Abstract
Wound healing is a sequester program that involves diverse cell signalling cascades. Notwithstanding, complete signal transduction pathways underpinning acidic milieu derived from cancer cells is not clear, yet. MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) assay, fluorescein diacetate/propidium iodide staining, and cell cycle flow cytometry revealed that acidic media decreased cell viability and cell number along with enhanced dead cells and S‐phase arrest in normal fibroblasts. Notably, the trends of intracellular reactive oxygen species production and lactate dehydrogenase release significantly increased with time. It seems the downregulation of Klf4 is in part due to acidosis‐induced DNA damage. It promoted cells towards S‐phase arrest and diminished cell proliferation. Klf4 downregulation had a direct correlation with the P53 level while acidic microenvironment promotes cells towards cell death mechanisms including apoptosis and autophagy. Noteworthily, the unchanged levels of Rb and Mlh1 indicated in those genes had no dominant role in the repairing of DNA damage in fibroblasts treated with the acidic microenvironment. Therefore, cells owing to not entering to mitosis and accumulation of DNA damage were undergone cell death to preserve cell homeostasis. Since acidic media decreased the level of tumour suppressor and DNA repair genes and altered the normal survival pathways in fibroblasts, caution should be exercised to not lead to cancer rather than wound healing.
Keywords: acidic microenvironment, autophagy, cancer, tumour suppressor, wound healing
1. INTRODUCTION
Wound healing is a sequester program that involves different cells and cell signalling cascades. Scientists divide it into several phases including haemostasis, inflammatory, proliferation, remodelling, granulation, and maturation phases, while others merge haemostasis phase and inflammatory phase and nominate proliferation as a granulation phase and remodelling as a maturation phase. It is worth noting that keratinocyte, melanocyte, fibroblast, and epithelial cells are the major cells in skin structure. Notwithstanding, wound healing is not limited to skin repair,1 and other types of wound healing can be seen in other tissues such as corneal epithelial,2 oral mucosal,3 and musculofacial tissues.4 In the present report, among different cells involved in wound repair, we focus on fibroblasts.
Fibroblasts are located in the dermis layer of skin and are responsible for wound contraction, producing extracellular matrix and collagen as well as biodegradation of fibrin clot. There are some reports indicating the positive role of acidic media on wound repair, and in this regard some commercial acidic wound dressings have been prepared to improve wound healing.5, 6 Unlike acute wounds, chronic wounds have alkaline pH milieu while the pH (5.5) gradually increases in re‐epithelisation phase of healing.7 Noteworthily, Sharpe et al disclosed that there is a direct relationship between the acidic media and healing process, in which burn wounds with pH 7.32 show significantly accelerated healing profile compared with pH 7.73.8 It appears that acidic media through altering protease activity, enhancing fibroblast and macrophage activities, epithelialisation, angiogenesis, releasing oxygen, destroying abnormal collagen, and antibacterial potential improve wound healing.5, 6 It is worth mentioning that there is no significant difference between the surface skin pH of men and women while pH increases in elderly,9 and it seems that the level of pH has a good correlation with ageing.
Besides, acidic medium is one of the most mysterious by‐product of cancer cells that acts as a Trojan horse, and it seems that through epigenetic and genetic alterations and failed reprogramming in normal cells adjacent cancer cells they convert into cancer cells.10 Tavakol and colleagues disclosed that acidic pH decreases necrosis and cell proliferation of cancer cells along with enhancement of autophagy.11 White et al denoted some part of acidic media in tumour cells to function and structure of pH sensors resident in cell plasma membrane and believed that the prominent behaviour of cancer cells will be attenuated with decreasing intracellular pH.12 Therefore, in the present study, the main question was that whether acidic pH of cancer cells changes the biological behaviour of their adjacent normal fibroblasts or not.
The important question is whether external acidic pH in the surrounding area of fibroblasts beside the wound healing effects changes the biological behaviour of fibroblasts or not. In other words, it is critical to be sure that the acidic microenvironment inducing an inverse pH (acidic extracellular pH; normal cells has the relatively acidic intracellular pH 7.2 than extracellular pH 7.412) does not have any adverse effect on fibroblasts.
To the best of our knowledge, there were no reports to collaterally investigate the effect of cancer pH on cell death mechanisms including autophagy and apoptosis of normal fibroblasts. Furthermore, the present study investigated the level of genes involved in DNA repair and tumour suppression in fibroblasts treated with acidic media. This study provides hints for the pH modulation of wound microenvironment by the focus on cell death mechanisms. Another important finding was with regard to this fact that wound healing is diminished in cancer tissues; therefore, the effect of extracellular cancer pH in normal cells was investigated.
2. MATERIALS AND METHODS
2.1. Cell treatment
Mouse subcutaneous connective tissue cell line, L929, was purchased from the Pasteur Institute of Iran. The cells was incubated in Dulbecco's modified Eagle's medium high glucose supplementation and then supplemented with 10% fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (BioIDEA, Tehran, Iran) at 37°C in 5% CO2 and 95% moisture. Fibroblasts (passage 3) were used for further investigations. To evaluate the effect of pH on cell behaviour, the pH of culture medium was adjusted to 7.4 and 6.7 and nominated L929 (N) and L929 (A), respectively.
2.2. Live‐dead staining by fluorescein diacetate/propidium iodide
Live‐dead analysis was performed using fluorescein diacetate/propidium iodide (FDA‐PI) staining, in which PI measures both late apoptotic and necrotic dead cells while FDA measures vital cells. In brief, fibroblasts (3 × 104 cells/well) at the three passages were seeded into a 24‐well plate and treated with normal and acidic pH media at 37°C in 5% CO2 and 95% moisture for 48 hours. To FDA‐PI staining, 5 mL staining solution containing 50 μL PI solution (1 mg/mL) and 8 μL FDA (5 mg/mL acetone) solution was prepared and added to the wells. Following, the cells were imaged under an inverted fluorescent microscope (Olympus AXE‐800, Shinjuku, Tokyo, Japan) coupled to a digital camera (Leica, DC200, Wetzlar, Germany). The assay was performed in triplicate, and the values provided are the normalised to mean ± SD of three independent experiments.
2.3. Cell viability assay using MTT assay
MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) assay is considered as a valuable analysis for cell viability measurement. L929 cells (1 × 104 cells/well) were seeded in triplicate in a 96‐well culture plate containing basal media for 24 hours. Then, the medium was completely exchanged with adjusted fresh medium to pHs 6.7 and 7.4 for 48 hours. When the time was over, media were completely removed and exchanged with 100 μL of MTT (Sigma, St. Louis, Michigan; 0.5 mg/mL) for 4 hours. Equal volume of dimethyl sulfoxide (Sigma) was then added to dissolve hydrazine crystal, and the absorbance was read at 570 nm using a microplate reader (BioTek, Winooski, Vermont) up to 20 minutes. The assay was performed in triplicate, and the values provided are the normalised mean ± SD of at least three independent experiments.
2.4. Cell membrane damage using lactate dehydrogenase release assay
To study the effect of pH on fibroblast cell membrane damage, released lactate dehydrogenase (LDH) into the surrounding environment is investigated. Fibroblasts in three passages were seeded into a 96‐well plate for 24 hours, and then the medium was completely refreshed with media at pHs 7.4 and 6.8 for 24 hours. LDH release was monitored using the LDH Elisa kit based on its protocol (Roche, Berlin, Germany). Briefly, 100 μL/well cell supernatant was transferred into the parallel well plates and mixed with equal volume of the reaction mixture for 30 minutes at 22°C. Absorbance was read at 490 nm using an Elisa Reader (BioTek). Analysis of LDH release was performed in triplicate and repeated three times in independent experiments, and the data were normalised with cell number and mean ± SD was reported.
2.4.1. Cell cycle by PI flow cytometry
Cell cycle analysis of fibroblasts treated with normal and acidic pH was performed using a PI flow cytometer. After incubation for 48 hours, fibroblasts were trypsinised, washed with phosphate buffer saline (PBS), and fixed with 70% ice‐cold ethanol at −20°C overnight. Then, fibroblast cells were washed with PBS and stained with 1 mg/mL PI solution (Sigma) and 50 μg/ mL RNase A (Roche) for 30 minutes in the dark at room temperature. To track the cell cycle, a FACScan cytometer (Becton Dickinson, Franklin Lakes, New Jersey) was used and then data were analysed using the FlowJo software (v7.6.1; FlowJo). The experiment was repeated three times and mean ± SD was calculated.
2.5. Intracellular reactive oxygen species analysis using 2′,7′‐dichlorofluorescein diacetate flow cytometry and microplate reader in kinetic mode
Fibroblasts (30 × 103 cells/well, passage 3) were seeded in the 24‐well culture plates for 24 hours. Then cells were treated by the fresh media adjusted to pH 7.4 and 6.8 for 24 and 48 hours. Fluorescent dye of 2′,7′‐dichlorofluorescein diacetate (DCFH‐DA; 10 μM) was added to the treated cells, and the kinetics of intracellular reactive oxygen species (ROS) in cells was evaluated using a fluorescence microplate reader for 45 minutes. The assay was performed in triplicate, and the values provided were normalised to cell number and mean ± SD of three independent experiments were reported. Furthermore, to track intracellular ROS production in fibroblast cells by 48 hours, treated cells in normal and acidic pH media were tracked using an inverted fluorescent microscope (Olympus AXE‐800) coupled to a digital camera (Leica, DC200) and flow cytometry.
2.6. Gene analysis using quantitative reverse transcription polymerase chain reaction
Rb, Klf4, Mlh1, and TP53 genes were tracked using quantitative reverse transcription polymerase chain reaction (qRT‐PCR) at the level of mRNA. Briefly, fibroblast cells (passage 3) were treated by acidic and normal media for 48 hours. Then, total RNA was extracted using RNX‐Plus kit (Sinaclon, Iran), and then DNAse 1 treatment was applied and random hexamer and oligo dt‐primed cDNA synthesis was carried out using a cDNA synthesis kit (Takara, Japan). cDNA was used for 45‐cycle RT‐PCR in Rotor‐Gene Q real‐time analyzer (Corbett, Australia) using the EvaGreen master mix. Each reaction was repeated three times, and relative fold change gene expression was quantified using the DDCt method. β‐Actin gene was selected as an internal gene. The duplicate experiments were repeated three times and mean ± SD was calculated. The primer sequences of genes were as follows:
Rb:
F: 5′‐AAGTGTACCGTCTAGCATATCTCC‐3
R: 5′‐TAGAGCACATCATAATCTGGTCCAA‐3′
Klf4:
F: 5′‐AGAACAGCCACCCACACTTG‐3′
R: 5′‐GTGGTAAGGTTTCTCGCCTGT‐3′
Mlh1:
F: 5′‐GGAAGTTGTTGGCAGGTATTCAATA‐3′
R: 5′‐CGAATGTTGTCCACGGTTGT‐3
Trp53:
F: 5′‐ TGGAGGAGTCACAGTCGGATA‐3′
R: 5′‐CAGTGAGGTGATGGCAGGAT‐3
2.7. Western blotting of apoptotic proteins
Fibroblasts (passage 3) were subcultured and were treated with acidic and normal media for 48 hours. Briefly, proteins were extracted using radioimmunoprecipitation assay buffer, and then 60 μg of the proteins was separated in SDS‐PAGE and electrotransferred onto a PVDF membrane. β‐Actin protein was selected as the internal control. Protein expression was evaluated using Bax (Cell Signaling, CAT N 2774S; 1:1000) and Bcl2 (Cell Signaling, CAT N 4223S; 1:1000) antibodies. Their protein levels were normalised to ß‐actin antibody (Padtan Zist Pajooh, Iran, 1:1000). The duplicate experiments were analysed and mean ± SD was calculated.
2.8. Autophagy measurement using acridine orange staining
Thomé et al reported that the red‐to‐green fluorescence intensity ratio (R/GFIR) of acridine orange (AO) is in agreement with the LC3‐I into LC3‐II conversion, late autophagy, and P62 decrement level.13 Fibroblasts (3 × 104 cells/well) were seeded in triplicate in a 24‐well plate for 24 hours, and then the medium was completely exchanged with fresh medium adjusted to pHs 7.4 and 6.7 for 24 and 48 hours. Then the fibroblast cells were stained with 1 μg/mL of AO (2.7 μM), and R/GFIR was analysed using a fluorometer (BioTek) at a fluorescence excitation wavelength of 488 nm and emission wavelengths of 530 (green) and 620 (red) nm. The assay was performed in triplicate and the values provided are the normalised mean ± SD of three independent experiments. However, images were acquired using an inverted fluorescent microscope (Olympus AXE‐800) coupled to a digital camera (Leica, DC200) for 48 hours.
2.8.1. Statistical analysis
The GraphPad software (GSL Biotech LLC) was applied to calculate and analyse LDH release, MTT assay, FDA‐PI, cell cycle, intracellular ROS kinetics, relative fold change gene, and protein expression in the cells treated with acidic and normal pH media. All triplicate experiments were repeated three times. Experiments were performed as mean ± SD. Unpaired and two‐tailed Student's t test was used for statistical analysis of two groups. A P value of <.05 was considered statistically significant.
3. RESULTS
3.1. Live‐dead staining by FDA‐PI
Live‐dead staining was performed to evaluate the percentage of live and dead cells treated with normal and acidic pH microenvironments in fibroblasts. Florescence microscopy images showed that acidic pH microenvironment induced higher dead fibroblast cells than the cells treated in normal media by 48 hours. Furthermore, quantitative data derived from the fluorometer revealed that cells treated with acidic media were significantly more than cells treated with normal media experienced cell death (Figure 1A). Another findings derived from FDA staining was related to cell proliferation, in which results disclosed that the cell proliferation potential of fibroblasts treated with acidic media was approximately 33.83% less than the cells treated with normal media.
Figure 1.
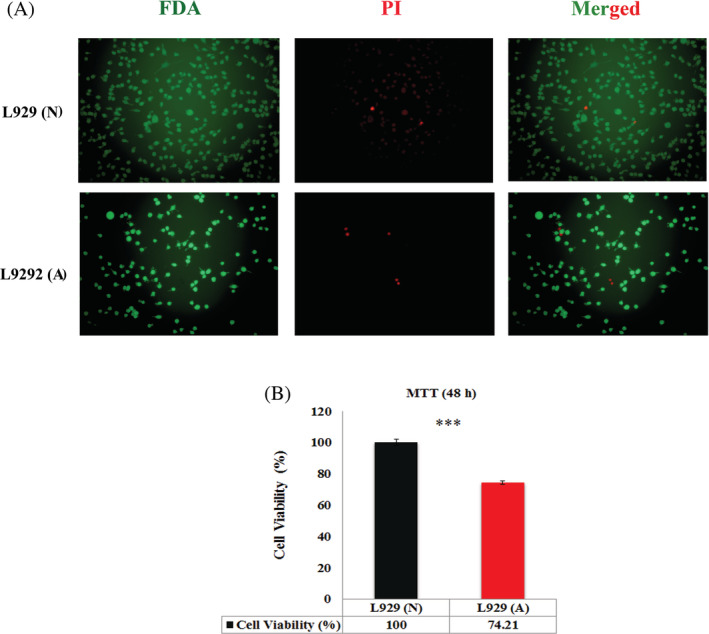
A, FDA‐PI staining of fibroblasts in face to acidic media. It was shown that the numbers of live cells was significantly less in fibroblasts treated with acidic media compared with the normal cells while the number of dead cells was higher in acidic media. B, MTT assay related to fibroblasts in face to acidic media by 48 hours. Results showed less percentage of cell viability in fibroblasts treated with acidic media compared with the normal cells. L929 (A) means fibroblasts in acidic media, L929 (N) means fibroblasts in normal pH media, (*** means P < .001). FDA‐PI, fluorescein diacetate/propidium iodide
3.2. Cell viability assay using MTT assay
Formazan reduction in MTT assay is a marker of live cells. The assay was performed to investigate the cell viability of fibroblast cells treated with acidic and normal media. Results disclosed that acidic pH significantly decreased the cell viability of fibroblasts compared with normal media by 48 hours (P < .001) (Figure 1B).
3.3. Cell membrane damage using LDH release assay
LDH release as a released enzyme derived from necrotic and cell membrane‐damaged cells was investigated. Results showed that fibroblasts treated with acidic media release significantly less LDH than those treated with normal media by 2 hours. The two‐tailed P value was <.0001 and was considered extremely significant. Furthermore, following 24‐hour treatment, normalised LDH release data derived from fibroblast cells treated with acidic media revealed significantly less LDH release than those treated with normal media (P < .0001). However, normalised intracellular LDH data derived from fibroblast cells disclosed that acidic media significantly enhanced LDH production than normal media in fibroblast cells by 48 hours. The two‐tailed P value is .0043, which was considered very significant (Figure 2A‐C).
Figure 2.

LDH release and production in fibroblasts treated with acidic media. A, LDH release derived from fibroblasts treated with acidic media for 2 hours. Acidic media induced significantly less LDH release in fibroblasts than normal pH media. B, LDH release derived from fibroblasts treated with acidic media for 24 hours. Acidic media induced significantly less LDH release in fibroblasts than normal pH media. C, LDH production in fibroblasts treated with acidic media by 48 hours. Acidic media induced significantly higher LDH production in fibroblasts than normal pH media. L929 (A) means fibroblasts in acidic media, L929 (N) means fibroblasts in normal pH media (** means P < .01, *** means P < .001). LDH, lactate dehydrogenase
3.4. Intracellular ROS analysis using DCFH‐DA flow cytometry and ELISA in kinetic mode
H2DCFDA is a non‐fluorescent dye that gets irradiated upon cleavage by oxidation and intracellular esterase. It was used as a marker of intracellular ROS production in fibroblast cells treated by normal and acidic media and tracked for 45 minutes using a fluorescence ELISA reader. Normalised data derived from the cells 24‐hour posttreatment disclosed that there was no significant difference between intracellular ROS production of cells treated with acidic media compared with the normal media (P = .6433; Figure 3A). However, flow cytometry and fluorescence microscopy results derived from the cells treated by normal and acidic media revealed that cell incubation with acidic media significantly increased intracellular ROS production compared with the normal media by 48 hours (P < .001; Figure 3B).
Figure 3.
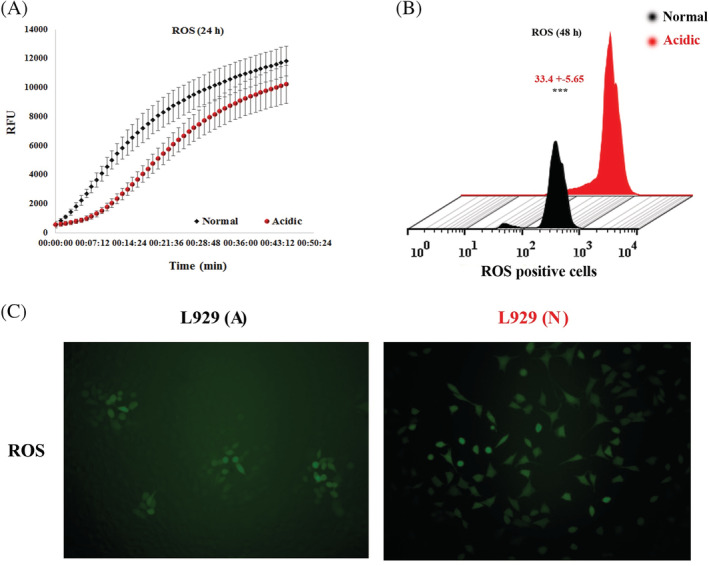
Intracellular ROS production in fibroblasts treated with acidic media. A, Kinetics of intracellular ROS production in fibroblasts treated with acidic media for 24 hours. There was no significant difference between the production of ROS in fibroblasts treated with acidic and normal media. B, Flow cytometry of intracellular ROS production in fibroblasts treated with acidic media for 48 hours. There was significant difference between the production of ROS in fibroblasts treated with acidic and normal media. C, Fluorescent inverted microscopy related to intracellular ROS production in fibroblasts treated with acidic media. There was significant difference between the production of ROS in fibroblasts treated with acidic and normal media. L929 (A) means fibroblasts in acidic media, L929 (N) means fibroblasts in normal pH media (*** means P < .001). ROS, reactive oxygen species
3.5. Cell cycle by PI flow cytometry
Results derived from cell cycle analysis evaluated by flow cytometry showed that acidic media induced S‐phase arrest compared with the normal media in fibroblast cells (Figure 4A‐C). In other words, acidic media induced significantly higher levels of S phase in fibroblast cells than normal media (P < .001).
Figure 4.
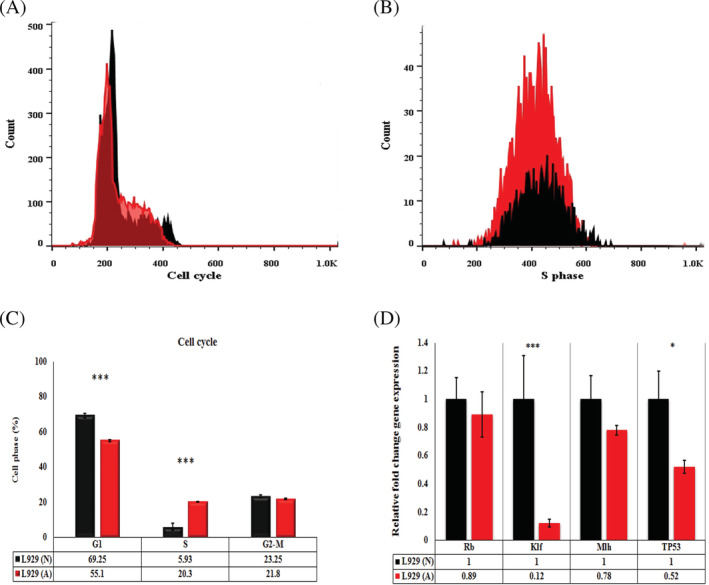
PI cell cycle flow‐cytometry. A, Cell cycle of fibroblasts treated with acidic media for 48 hours. B, S‐phase cell cycle showed arrested S phase in fibroblasts treated with acidic media for 48 hours compared with the normal media. C, The level of different phases of cell cycle in fibroblasts treated with acidic and normal media. The less percent of G1‐phase and higher percent of S‐phase cell cycle has been shown in fibroblasts treated with acidic media compared with normal media. D, The level of tumour suppressive and DNA repair genes in fibroblasts treated with acidic media. The level of Rb and Mlh1 unchanged while the levels of P53 and Klf4 significantly decreased. L929 (A) means fibroblasts in acidic media, L929 (N) means fibroblasts in normal pH media (* means P < .05, *** means P < .001). PI, propidium iodide
3.6. Gene analysis using qRT‐PCR
To investigate the Klf4 and Trp53 genes, qRT‐PCR was performed. qRT‐PCR data revealed that acidic media induced significant KLF4 gene expression than normal media in fibroblast cells (P = .006914682). Furthermore, acidic media induced significant Trp53 gene expression than normal media in fibroblast cells (P = .01343602). There was no significant difference between Rb and Mlh1 gene expressions in fibroblasts treated with acidic and normal media (P > .05; Figure 4D).
3.7. Autophagy measurement using AO staining
Autophagy may be studied using the evaluation of the R/GFIR in fibroblasts stained with AO. R/GFIR data obtained using a fluorescence microplate reader revealed that acidic pH microenvironment induced significantly late autophagosomes compared with normal media by 24 hours (P < .05). Furthermore, acidic media retained enhanced late autophagy in fibroblasts by 48 hours. In other words, acidic media improved the formation of late autophagosomes higher than 25% and 54% in fibroblasts during 24 and 48 hours, accordingly. Moreover, fibroblasts treated by acidic pH media were imaged by fluorescence microscopy after 24 and 48 hours and were compared with the cells treated with normal media. The figures disclosed that acidic media induced more late autophagy vacuoles in the red colour in fibroblast cells under the fluorescent microscope (Figure 5A,B).
Figure 5.
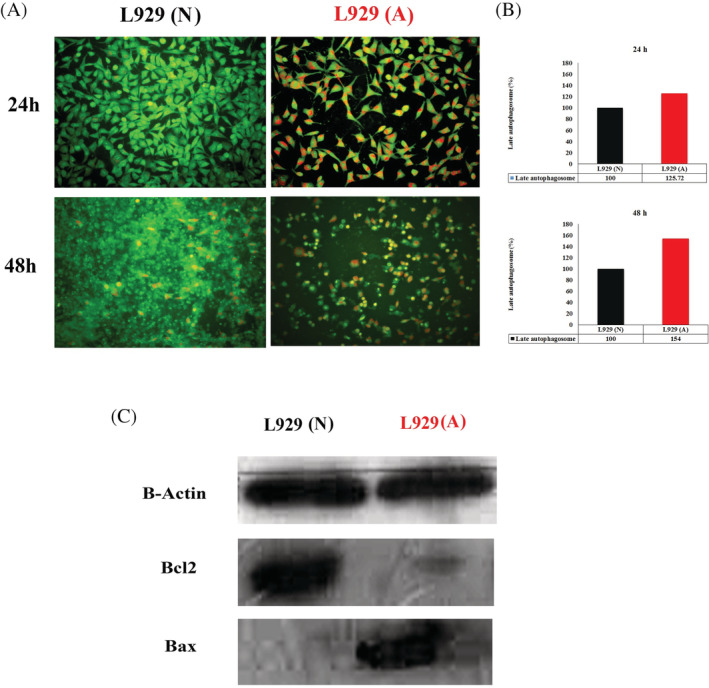
A, Acridine orange staining of fibroblasts treated with acidic media by 24 and 48 hours. B, The quantitative analysis of red‐to‐green fluorescence intensity ratio in fibroblasts treated with acidic media. C, Western blotting of Bax and Bcl2 proteins in fibroblasts treated with acidic media. L929 (A) means fibroblasts in acidic media; L929 (N) means fibroblasts in normal pH media
3.8. Western blotting of apoptotic proteins
The relative fold change Bcl2 protein as a survival protein in fibroblasts showed that acidic media significantly decreased the level of Bcl2 protein in fibroblasts treated with acidic media compared with normal media. Furthermore, evaluation of Bax protein as a marker of apoptosis disclosed that acidic media enhanced the Bax protein level in fibroblasts compared with normal media. Therefore, the level of Bax/Bcl2 ratio as a marker of apoptosis showed that the Bax/Bcl2 ratio was significantly higher in cells treated with acidic media compared with normal media (Figure 5C).
4. DISCUSSION
Fibroblasts are one of the major cells that play the critical role in wound healing through the wound contraction, producing extracellular matrix and collagen, and biodegrading fibrin clot. Noteworthily, the acidic microenvironment of cancer cells may affect normal fibroblasts. Our results demonstrated the acidic microenvironment derived from cancer cells, acidosis, and other metabolic disturbances leads to diminishing of cell viability and cell number, S‐phase arrest, and enhancement of dead cells along with increasing apoptosis and autophagy. Although acidic microenvironment did not damage the cell membranes of fibroblasts at the first place, it gradually damaged the cell membranes by 48 hours. These events were in accordance with intracellular ROS production and downregulation of P53 and Klf4, as well.
As mentioned earlier, fibroblasts treated with acidic media were produced and the LDH level in the cells was increased by 48 hours. These data were in good agreement with earlier studies indicating wound healing and are in concomitant with acidosis in the microenvironment due to the increase of lactic acid, organic acid, pCO2, O2, and so on.14 It seems that during the healing process, collagen synthesis forces cells to undergo greater glycolysis resulting in gradually enhancement in LDH production in cells.15 However, increase in the level of available O2 in the margin of wound in face to acidic microenvironment is due to Bohr's effect.16 As seen in Figure 3A,B, the level of intracellular ROS production gradually increased and was higher than the cells treated with pH 7.4 by 48 hours. Gupta et al demonstrated that acidic induced‐ ROS production is resulting from nicotinamide adenine dinucleotide phosphate oxidase activation and has the potential to oxidase some catalytic domain in some tumour suppressors such as PTEN.17 Furthermore, it has been shown that intracellular ROS production promotes transdifferentiation of fibroblast to myofibroblast through angiotensin II, JNK, and P38 signalling cascades.18
Several signalling pathways are involved in cell survival derived from acidic milieu in normal fibroblasts. The optimum pH for the proper functioning of fibroblasts—cell migration and DNA synthesis—is 7.2‐7.519 while our data showed that acidic media induced S‐phase arrest. This finding was in good agreement with the cell number and viability data derived from MTT assay as well as FDA staining, respectively. In other words, MTT assay and FDA‐PI staining data revealed diminishing of cell viability and cell number along with the increase of dead cells in face to the acidic media. However, Liu et al disclosed that cell proliferation assessed by MTT assay gradually increases from 7.4 to 5 pH.20 Furthermore, Lengheden et al reported that DNA synthesis in fibroblasts gradually decreased from 7.2 to 8.4 pH.19 Notably, Tavakol and colleagues reported that acidic media decrease cell viability of cancer cells.11 It was demonstrated that chronic wound decreases cell proliferation along with the alteration in phenotype and cytosin and decreased MMP and increased TIMP activity.21 Besides, Liu et al reported that cell proliferation significantly deceased in null Klf4 cells.22 These findings were in good agreement with our data, showing the decreased level of cell proliferation and Klf4 gene level in face to acidic milieu.
Besides, it seems that DNA damage by acidic media (data not shown) results in S‐phase arrest. However, Zhu et al demonstrated that the increase of S‐phase cycle in the cells had good correlation with the increase of apoptosis.23 Our data also showed that acidic media increase S‐phase cycle and cell lethality. A critical checkpoint regulator of S‐phase cycle is retinoblastoma (Rb).24 Our data showed that in spite of the S‐phase arrest, there was no significant difference in the level of Rb. However, eventually other markers such as ribosomal proteins and Mdm2‐p53 axis are responsible for25 another important gene involved in DNA mismatch repair is Mlh1.26 Noteworthily, the unchanged level of Rb and Mlh1 and the increase of S phase revealed that Rb has no significant role in DNA repair under acidic conditions and did not try to hinder cells to enter into the S‐phase cycle in spite of DNA damage. Therefore, some S phase checkpoints are involved in inhibiting the entering of cells towards G2 and mitotic phases eventually due to DNA breakage.
It seems that DNA synthesis under the non‐permissive process leads to DNA damage and thereby triggers DNA‐damage response (DDR) and death.27 However, Trp53 plays a critical role in DDR as well. Entering S phase without a mitogenic signal leads to apoptosis (Bax/Bcl2 ratio)27 as seen in our study. Bcl2 and Bax are two genes involved in cell survival and death, respectively. Our data showed that acidic medium not only led to a decrease of cell survival through Bcl2 downregulation but also led to cell death through the enhancement of Bax upregulation.
Interestingly, DNA damage, S‐phase arrest, and decreased cell viability were in concomitant with P53 and Klf4 downregulation. These findings were controversy because of the fact that both of these genes must increase in face to DNA damage to diminish cell cycle progression. Notably, P53 and Klf4 collaterally work together to control G1/S checkpoint progression,28 and in our study both genes collaterally decreased. Zhou et al revealed that the level of apoptosis induced by DNA damage has an inverse correlation with the level of Klf4, and in the case of cytostatic DNA damage, it changes the function of P53 from cell repair towards cell death. They believed that the decrease in the level of Klf4 is due to the increase in the mRNA turnover caused by the dissociation from Hur as a stabilising agent.29 We also showed that the level of Klf4 had an inverse relationship with DNA damage in cells treated with acidic media and also had a direct correlation with the level of P53, and these processes promote cells towards cell death.
It seems that cell mortality derived from acidic media is in part due to apoptosis. To sum up, it might be said that acidic medium induces DNA damage, excessive intracellular ROS production in fibroblasts, and is the reason for the S‐phase cell cycle arrest and apoptosis. An important point derived from our investigations is related to the dual role of acidic media in normal and cancer cells. On the other hand, acidic milieu decreased apoptosis in cancer cells and was increased in normal fibroblasts.
Besides apoptosis, another cell death mechanism that has a dual role in survival and mortality is autophagy. Reports indicated that autophagy is necessary for skin pigmentation and melanin synthesis. In other words, in the autophagy disturbance in skin cells, cells undergo ageing, P53 upregulation, and ichthyosis.30 These findings were in good agreement with our data that showed acidic media enhanced autophagy and P53 downregulation in fibroblasts. An important controversial finding is related to the level of P53 and apoptosis. It was disclosed that acidic media induced autophagy in fibroblasts. In correlation with our data, Tavakol and colleagues showed that acidic media increase autophagy in cancer cells treated with acidic media.11 They believed that intracellular ROS production is responsible for it. The level of autophagy drastically decreases with age that may lead to diminishing of lifespan.31 Notably, it was indicated that the level of Klf4 has a direct correlation with autophagy and inverse with ageing.31 Klf4 modulates autophagy through binding the promoter of SQSTM1.32, 33 Therefore, it seems that increase of intracellular ROS production, DNA damage, and some other markers more stronger than Klf4 are involved in autophagy in fibroblasts treated by acidic media.
Noteworthily, it was disclosed that acidic extracellular pH destabilises focal adhesions34 from one side, helps to promote wound healing and fibroblast migration, and from the other side, if the acidic media turn normal cells into failed reprogramming cells, the altered cells may initiate and progress cancer. Therefore, if the cells do not turn into cancer cells, this process will help them to enhance wound healing.
The acidic media increase MMP9 as a well‐known MMP for cell migration and invasion.35 The signal transduction pathway that leads to acidic extracellular‐induced MMP9 overexpression is through the activation of phospholipase D and phosphorylation of p38 and MAPK1/2. Park et al showed that cell treatment at pHs 6.04‐6.48 and 7.64‐7.99 leads to MMP‐1 and p38 gene expressions and ROS production.36 Our data also showed an enhancement of ROS production in face to acidic media. The MMP activity gradually decreases from the non‐healing state to the healing state while its tissue inhibitor, TIMP‐1, increases.37, 38 Interestingly, the promoter of MMP9 is located into the NFκB binding site and the acidic pH affects NFκB as well.35
5. CONCLUSIONS
To sum up, it might be said that acidic medium induces ROS production and eventually results in NFκB gene overexpression that is a prominent marker in aging.39, 40 Moreover, we previously reported that acidic media can trigger failed reprogramming in cells and turn cells into cancer cells. Unpleasant finding was that acidic media decreased Klf4 and TP53 genes: two important genes involved in tumour suppressor and DNA repair. In other words, cells look like cancer cells and were prone to tumorigenesis with DNA damage, S phase arrest, excessive ROS production, and downregulation of Klf4 and P53. It seems that downregulation of Klf4 eventually derived from acidosis‐induced DNA damage leads to induce the cells towards S‐phase arrest and diminish cell proliferation. However, cell owing to not entering to mitosis undergo cell death including apoptosis and autophagy. These findings can be an important alarm for scientists who encourage acidic bandage for wound healing and also describe one of the mechanism involved in diminishing wound healing in cancer cells. However, it seems that acidic medium has a positive role in skin wound healing, our data showed that acidic media induce abnormal genotype with diminished cell survival in fibroblasts. Caution should be exercised in wound healing therapy with acidic media for fibroblasts located inside the body due to this fact that acidic media decreased the level of tumour suppressor and DNA repair genes and alter the normal survival pathways in fibroblasts.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by a grant from Iran National Science Foundation Science Deputy of Presidency, Tehran, Iran (Grant number: 97007668) and a grant from Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran (Grant number: 97‐03‐117‐32728).
Rabiee Motmaen S, Tavakol S, Joghataei MT, Barati M. Acidic pH derived from cancer cells as a double‐edged knife modulates wound healing through DNA repair genes and autophagy. Int Wound J. 2020;17:137–148. 10.1111/iwj.13248
Funding information Iran National Science Foundation Science deputy of presidency, Grant/Award Number: 97007668; Cellular and Molecular Research Center, Iran University of Medical Sciences, Grant/Award Number: 97‐03‐117‐32728
REFERENCES
- 1. Tetley RJ, Staddon MF, Heller D, Hoppe A, Banerjee S, Mao Y. Tissue fluidity promotes epithelial wound healing. Nat Phys. 2019;1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zehra M, Mushtaq S, Musharraf SG, Ghani R, Ahmed N. Association of cyclin dependent kinase 10 and transcription factor 2 during human corneal epithelial wound healing in vitro model. Sci Rep. 2019;9:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simões A, Chen L, Chen Z, et al. Differential microRNA profile underlies the divergent healing responses in skin and oral mucosal wounds. Sci Rep. 2019;9:7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Zhang Q, Wu Y, Branch‐Brooks CD, Butler CE. Short‐term influences of radiation on musculofascial healing in a laparotomy rat model. Sci Rep. 2019;9:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wadher B, Shende K, Joshi A, Naranje N, Magar S. Acidic environment and wound healing: a review. Wounds. 2015;27:5‐11. [Google Scholar]
- 6. Seal LA, Robson MC. The influence of pH on chronic wound healing and the antimicrobial activity of chlorine. Ostomy Wound Manage. 2018;64:8‐10. [Google Scholar]
- 7. Osti E. Skin ph variations from the acute phase to re‐epithelialization in burn patients treated with new materials (burnshield®, semipermeable adhesive film, dermasilk®, and hyalomatrix®). Non‐invasive preliminary experimental clinical trial. Ann Burns Fire Disasters. 2008;21:73. [PMC free article] [PubMed] [Google Scholar]
- 8. Sharpe JR, Booth S, Jubin K, Jordan NR, Lawrence‐Watt DJ, Dheansa BS. Progression of wound pH during the course of healing in burns. J Burn Care Res. 2013;34:e201‐e208. [DOI] [PubMed] [Google Scholar]
- 9. Zlotogorski A. Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res. 1987;279:398‐401. [DOI] [PubMed] [Google Scholar]
- 10. Tavakol S. Acidic pH derived from cancer cells may induce failed reprogramming of normal differentiated cells adjacent tumor cells and turn them into cancer cells. Med Hypotheses. 2014;83:668‐672. [DOI] [PubMed] [Google Scholar]
- 11. Rabiee S, Tavakol S, Barati M, Joghataei MT. Autophagic, apoptotic, and necrotic cancer cell fates triggered by acidic pH microenvironment. J Cell Physiol. 2019;234:12061‐12069. [DOI] [PubMed] [Google Scholar]
- 12. White KA, Grillo‐Hill BK, Barber DL. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 2017;130:663‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomé MP, Filippi‐Chiela EC, Villodre ES, et al. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J Cell Sci. 2016;129:4622‐4632. [DOI] [PubMed] [Google Scholar]
- 14. Roberts G, Hammad L, Collins C, Shearman C, Mani R. Some effects of sustained compression on ulcerated tissues. Angiology. 2002;53:451‐456. [DOI] [PubMed] [Google Scholar]
- 15. Lampiaho K, Kulonen E. Metabolic phases during the development of granulation tissue. Biochem J. 1967;105:333‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunt TK, Twomey P, Zederfeldt B, Dunphy JE. Respiratory gas tensions and pH in healing wounds. Am J Surg. 1967;114:302‐307. [DOI] [PubMed] [Google Scholar]
- 17. Gupta SC, Singh R, Pochampally R, Watabe K, Mo Y‐Y. Acidosis promotes invasiveness of breast cancer cells through ROS‐AKT‐NF‐κB pathway. Oncotarget. 2014;5:12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)–a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012;24:e65. [DOI] [PubMed] [Google Scholar]
- 19. Lengheden A, Jansson L. pH effects on experimental wound healing of human fibroblasts in vitro. Eur J Oral Sci. 1995;103:148‐155. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Kalén A, Risto O, Wahlström O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen. 2002;10:336‐340. [DOI] [PubMed] [Google Scholar]
- 21. Cook H, Stephens P, Davies KJ, Thomas DW, Harding KG. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP‐1, TIMP‐2, and MMP‐2 activity. J Investig Dermatol. 2000;115:225‐233. [DOI] [PubMed] [Google Scholar]
- 22. Liu C, EP DR, Stecyk C, Wolsey M, Szuchnicki M, Hagos EG. Impaired autophagy in mouse embryonic fibroblasts null for Krüppel‐like factor 4 promotes DNA damage and increases apoptosis upon serum starvation. Mol Cancer. 2015;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Q, Hu J, Meng H, Shen Y, Zhou J, Zhu Z. S‐phase cell cycle arrest, apoptosis, and molecular mechanisms of aplasia ras homolog member I–induced human ovarian cancer SKOV3 cell lines. Int J Gynecol Cancer. 2014;24:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knudsen KE, Booth D, Naderi S, et al. RB‐dependent S‐phase response to DNA damage. Mol Cell Biol. 2000;20:7751‐7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu X, Xiong X, Sun Y. The role of ribosomal proteins in the regulation of cell proliferation, tumorigenesis, and genomic integrity. Sci China Life Sci. 2016;59:656‐672. [DOI] [PubMed] [Google Scholar]
- 26. O'Brien V, Brown R. Signalling cell cycle arrest and cell death through the MMR system. Carcinogenesis. 2005;27:682‐692. [DOI] [PubMed] [Google Scholar]
- 27. Benedict B, van Harn T, Dekker M, et al. Loss of p53 suppresses replication‐stress‐induced DNA breakage in G1/S checkpoint deficient cells. Elife. 2018;7:e37868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon HS, Chen X, Yang VW. Krüppel‐like factor 4 mediates p53‐dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101‐2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Q, Hong Y, Zhan Q, Shen Y, Liu Z. Role for Krüppel‐like factor 4 in determining the outcome of p53 response to DNA damage. Cancer Res. 2009;69:8284‐8292. [DOI] [PubMed] [Google Scholar]
- 30. Sil P, Wong S‐W, Martinez J. More than skin deep: autophagy is vital for skin barrier function. Front Immunol. 2018;9:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hsieh PN, Zhou G, Yuan Y, et al. A conserved KLF‐autophagy pathway modulates nematode lifespan and mammalian age‐associated vascular dysfunction. Nat Commun. 2017;8:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riz I, Hawley TS, Hawley RG. KLF4‐SQSTM1/p62‐associated prosurvival autophagy contributes to carfilzomib resistance in multiple myeloma models. Oncotarget. 2015;6:14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salmon M, Spinosa M, Zehner ZE, Upchurch GR, Ailawadi G. Klf4, Klf2, and Zfp148 activate autophagy‐related genes in smooth muscle cells during aortic aneurysm formation. Physiol Rep. 2019;7:e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Srivastava J, Barreiro G, Groscurth S, et al. Structural model and functional significance of pH‐dependent Talin–Actin binding for focal adhesion remodeling. Proc Natl Acad Sci. 2008;105:14436‐14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato Y, Lambert CA, Colige AC, et al. Acidic extracellular pH induces matrix metalloproteinase‐9 expression in mouse metastatic melanoma cells through the phospholipase D‐mitogen‐activated protein kinase signaling. J Biol Chem. 2005;280:10938‐10944. [DOI] [PubMed] [Google Scholar]
- 36. Park G, Oh D‐S, Kim Y‐u, Park M‐K. Acceleration of collagen breakdown by extracellular basic pH in human dermal fibroblasts. Skin Pharmacol Physiol. 2016;29:204‐209. [DOI] [PubMed] [Google Scholar]
- 37. Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442‐452. [DOI] [PubMed] [Google Scholar]
- 38. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound‐healing: a new perspective for wound‐therapy? Arch Dermatol Res. 2007;298:413‐420. [DOI] [PubMed] [Google Scholar]
- 39. Tavakol S, Zare S, Hoveizi E, Tavakol B, Rezayat SM. The impact of the particle size of curcumin nanocarriers and the ethanol on beta_1‐integrin overexpression in fibroblasts: a regenerative pharmaceutical approach in skin repair and anti‐aging formulations. DARU J Pharm Sci. 2019;27:159‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kriete A, Mayo KL, Yalamanchili N, et al. Cell autonomous expression of inflammatory genes in biologically aged fibroblasts associated with elevated NF‐kappaB activity. Immun Ageing. 2008;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


