course of TEN and SJS consists of two phases. Only after the stage of fever, headache, cough, conjunctivitis and general malaise with accompanying upper respiratory tract infection, bullous changes appear on the skin and mucous membranes. The lesions first appear on the face, neck and upper torso, and then in other places. They have the character of large flabby blebs. The patient may also lose nails and eyebrows. In severe cases, large fragments of the epidermis detach themselves in places of light pressure (above‐mentioned Nikolsky's sign). These changes are accompanied by painful scabs in the mouth, erosions, conjunctivitis and keratitis. There is also bronchial oedema causing cough, dyspnoea, pneumonia, pulmonary oedema and hypoxemia. Moreover, glomerulonephritis and hepatitis are repeatedly observed.82
Although TEN and SJS are rare, they are characterised by high probability of complications and high mortality. Mortality is 3% for SJS and 25% to 40% for TEN.81 The TEN pathophysiology is not fully understood, it is believed that the patient's immune system (primarily T‐lymphocytes) is responsible for the epithelial cells apoptosis, which resembles graft‐versus‐host disease (GVHD).82 The presence of the HLA‐B*15:02 allele is also considered as a risk factor for SJS and TEN development in certain ethnic groups.83, 84 It is obvious that TEN and SJS arise from the complicated interaction of many processes and risk factors such as: genetic predisposition, environmental factors, immune response, inflammatory mediators activity or the aforementioned cell apoptosis rate. However, medications are the most common cause of TEN/SJS induction. In 77% to 94% of the cases, diseases are caused by antimicrobials, anticonvulsants, analgesics and nonsteroidal antiinflammatory agents. The profile of drugs causing TEN/SJS varies, depending on the population or popularity of a particular medicine in a given population. The recognition of illnesses is a serious problem. In the second phase of TEN development, after covering over 30% of the body surface, the symptomatic treatment is the standard. It has been proven that the best results are obtained when it is conducted at the intensive care unit in specialised burn centers. This treatment should be based on maintaining the body's normal temperature and appropriate water–electrolyte balance and on proper nutrition. Surgical interventions carried out in case of TEN (as well as SJS) involve most of all cleaning the necrotic epidermis and covering a large wound surface with a biological or synthetic dressing.85, 86, 87
5.2. Ocular complications in TEN and SJS
In the treatment of TEN, it is very important to implement ophthalmic treatment in the early stage of the disease, because in less than 24 hours, adhesions between conjunctiva and cornea can occur. Therefore, ophthalmologic treatment should focus on prevention, adhesion prevention and elimination of inflammatory process effects. Ocular complications develop in 69% to 84% of hospitalised patients in the acute phase of SJS and TEN.78 If the inflammation and the eye surface ulceration are not eliminated in the short time, the healing process of these lesions usually leads to scarring. Inflammations and ulcerations of conjunctiva or cornea can contribute to epithelial stem cells damage in the limbus, leading to a pathological condition known as corneal limbal stem cell deficiency. However, the deficiency of these stem cells cannot be treated by a conventional corneal transplantation—despite the use of allogenic limbal stem cells, prognosis in patients is very bad.78 SJS and TEN (especially TEN) as life‐threatening diseases often require artificial ventilation of patients. In intubated patients, eyes are often closed, so they do not feel any discomfort caused by inflammation or ulceration, which may give the false impression, that the eyes are not affected by the disease process.81 Taking into account the high prevalence of ocular complications in TEN and SJS, patients should be often consulted by the ophthalmologist and if any changes appear, ophthalmological treatment should be implemented as soon as possible. Rinsing the affected eyes with a sterile saline solution that removes all inflammatory residues from the eye surface contributes to reduction of infection risk. The preventive use of topical antibiotics also seems justified.88 Because the treatment of long‐term scarring consequences is difficult and often unsuccessful, it is necessary to implement therapeutic procedures to prevent their occurrence. Among others, hard contact lenses are used, which allows regeneration of the damaged eye surface and reduction of symptoms in patients. They also prevent scarring and reduce the effects of acute inflammation. However, patients must be dependent on lenses for the rest of their lives. Therefore, the use of this type of treatment is particularly unsuitable for children.
5.3. The use of human amnion in ocular complications in patients with TEN and SJS
The above‐mentioned inconveniences regarding the use of hard contact lenses in the treatment of ocular complications in patients with TEN and SJS contributed to the search for more appropriate and effective therapy in the acute phase of the disease.88 Transplantation of cryopreserved amniotic membrane is a very promising treatment method. Most of all, it reduces scar formation (mentioned influence on TGF‐β) and exerts a direct analgesic effect acting on the fibroblasts of the ocular surface.7, 41 These two properties are of particular importance in the eye complications accompanying TEN/SJS. The use of amnion also inhibits inflammation, prevents ulceration and stimulates healing in the acute phase of these diseases. However, there is the need for such treatment in the first 2 weeks after the onset of eye lesions. Then, the amniotic membrane contributes to the rapid epithelium healing and as a result prevents the eye surface scarring. If the transplantation is performed later, it is possible to heal the damaged corneal surface, but it is impossible to stop the progressive conjunctiva scarring.78
In prospective, randomised, controlled clinical trials involving 25 patients (50 eyes affected by disease), the efficacy of standard therapy was compared with treatment supported by amniotic membrane transplantation. Six months after the therapy, visual acuity was significantly better in the amniotic membrane‐treatment group (compared to the standard ophthalmic treatment group). The conjunctival oedema maintained in 44% of the eyes of patients with standard treatment, and only in 4% of the eyes treated with amniotic membrane. Moreover, no patient in the amnion‐treated group suffered from corneal opacity, limbal stem cell deficiency, symblepharon, ankyloblepharon or eyelid‐related complications. Among patients undergoing standard treatment, corneal opacity occurred in 44%, corneal vascularisation in 24%, symblepharon in 16%, ankyloblepharon in 4% and ectropion/entropion in 8%. These results clearly indicate the efficacy of amniotic membrane transplantation in the treatment and complications prevention in patients with ocular lesions in the course of SJS/TEN.89 The same conclusions were drawn by researchers describing a series of 10 cases of patients who underwent amniotic membrane transplantation in the acute stage of SJS or TEN. All patients restored very good visual acuity and in all patients, the severity of the dry eye was moderate or low and scarring of the eye surface and eyelids was mild to moderate.90 These results also indicate that in the acute phase of SJS and TEN, amniotic membrane transplantation is an effective treatment of severe eye and eyelid inflammations. It also significantly reduces the risk of severe ocular and visual complications. A number of other case reports have been published, in which the use of amniotic membrane in the treatment of ocular complications in TEN/SJS is an effective method.91 Various techniques of amniotic membrane transplantation on eyes have also been described—they use either several amniotic layers (simultaneously) or single large membrane sheets (5 × 10 cm).78, 92 What is more, both the methods in which the membrane is sewn on to the eyelids are developed, as well as the sutureless technique using fibrin tissue glue. Special rings from rubber tubes are also used, which facilitate the placement of the amnion in the desired point and its proper adherence to wounds.78, 92
Based on the treatment analysis of seventy‐nine patients (158 eyes), a new classification system (and related therapeutic guidelines) regarding eye complications in TEN and SJS was developed. This system facilitates making therapeutic decisions in case of acute ocular symptoms occurrence in TEN/SJS. Mild and moderate cases have a low risk of significant scarring or reduced visual acuity, so they can be monitored and treated with standard methods (provided that the condition does not deteriorate). Severe cases should receive an amniotic membrane transplant as soon as possible, in order to reduce the risk of scarring and visual acuity reduction. It is necessary because such cases (with extensive eye lesions) are endangered by permanent eye surface and eyelid damage. These patients suffer often from severe eye pain, photophobia and visual impairment.93
5.4. The use of human amnion in the topical treatment of skin lesions in TEN and SJS
The use of proper topical treatment of skin lesions in TEN/SJS contributes to increased survival rate and accelerates wound healing.62 However, there are no unambiguous standards for topical treatment in TEN/SJS, as there is very little scientific data describing such treatment of skin lesions developed in the course of these diseases. In the surgical treatment of TEN/SJS, necrotic epidermis should be removed, leaving most skin blebs that act as natural dressings and promote re‐epithelisation (Figure 3). Then, the cleaned wounds can be covered with a biological or synthetic dressing, for example, Biobrane. When using Biobrane and similar skin substitutes, care should be taken when covering areas larger than 40% of total body surface area (TBSA). In these cases, a secondary infection of wounds (at the site of graft implantation) often occurs. In addition to synthetic dressings such as Biobrane, biological dressings are also used in topical treatment of TEN/SJS. It has been proven that both homograft (cadaveral allograft) and porcine xenograft significantly reduce wound pain and loss of fluids as well as stimulate epithelialisation in patients with TEN. Xenograft obtained from decellularised pig skin (Xe‐Dermy) has been successfully used in topical treatment of skin lesions in TEN, whereas the homograft use carries the risk of its vascularisation, which may cause difficulties in removing the dressing.94, 95
Figure 3.
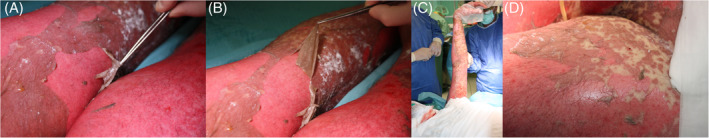
Removal of necrotic epidermis (A,B), leaving blebs (C,D)
In our hospital, after surgical purification, wounds are treated with leaving most of the blebs on the skin, which act as natural dressings probably facilitating re‐epithelialisation. Amnion is applicated after a very delicate wound cleansing. By implementing this type of dressing in 26 patients diagnosed with TEN, a special clinical usefulness of the amniotic membrane in the treatment of this disease was observed. Therefore, the use of amnion was introduced in our hospital as a new standard of TEN treatment. Its validity was confirmed by very good effectiveness in accelerating healing and by very good cosmetic effects after application (Figure 4). It has been unequivocally proven that the use of appropriate topical TEN treatment contributes to increased survival rate and accelerates wound healing.62
Figure 4.
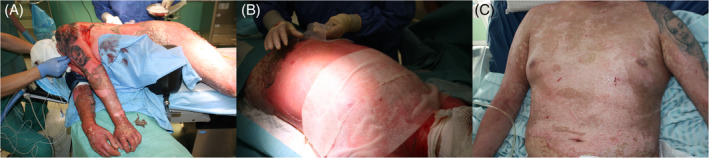
Clinical effect of the use of amniotic transplants in TEN: A, condition of the patient during the first days of hospitalisation. B, allogenic amnion transplantation. C, condition of the patient on the day of discharge from the intensive care unit
6. NEW AND UNUSUAL APPLICATIONS OF AMNIOTIC MEMBRANE IN THE WOUND TREATMENT
In recent years, as the result of increasing knowledge about the amniotic membrane and placenta‐derived stem cells, new and unusual clinical applications of amniotic transplants have appeared.
Amniotic membrane, AMSCs and AECs are often used in skin tissue engineering as a drug reservoir or to develop skin substitutes.27 Human amniotic cells and amniotic membrane's matrix have the ability to store drugs and release them in the body, which is clinically useful, because at the same time they show resistance to many drugs, for example, to paclitaxel (PTX).96 To form skin substitute, AECs are cultured on de‐epidermised dermis or on AMSCs seeded fibrin scaffold, which results in stratified epithelium formation.28, 97 AMSCs alone have also turned to be effective in wound healing. When different types of AMSCs were topically applied to murine wounded skin (on dermal substitute as a carrier), they accelerated healing process and increased the number of blood vessels in the wound in comparison to controls.98
AECs‐derived exosomes (extra‐cellular nano‐vesicles transferring active cargoes between the cells) are increasingly used in clinic, as they were shown to exert anti‐inflammatory and anti‐fibrotic properties. Moreover, exosomes have the ability to influence different cell signalling pathways because they include microRNAs (controlling, eg, the fibrosis signalling) as well as various proteins involved in apoptosis and cell development modulation.99, 100
In addition to the classical use of amnion for the treatment of skin wounds, there are examples of its effective application in therapies of fibrotic diseases, for example, in lungs and liver. Fragments of amnion placed on the rat liver significantly reduced the severity of liver fibrosis in the model of bile duct ligation,101 whereas human AECs transplantation resulted in decreased fibrosis and collagen deposition as well as increased collagen degradation in the injured murine lungs.102, 103 Similar results were obtained using conditioned media from amnion‐derived cells, so it is hypothesized, that mentioned anti‐fibrotic effects are mostly due to paracrine activity of molecules secreted by these cells.22, 104 It is also possible to use amnion to treat adhesion‐related pathologies. It was proven that it reduced adhesions, inflammation and fibrosis in experimental, animal models.105, 106
Amniotic membrane has also a potential to be used in orthopaedic, urological and periapical surgery. It was successfully implanted together with a tendon graft during anterior cruciate ligament (ACL) reconstruction, which reduced pain and decreased complication rates.107 In another study, it was effective following nerve‐sparing robot‐assisted radical prostatectomy, when it was placed around the prostatic neurovascular bundle. In patients who received amniotic transplants, the recovery of urinary continence and potency was faster than in control group, with no adverse effect of the therapy.108 Interestingly, the usefulness of amniotic membrane in regenerative periapical endodontic surgery was shown when it was combined with bone graft and platelet‐rich fibrin (PRF). Such combination enhanced the radiographic healing outcome and decreased post‐operative discomfort in patients suffering from radicular cysts.109
There are also some reports about protective effects of amniotic membrane proteins (AMPs) against hypoxia‐induced injuries of cardiomiocytes, which was proven on the H9c2 rat cell line pre‐treated with AMPs and subjected to 24 hours hypoxia. This procedure caused nuclear factor erythroid‐2‐related factor 2 (Nrf2) a heme‐oxygenase 1 (HO‐1) expression upregulation with concomitant downregulation of inflammatory cascade‐related proteins levels. It gives hope for a new strategy to prevent hypoxia‐associated myocardial dysfunction.110
Another unusual application of amnion is its use in vascular reconstructions, for example as a substitute in the external jugular vein. The experiment was performed on juvenile sheep—the amniotic membrane was rolled around the tube and such conduits were interposed to the external jugular vein. It turned out that after about 1 year, amnion grafts did not show any signs of dilation. The monolayer of endothelial cells was formed on the explanted grafts, and there were no signs of inflammation and fibrosis.111 Similarly, a year after wrapping the brachial plexus (BP) with amniotic membrane during scalenectomy, the membrane was intact, whereas BP was protected against scar formation around it.112
Finally, amniotic membrane was proven to exert some anti‐cancer properties, as AECs produce anti‐angiogenic factors that induce apoptosis in cancer cells (via caspase‐8 and caspase‐3 secretion).113, 114, 115
7. CONCLUSIONS
Amniotic membrane and other placental tissues have the potential for increased clinical use as a treatment option for different types of non‐healing wounds. They are also successfully applied in growing number of other medicine fields, including treatment of rare and fatal diseases. Thus, it is very important to test and improve the methods of placental tissue preparation and storage, which can result in better clinical outcomes.
Recently, more and more experiments have revealed detailed mechanisms of wound healing promotion by amniotic membrane as well as amnion‐derived cells and proteins However, further, large‐size studies are needed in order to increase the therapeutic effectiveness of placental tissue transplants and to ensure their optimal application methods, standardised for different medical cases.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Klama‐Baryła A, Rojczyk E, Kitala D, et al. Preparation of placental tissue transplants and their application in skin wound healing and chosen skin bullous diseases ‐ Stevens‐Johnson syndrome and toxic epidermal necrolysis treatment. Int Wound J. 2020;17:491–507. 10.1111/iwj.13305
REFERENCES
- 1. Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. New York, NY: Springer‐Verlag Berlin Heidelberg; 2012. [Google Scholar]
- 2. Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397‐407. [DOI] [PubMed] [Google Scholar]
- 3. Chen CP, Aplin JD. Placental extracellular matrix: gene expression, deposition by placental fibroblasts and the effect of oxygen. Placenta. 2003;24(4):316‐325. [DOI] [PubMed] [Google Scholar]
- 4. Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341‐348. [DOI] [PubMed] [Google Scholar]
- 5. Nyman E, Huss F, Nyman T, Junker J, Kratz G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: in vitro studies of re‐epithelialisation in human skin wounds. J Plast Surg Hand Surg. 2013;47(2):89‐92. [DOI] [PubMed] [Google Scholar]
- 6. Parry S, Strauss JF III. Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663‐670. [DOI] [PubMed] [Google Scholar]
- 7. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51‐77. [DOI] [PubMed] [Google Scholar]
- 8. Bankowski E, Sobolewski K, Romanowicz L, Chyczewski L, Jaworski S. Collagen and glycosaminoglycans of Wharton's jelly and their alterations in EPH‐gestosis. Eur J Obstet Gynecol Reprod Biol. 1996;66(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 9. Franc S, Rousseau JC, Garrone R, van der Rest M, Moradi‐Ameli M. Microfibrillar composition of umbilical cord matrix: characterization of fibrillin, collagen VI and intact collagen V. Placenta. 1998;19(1):95‐104. [DOI] [PubMed] [Google Scholar]
- 10. Sobolewski K, Malkowski A, Bankowski E, Jaworski S. Wharton's jelly as a reservoir of peptide growth factors. Placenta. 2005;26(10):747‐752. [DOI] [PubMed] [Google Scholar]
- 11. Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330‐1337. [DOI] [PubMed] [Google Scholar]
- 12. Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141(6):715‐724. [DOI] [PubMed] [Google Scholar]
- 13. Koob TJ, Lim JJ, Massee M, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10(5):493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maan ZN, Rennert RC, Koob TJ, Januszyk M, Li WW, Gurtner GC. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res. 2015;193(2):953‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massee M, Chinn K, Lei J, Lim JJ, Young CS, Koob TJ. Dehydrated human amnion/chorion membrane regulates stem cell activity in vitro. J Biomed Mater Res B Appl Biomater. 2016;104(7):1495‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aller MA, Arias N, Martinez V, Vergara P, Arias J. The gestational power of mast cells in the injured tissue. Inflamm Res. 2018;67(2):111‐116. [DOI] [PubMed] [Google Scholar]
- 18. Nazari M, Ni NC, Ludke A, et al. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J Mol Cell Cardiol. 2016;94:32‐42. [DOI] [PubMed] [Google Scholar]
- 19. Siegel N, Rosner M, Hanneder M, Freilinger A, Hengstschlager M. Human amniotic fluid stem cells: a new perspective. Amino Acids. 2008;35(2):291‐293. [DOI] [PubMed] [Google Scholar]
- 20. Uberti MG, Pierpont YN, Ko F, et al. Amnion‐derived cellular cytokine solution (ACCS) promotes migration of keratinocytes and fibroblasts. Ann Plast Surg. 2010;64(5):632‐635. [DOI] [PubMed] [Google Scholar]
- 21. Franz MG, Payne WG, Xing L, et al. The use of amnion‐derived cellular cytokine solution to improve healing in acute and chronic wound models. Eplasty. 2008;8:e21. [PMC free article] [PubMed] [Google Scholar]
- 22. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion‐derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6‐14. [DOI] [PubMed] [Google Scholar]
- 23. Steed DL, Trumpower C, Duffy D, et al. Amnion‐derived cellular cytokine solution: a physiological combination of cytokines for wound healing. Eplasty. 2008;8:e18. [PMC free article] [PubMed] [Google Scholar]
- 24. Kesting MR, Wolff KD, Hohlweg‐Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29(6):907‐916. [DOI] [PubMed] [Google Scholar]
- 25. Vinketova K, Mourdjeva M, Oreshkova T. Human Decidual stromal cells as a component of the implantation niche and a modulator of maternal immunity. J Pregnancy. 2016;2016:8689436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300‐311. [DOI] [PubMed] [Google Scholar]
- 27. Farhadihosseinabadi B, Farahani M, Tayebi T, et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol. 2018;46(supp 2):431‐440. [DOI] [PubMed] [Google Scholar]
- 28. Fatimah SS, Chua K, Tan GC, Azmi TI, Tan AE, Abdul Rahman H. Organotypic culture of human amnion cells in air‐liquid interface as a potential substitute for skin regeneration. Cytotherapy. 2013;15(8):1030‐1041. [DOI] [PubMed] [Google Scholar]
- 29. Cheng T, Yang B, Li D, et al. Wharton's jelly transplantation improves neurologic function in a rat model of traumatic brain injury. Cell Mol Neurobiol. 2015;35(5):641‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moll G, Alm JJ, Davies LC, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nevala‐Plagemann C, Lee C, Tolar J. Placenta‐based therapies for the treatment of epidermolysis bullosa. Cytotherapy. 2015;17(6):786‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu KH, Mo XM, Han ZC, Zhou B. Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg. 2011;92(5):1917‐1925. [DOI] [PubMed] [Google Scholar]
- 35. Fidel PL Jr, Romero R, Ramirez M, et al. Interleukin‐1 receptor antagonist (IL‐1ra) production by human amnion, chorion, and decidua. Am J Reprod Immunol. 1994;32(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 36. Higa K, Shimmura S, Shimazaki J, Tsubota K. Hyaluronic acid‐CD44 interaction mediates the adhesion of lymphocytes by amniotic membrane stroma. Cornea. 2005;24(2):206‐212. [DOI] [PubMed] [Google Scholar]
- 37. Kang JW, Koo HC, Hwang SY, et al. Immunomodulatory effects of human amniotic membrane‐derived mesenchymal stem cells. J Vet Sci. 2012;13(1):23‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li H, Niederkorn JY, Neelam S, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900‐907. [DOI] [PubMed] [Google Scholar]
- 39. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88‐99. [DOI] [PubMed] [Google Scholar]
- 40. Lee NJ, Wang SJ, Durairaj KK, Srivatsan ES, Wang MB. Increased expression of transforming growth factor‐beta1, acidic fibroblast growth factor, and basic fibroblast growth factor in fetal versus adult fibroblast cell lines. Laryngoscope. 2000;110(4):616‐619. [DOI] [PubMed] [Google Scholar]
- 41. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor‐beta isoforms, TGF‐beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179(3):325‐335. [DOI] [PubMed] [Google Scholar]
- 42. Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT, EpiFix VLUSG . A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):688‐693. [DOI] [PubMed] [Google Scholar]
- 43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kogan S, Sood A, Granick MS. Amniotic membrane adjuncts and clinical applications in wound healing: a review of the literature. Wounds. 2018;30(6):168‐173. [PubMed] [Google Scholar]
- 45. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi‐Centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lavery LA, Fulmer J, Shebetka KA, et al. Grafix diabetic foot ulcer study G. the efficacy and safety of Grafix([R]) for the treatment of chronic diabetic foot ulcers: results of a multi‐Centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mohajeri‐Tehrani MR, Variji Z, Mohseni S, et al. Comparison of a bioimplant dressing with a wet dressing for the treatment of diabetic foot ulcers: a randomized, controlled clinical trial. Wounds. 2016;28(7):248‐254. [PubMed] [Google Scholar]
- 48. Snyder RJ, Shimozaki K, Tallis A, et al. Randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of Care for the Closure of chronic diabetic foot ulcer. Wounds. 2016;28(3):70‐77. [PubMed] [Google Scholar]
- 49. Laurent I, Astere M, Wang KR, Cheng QF, Li QF. Efficacy and time sensitivity of amniotic membrane treatment in patients with diabetic foot ulcers: a systematic review and meta‐analysis. Diabetes Ther. 2017;8(5):967‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Serena TE, Yaakov R, DiMarco D, et al. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: correlation between 4‐week and 24‐week outcomes. J Wound Care. 2015;24(11):530‐534. [DOI] [PubMed] [Google Scholar]
- 51. Sabella N. Use of fetal membranes in skin grafting. Med Rec NY. 1913;83:478. [Google Scholar]
- 52. Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns. 1989;15(5):339‐342. [DOI] [PubMed] [Google Scholar]
- 53. Lorusso R, Geraci V, Masellis M. The treatment of superficial burns with biological and synthetic material: frozen amnion and biobrane. Ann Med Burn Club. 1989;2:79‐84. [Google Scholar]
- 54. Ugar N, Haberal M. Comparison of various dressing materials used for out‐patient burn treatment at our Centre. Ann Med Burn Club. 1994;7:147‐149. [Google Scholar]
- 55. Singh R, Purohit S, Chacharkar MP, Bhandari PS, Bath AS. Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second‐degree burns. Burns. 2007;33(4):505‐510. [DOI] [PubMed] [Google Scholar]
- 56. Branski LK, Herndon DN, Celis MM, Norbury WB, Masters OE, Jeschke MG. Amnion in the treatment of pediatric partial‐thickness facial burns. Burns. 2008;34(3):393‐399. [DOI] [PubMed] [Google Scholar]
- 57. Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol. 2018;12:1105‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf. 2004;2(3):201‐211. [DOI] [PubMed] [Google Scholar]
- 60. Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12(4):269‐281. [DOI] [PubMed] [Google Scholar]
- 61. Tseng SC. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep. 2001;21(4):481‐489. [DOI] [PubMed] [Google Scholar]
- 62. Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012;9:CD009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18(2):193‐204. [DOI] [PubMed] [Google Scholar]
- 64. Rock T, Bartz‐Schmidt KU, Landenberger J, Bramkamp M, Rock D. Amniotic membrane transplantation in reconstructive and regenerative ophthalmology. Ann Transplant. 2018;23:160‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Westekemper H, Figueiredo FC, Siah WF, Wagner N, Steuhl KP, Meller D. Clinical outcomes of amniotic membrane transplantation in the management of acute ocular chemical injury. Br J Ophthalmol. 2017;101(2):103‐107. [DOI] [PubMed] [Google Scholar]
- 66. Liu HY, Chen YF, Chen TC, Yeh PT, Hu FR, Chen WL. Surgical result of pterygium extended removal followed by fibrin glue‐assisted amniotic membrane transplantation. J Formos Med Assoc. 2017;116(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 67. L JL, Hall L, Liu J. Improving glaucoma surgical outcomes with adjunct tools. J Curr Glaucoma Pract. 2018;12(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baradaran‐Rafii A, Asl NS, Ebrahimi M, et al. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul Surf. 2018;16(1):146‐153. [DOI] [PubMed] [Google Scholar]
- 69. Bonci PO, Bonci PA, Lia A. Suspension made with amniotic membrane: clinical trial. Eur J Ophthalmol. 2005;15(4):441‐445. [DOI] [PubMed] [Google Scholar]
- 70. Kang M, Choi S, Cho Lee AR. Effect of freeze dried bovine amniotic membrane extract on full thickness wound healing. Arch Pharm Res. 2013;36(4):472‐478. [DOI] [PubMed] [Google Scholar]
- 71. Liang L, Li W, Ling S, et al. Amniotic membrane extraction solution for ocular chemical burns. Clin Experiment Ophthalmol. 2009;37(9):855‐863. [DOI] [PubMed] [Google Scholar]
- 72. Shahriari HA, Tokhmehchi F, Reza M, Hashemi NF. Comparison of the effect of amniotic membrane suspension and autologous serum on alkaline corneal epithelial wound healing in the rabbit model. Cornea. 2008;27(10):1148‐1150. [DOI] [PubMed] [Google Scholar]
- 73. Sheha H, Liang L, Hashem H, Ramzy M, Zaki A. Amniotic membrane extract for acute ocular chemical burns. Tech Ophthalmol. 2010;8(4):146‐150. [Google Scholar]
- 74. Ghetti M, Bondioli E, Purpura V, Cenacchi G, Ruscelli P, Melandri D. Decellularized human dermal matrix produced by a skin bank a new treatment for abdominal wall defects. Ann Ital Chir. 2017;5:443‐448. [PubMed] [Google Scholar]
- 75. Milan PB, Pazouki A, Joghataei MT, et al. Decellularization and preservation of human skin: a platform for tissue engineering and reconstructive surgery. Methods. 2019. 10.1016/j.ymeth.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 76. Tyszkiewicz JT, Uhrynowska‐Tyszkiewicz IA, Kaminski A, Dziedzic‐Goclawska A. Amnion allografts prepared in the central tissue Bank in Warsaw. Ann Transplant. 1999;4(3–4):85‐90. [PubMed] [Google Scholar]
- 77. Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299‐307. [PubMed] [Google Scholar]
- 78. Shanbhag SS, Chodosh J, Saeed HN. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens‐Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. 2019;17:560‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and opthalmitis. Report of two cases in children. Am J Dis Child. 1922;24(6):526‐533. [Google Scholar]
- 80. Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68(11):355‐361. [DOI] [PubMed] [Google Scholar]
- 81. Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens‐Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. 2009;54(6):686‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mawson AR, Eriator I, Karre S. Stevens‐Johnson syndrome and toxic epidermal necrolysis (SJS/TEN): could retinoids play a causative role? Med Sci Monit. 2015;21:133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahmadi M, van Hoeven L, Bokhoven K. From facial pain to toxic epidermal necrolysis: a rare complication of carbamazepine usage. Ned Tijdschr Geneeskd. 2017;161:D1895. [PubMed] [Google Scholar]
- 84. Devi K. The association of HLA B*15:02 allele and Stevens‐Johnson syndrome/toxic epidermal necrolysis induced by aromatic anticonvulsant drugs in a south Indian population. Int J Dermatol. 2018;57(1):70‐73. [DOI] [PubMed] [Google Scholar]
- 85. Adzick NS, Kim SH, Bondoc CC, Quinby WC, Remensnyder JP. Management of toxic epidermal necrolysis in a pediatric burn center. Am J Dis Child. 1985;139(5):499‐502. [DOI] [PubMed] [Google Scholar]
- 86. Fischer M, Fiedler E, Marsch WC, Wohlrab J. Antitumour necrosis factor‐alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146(4):707‐709. [DOI] [PubMed] [Google Scholar]
- 87. Halebian PH, Shires GT. Burn unit treatment of acute, severe exfoliating disorders. Annu Rev Med. 1989;40:137‐147. [DOI] [PubMed] [Google Scholar]
- 88. Gregory DG. The ophthalmologic management of acute Stevens‐Johnson syndrome. Ocul Surf. 2008;6(2):87‐95. [DOI] [PubMed] [Google Scholar]
- 89. Sharma N, Thenarasun SA, Kaur M, et al. Adjuvant role of amniotic membrane transplantation in acute ocular Stevens‐Johnson syndrome: a randomized control trial. Ophthalmology. 2016;123(3):484‐491. [DOI] [PubMed] [Google Scholar]
- 90. Gregory DG. Treatment of acute Stevens‐Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology. 2011;118(5):908‐914. [DOI] [PubMed] [Google Scholar]
- 91. Barua A, McKee HD, Barbara R, Carley F, Biswas S. Toxic epidermal necrolysis in a 15‐month‐old girl successfully treated with amniotic membrane transplantation. J AAPOS. 2012;16(5):478‐480. [DOI] [PubMed] [Google Scholar]
- 92. Ma KN, Thanos A, Chodosh J, Shah AS, Mantagos IS. A novel technique for amniotic membrane transplantation in patients with acute Stevens‐Johnson syndrome. Ocul Surf. 2016;14(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 93. Gregory DG. New grading system and treatment guidelines for the acute ocular manifestations of Stevens‐Johnson syndrome. Ophthalmology. 2016;123(8):1653‐1658. [DOI] [PubMed] [Google Scholar]
- 94. Klosova H, Nemeckova Crkvenjas Z, Petras L, Stetinsky J. The use of acellular biological xenografts in local treatment of Lyells syndrome. Rozhl Chir. 2014;93(2):76‐81. [PubMed] [Google Scholar]
- 95. Lipovy B, Rihova H, Kaloudova Y, Mager R, Suchanek I. Use of Xe‐derma([R]), a novel biological cover, in a female patient with toxic epidermal necrolysis. Ann Burns Fire Disasters. 2014;27(3):136‐140. [PMC free article] [PubMed] [Google Scholar]
- 96. Bonomi A, Silini A, Vertua E, et al. Human amniotic mesenchymal stromal cells (hAMSCs) as potential vehicles for drug delivery in cancer therapy: an in vitro study. Stem Cell Res Ther. 2015;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jiang LW, Chen H, Lu H. Using human epithelial amnion cells in human de‐epidermized dermis for skin regeneration. J Dermatol Sci. 2016;81(1):26‐34. [DOI] [PubMed] [Google Scholar]
- 98. Ertl J, Pichlsberger M, Tuca AC, et al. Comparative study of regenerative effects of mesenchymal stem cells derived from placental amnion, chorion and umbilical cord on dermal wounds. Placenta. 2018;65:37‐46. [DOI] [PubMed] [Google Scholar]
- 99. Alhomrani M, Correia J, Zavou M, et al. The human amnion epithelial cell Secretome decreases hepatic fibrosis in mice with chronic liver fibrosis. Front Pharmacol. 2017;8:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tan JL, Lau SN, Leaw B, et al. Amnion epithelial cell‐derived Exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7(2):180‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant. 2011;20(3):441‐453. [DOI] [PubMed] [Google Scholar]
- 102. Moodley Y, Ilancheran S, Samuel C, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med. 2010;182(5):643‐651. [DOI] [PubMed] [Google Scholar]
- 103. Murphy S, Lim R, Dickinson H, et al. Human amnion epithelial cells prevent bleomycin‐induced lung injury and preserve lung function. Cell Transplant. 2011;20(6):909‐923. [DOI] [PubMed] [Google Scholar]
- 104. Cargnoni A, Ressel L, Rossi D, et al. Conditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin‐induced lung fibrosis. Cytotherapy. 2012;14(2):153‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kelekci S, Uygur D, Yilmaz B, Sut N, Yesildaglar N. Comparison of human amniotic membrane and hyaluronate/carboxymethylcellulose membrane for prevention of adhesion formation in rats. Arch Gynecol Obstet. 2007;276(4):355‐359. [DOI] [PubMed] [Google Scholar]
- 106. Petter‐Puchner AH, Fortelny RH, Mika K, Hennerbichler S, Redl H, Gabriel C. Human vital amniotic membrane reduces adhesions in experimental intraperitoneal onlay mesh repair. Surg Endosc. 2011;25(7):2125‐2131. [DOI] [PubMed] [Google Scholar]
- 107. Woodall BM, Elena N, Gamboa JT, et al. Anterior cruciate ligament reconstruction with amnion biological augmentation. Arthrosc Tech. 2018;7(4):e355‐e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Patel VR, Samavedi S, Bates AS, et al. Dehydrated human amnion/Chorion membrane allograft nerve wrap around the prostatic neurovascular bundle accelerates early return to continence and potency following robot‐assisted radical prostatectomy: propensity score‐matched analysis. Eur Urol. 2015;67(6):977‐980. [DOI] [PubMed] [Google Scholar]
- 109. Uppada UK, Kalakonda B, Koppolu P, et al. Combination of hydroxyapatite, platelet rich fibrin and amnion membrane as a novel therapeutic option in regenerative periapical endodontic surgery: case series. Int J Surg Case Rep. 2017;37:139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Faridvand Y, Nozari S, Vahedian V, et al. Nrf2 activation and down‐regulation of HMGB1 and MyD88 expression by amnion membrane extracts in response to the hypoxia‐induced injury in cardiac H9c2 cells. Biomed Pharmacother. 2019;109:360‐368. [DOI] [PubMed] [Google Scholar]
- 111. Peirovi H, Rezvani N, Hajinasrollah M, Mohammadi SS, Niknejad H. Implantation of amniotic membrane as a vascular substitute in the external jugular vein of juvenile sheep. J Vasc Surg. 2012;56(4):1098‐1104. [DOI] [PubMed] [Google Scholar]
- 112. Sanders RJ, Annest SJ. Amnion membrane improves results in treating neurogenic thoracic outlet syndrome. J Vasc Surg Cases Innov Tech. 2018;4(2):163‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Niknejad H, Khayat‐Khoei M, Peirovi H, Abolghasemi H. Human amniotic epithelial cells induce apoptosis of cancer cells: a new anti‐tumor therapeutic strategy. Cytotherapy. 2014;16(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 114. Niknejad H, Yazdanpanah G. Anticancer effects of human amniotic membrane and its epithelial cells. Med Hypotheses. 2014;82(4):488‐489. [DOI] [PubMed] [Google Scholar]
- 115. Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell‐cycle arrest and inhibition of angiogenesis make human amnion‐derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016;363(3):599‐608. [DOI] [PubMed] [Google Scholar]


