Abstract
Neonatal herpes simplex virus (HSV) infection is rare, with an estimated incidence of 3.58 per 100 000 live births in the UK and should be suspected in any newborn with fever and bacterial culture-negative sepsis. We describe a case of a previously well full-term male neonate who presented with persistent fever and elevated ferritin level that was carried out during the era of the COVID-19 pandemic as part of SARS-CoV-2 panel investigations. Despite the initial negative HSV serology, HSV-1 PCR from a scalp lesion returned positive. He made a full recovery after acyclovir therapy. This case highlights the importance of maintaining a high clinical index of suspicion of HSV infection in any febrile neonate even with absence of maternal history and negative serology, particularly if associated with hyperferritinaemia. We also address the challenge of interpreting inflammatory biomarkers’ results for SARS-CoV-2 infection in neonates.
Keywords: neonatal intensive care, paediatrics, neonatal health, infectious diseases, COVID-19
Background
The incidence of neonatal herpes simplex virus (HSV) infection in the UK is rare though, increased from 1.65 to 3.58/100 000 live births between the first national British Paediatric Surveillance Unit (BPSU) study (1986–1991) and the second (2004–2006). There are currently insufficient data about the number of cases of this disease in the UK. A new BPSU surveillance of HSV disease in infants less than 90 days of ages commenced in July 2019 and will run for 2 years.1
Neonatal HSV infection still poses a diagnostic dilemma for clinicians considering advances in diagnostic methods.2 It is challenging to diagnose neonatal herpes infection when typical skin lesions are absent. Moreover, neonatal infection is usually contracted through vertical transmission from asymptomatic mothers with no known history of genital herpes infection or lesions that may aid in earlier identification.3
Neonatal HSV infection can be classified into three main types: localised skin, eye and mouth (SEM); Central Nervous System (CNS) with or without SEM and disseminated infection. Disseminated type is considered when neonates present with negative culture sepsis-like syndrome associated with severe hepatic impairment and or consumptive coagulopathy.4
Hyperferritinaemia can be found in particular inflammatory disorders such as haemophagocytic lymphohistiocytosis (HLH) that could be induced by infection with HSV. Disseminated infection or severe organ dysfunction may also be associated with hyperferritinaemia.5
We report the case of disseminated HSV infection in a clinically well-term neonate who presented with a persistent unexplained fever that could not be attributed to neonatal sepsis because of the absence of other manifestations of sepsis and negative cultures. Extensive screening of SARS-CoV-2 inflammatory biomarkers was carried out during the first wave of COVID-19 pandemic. Our infant had substantially elevated levels of ferritin, lactate dehydrogenase and D-dimers in this context. These findings initially based our thinking on neonatal COVID-19 infection before having a positive HSV-1 DNA PCR result. We explore the learning points in this case.
Case presentation
A male neonate born to a primigravida mother at term by vaginal delivery with ventouse assist following an uncomplicated pregnancy with no risk factors for sepsis. All mother’s antenatal serology was normal. Her membranes ruptured 10 hours before delivery. Both the mother and her partner denied any history of genital herpes or cold sores and, there was no history of prior genital ulcers during pregnancy or at delivery. The baby’s antenatal scan showed moderate left-sided hydronephrosis. No fetal scalp electrodes were inserted, nor fetal blood was sampled during labour. The infant was born in good condition with Apgar scoring 9, 9 at 1 and 5 min, respectively.
The infant was admitted to our level two neonatal unit in a district general hospital following ongoing episodes of fever, which were first noted on day 4 of life. He was treated for suspected neonatal sepsis, including a full-septic screen and administration of empiric intravenous antibiotics, in accordance with local policy.
Physical examination revealed normal respiratory, cardiac and neurological function including no organomegaly.
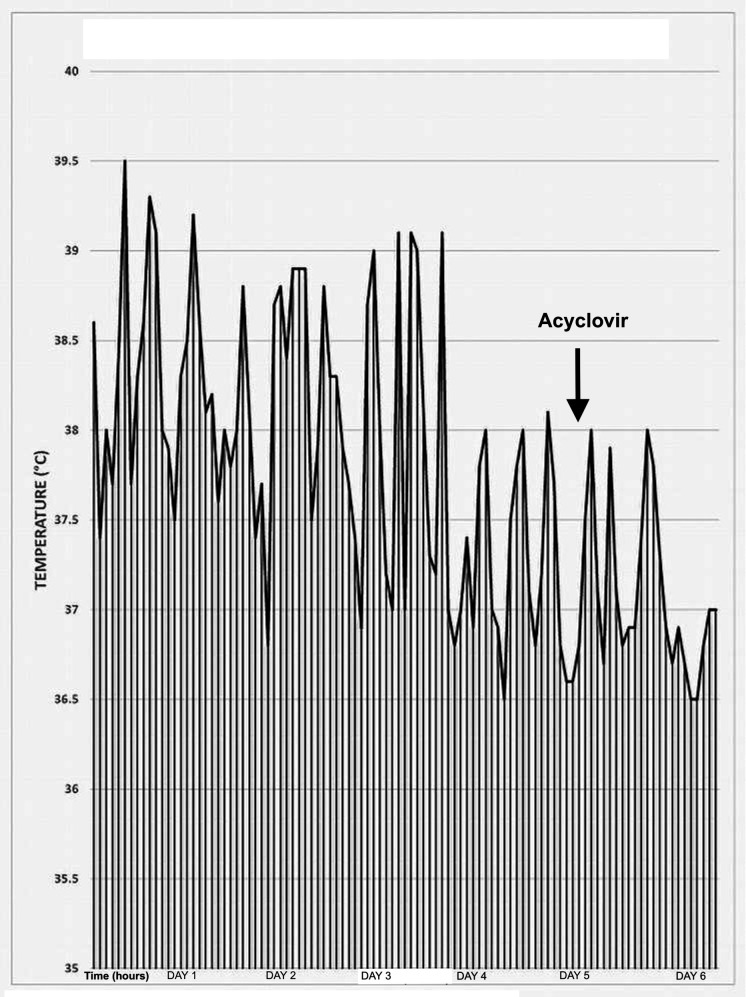
The infant continued to have feverish episodes (>38°C) for 6 days after admission. More than five episodes of high temperature above 38.5°C recorded on the first 3 days of hospital stay with a maximum temperature of 39.5°C. Over the next 3 days, the fever frequency was reduced to 2–3 episodes per day with a temperature around 38°C. The temperature pattern is illustrated in figure 1. Blood, cerebrospinal fluid (CSF) and urine cultures were reported negative for bacterial infection. Initial C-reactive protein was within the normal range at 5 mg/L on admission (day 4 of life) and continued to rise to a maximum peak at 50 mg/L (day 8 of life). Parameters of full blood count (FBC) did not show any abnormality apart from low lymphocyte count at 1.17×109/L, 1.67×109/L on the first and second FBC, respectively (table 1). Retrospectively, this lymphopaenia has raised suspicion of a possible ongoing viral infection, especially with the persistence of fever despite antibiotic therapy.
Figure 1.
Temperature trend over the first 6 days of hospital stay.
Table 1.
Laboratory values during the hospital stay
| Investigation | Range | Day 4 | Day 6 | Day 8 | Day 9 | Day 11 | Day 15 | Day 17 |
| Hb | 125–205 g/L | 161 | 141 | 144 | 139 | 135 | 114 | |
| WCC | 6–18 109/L | 8.7 | 5.6 | 6.5 | 6.3 | 14.1 | 13.3 | |
| Neut | 1–8.5 109/L | 5.91 | 3.52 | 1.78 | 1.64 | 4.91 | 5.52 | |
| Lymph | 3–13.5 109/L | 1.17 | 1.67 | 3.94 | 3.78 | 5.84 | 4.84 | |
| Plt | 150–400 109/L | 246 | 190 | 176 | 193 | 260 | 382 | |
| PT | 11.7–19.7 s | 12.5 | 10.7 | 10.7 | ||||
| APTT | 27.2–49.6 s | 45.9 | 46.6 | 33.9 | ||||
| Fib | 1.8–4.6 g/L | 3.48 | 1.98 | 4.8 | ||||
| D-dimer | <243 ng/mL | 944 | 2238 | 541 | ||||
| ALT | <50 U/L | 90 | 154 | 203 | 150 | 56 | 17 | |
| CRP | 5 mg/L | 5 | 19 | 50 | 11 | 3 | 1 | 3 |
| Ferritin | 15–300 ng/mL | 6653 | 524 | 329 | ||||
| LDH | 240–480 U/L | 1932 | 833 | 551 | ||||
| Trop | <14 ng/L | 33 | 53 | 93 | 63 |
Blank cell indicates laboratory study was not performed.
ALT, alanine aminotransferase; APTT, activated partial thromoplastin time; CRP, C-reactive protein; Fib, fibrinogen; Hb, haemoglobin; LDH, lactate dehydrogenase; lymph, lymphocytes; Neut, neutrophils; Plt, platelets; PT, prothrombin time; Trop, troponin; WCC, white cell count.
Local policy during the first pandemic of COVID-19 advocated additional screening for the possibility of SARS-CoV-2 due to the widespread prevalence of the disease in the UK at this time. COVID-19 swabs and a predefined COVID-19 blood panel investigations (table 1) were requested due to ongoing fever, failure of antibiotic therapy, lymphopaenia and a questionable diagnosis of neonatal sepsis in view of negative cultures.
Alanine aminotransferase (ALT) was 203 U/L, troponin 93 ng/L, D-dimer 2238 ng/mL, ferritin 6653 ng/mL and lactate dehydrogenase 3526 U/L. Laboratory values are outlined in table 1. The nasal swab for SARS-CoV-2 was negative, as was serology.
Triglyceride level was normal and there was no evidence of any cytopenia. HLH was felt to be unlikely and no further investigation was undertaken.
Toxoplasmosis, congenital cytomegalovirus, rubella and herpes simplex screen was requested in view of the raised ALT result, which was negative.
On meticulous physical examination after admission (day 5 of life), two tiny erythematous non-vesicular scaly crusted lesions were noted on the back of the infant’s scalp. Both lesions were approximately 1 mm×2 mm in diameter and around 1 cm apart from each other. It was thought that these lesions were healing injury marks at the ventouse application site during the time of delivery. Although the scaly spots were not consistent with probable HSV skin lesions, they were scraped for viral PCR and samples from one lesion were obtained for HSV DNA PCR testing (nucleic acid amplification). Given the uncertainty of diagnosis and fever pattern, empiric intravenous acyclovir was commenced on the same day, results pending, even in view of negative serology for HSV and negative maternal history. Seven days later, PCR results for HSV type 1 returned positive from the lesion.
The case was discussed with the tertiary infectious diseases team who advised treatment of disseminated HSV infection based on evidence of hepatitis with raised ALT level and positive HSV-1 PCR.
The ophthalmology team reviewed the infant for signs of herpes-related eye complications; the examination was unremarkable.
Investigations
Lumbar puncture was clear on two occasions with no evidence of HSV PCR positivity in the CSF. Results are shown in table 2.
Table 2.
CSF studies during the hospital stay
| Age (days) | Day 5 | Day 8 |
| CSF appearance | Clear | Clear |
| CSF glucose (mmol/L)* | 2.8 | 2.7 |
| CSF protein (0.15–1.30 g/L) | 0.86 g/L | 0.59 g/L |
| White cell count (cells/mm3) | <1 | 2 |
| Red blood cells (cells/mm3) | 30 | 55 |
| Gram stain | No organism | No organism |
| CSF culture | No growth | No growth |
| HSV type 1 DNA | Not detected | Not detected |
| HSV type 2 DNA | Not detected | Not detected |
| Varicella-zoster virus DNA | Not detected | Not detected |
| Enterovirus RNA | Not detected | Not detected |
| Parechovirus RNA | Not detected | Not detected |
*Normal CSF glucose is approximately two-thirds of plasma glucose level (4.5, 4.2 mmol/L respectively).
CSF, cerebrospinal fluid; HSV, herpes simplex virus.
Neuroimaging with MRI was normal. An abdominal ultrasound scan confirmed left-sided hydronephrosis with no acute changes. An ECG and an echocardiogram showed no abnormality.
Treatment
A 3-week course of intravenous acyclovir followed by oral prophylaxis for 1 year.
Outcome and follow-up
Neonatal HSV infection is lifelong with need for ongoing vigilance concerning recurrent infection. The infant responded well and was discharged home on prophylactic acyclovir and trimethoprim prophylaxis for hydronephrosis.
He was discharged on day 27 of life, following a 23-day stay in the neonatal unit.
Monthly FBC and liver function tests were arranged in view of a prolonged acyclovir course that, to date have resulted normal.
Discussion
Neonatal sepsis usually presents with hypothermia or fever, apnoea or tachypnoea, bradycardia or tachycardia, irritability or lethargy, poor feeding, prolonged sleepiness, or becoming fussy. The physical examination may be normal or reveal non-specific signs.6
Fever in neonates is uncommon, resulting in full-septic screening and initiation of empiric antimicrobial treatment, typically for 3–5 days.7 Viraemia secondary to HSV infection may induce fever, however, HSV is not routinely investigated for in febrile neonates without suggestive clinical history. Given the frequency of asymptomatic maternal herpes infection, often primary infection, history is nearly always absent. Hence, HSV should be considered when evaluating any febrile infant up to 6 weeks of age.8
HSV has two main types, 1 and 2. Both can cause neonatal infection, which usually occurs when the baby is exposed to infected maternal secretions at the time of delivery, post-natal or less commonly in-utero. The majority of women who acquire genital herpes during pregnancy have no lesions or symptoms suggestive of HSV infection. Thus it is typical that most women who deliver an HSV-infected baby may have no evidence of prior HSV infection.9 This is consistent with our report as our baby’s mother did not have any skin lesions indicative of herpes infection nor any suggestive previous history.
This situation makes the diagnosis even more challenging as newborns often present with non-specific and subtle clinical findings, making a timely and accurate diagnosis of infection critical, particularly as disseminated neonatal HSV is associated with almost 30% mortality.10 11
In our case, persistent fever despite antibiotics led to a search for other causes. Though not typical, the scalp lesions raised suspicion of HSV infection and viral DNA PCR confirmed the diagnosis later. Kotzbauer et al described the presence of neonatal fever in one-half of their studied cohort of neonates diagnosed with disseminated HSV infection.12
Our patient had negative HSV serology, however, if there is hepatic dysfunction of unclear aetiology, empiric therapy with acyclovir is reasonable to be initiated due to the possible severity of HSV hepatitis. Soliman et al reported a case of disseminated HSV infection in a pregnant teenager, who presented with acute hepatic failure with negative HSV serology before her PCR results confirmed HSV-2 infection. The negative HSV serology was explained by the fact that it takes about 2–3 weeks for antibodies to develop against HSV, resulting in serology being negative at that period.13
Elevated ferritin level has a number of causes, including neonatal HLH. Although the specific mechanism remains unknown, disseminated HSV infection has been identified as a trigger of neonatal HLH.14
Diagnostic criteria for HLH include five of the eight following characteristics: fever ≥38.5°C, splenomegaly, cytopenia in at least two cell lines, hypertriglyceridaemia (fasting >265 mg/dL) or hypofibrinogenaemia (<150 mg/dL), hyperferritinaemia >500 ng/mL, elevated soluble interleukin-2 receptor, decreased or absent natural killer cell activity and evidence of haemophagocytosis in bone marrow, CSF or lymph nodes.15 Our patient met only two of these criteria (fever and hyperferritinaemia); we did not test for soluble interleukin-2 receptor, natural killer cell activity or haemophagocytosis on either bone marrow or liver biopsy. There were no other features suggestive of HLH and the ferritin levels dropped spontaneously during treatment for HSV.
Following a search of the PubMed database, seven case reports on neonates with HSV-associated hyperferritinaemia were found in the English literature (table 3). In these cases, ferritin values ranged from >15 000 to 241 000 ng/mL. Four cases presented with fever as the initial symptom. Two cases did not fulfil the criteria for diagnosing HLH.
Table 3.
Neonatal HSV-associated hyperferritinaemia reported in the literature
| Source | HSV serotype | Initial symptom | DOL | Ferritin level (ng/mL) |
HLH criteria fulfilled | Outcome |
| Yamada et al16 | HSV-1 | Fever | 4 | >15 000 | Yes | Survived |
| Vladescu et al17 | ||||||
| Case1 | HSV-2 | RD | 6 | 68 090 | No | Died |
| Case2 | HSV-1 | Fever | 10 | >40 000 | Yes | Survived |
| Case3 | HSV-1 | Lethargy | 9 | >40 000 | No | Died |
| Halstead et al18 | HSV-2 | Apnoea | 5 | 241 000 | Yes | Died |
| Takehara et al19 | HSV-1 | Fever | 1 | 113 200 | Yes | Died |
| Averitt et al20 | HSV-2 | Fever | 6 | >100 000 | Yes | Died |
DOL, day of life; HLH, haemphagocytic lymphohistiocytois; HSV, herpes simplex virus; RD, respiratory distress.
To conclude, neonatal HSV infection can have various presentations from local to disseminated, which can overlap with HLH clinically and biochemically. The incidental finding of hyperferritinaemia, which would not usually be tested for, was revealed as a consequence of a standardised COVID-19 blood panel, which along with the raised ALT were simply the consequence of hepatocyte damage due to disseminated infection.
Unexplained fever should prompt a search for other diagnoses and not only sepsis in neonates and localised herpes infection should urge a search for features of disseminated disease.
Careful interpretation of the COVID-19 inflammatory biomarker investigations is needed within the clinical context to avoid missing other serious diagnoses.
Patient’s perspective.
Patient’s mother’s perspective
Being a mother is always something that I have dreamed of. I have always wanted children and loved children, my job as a primary school teacher reflects this. When my boyfriend and I found out I was pregnant it was the most wonderful feeling in the world. I had a brilliant, low-risk pregnancy and then a week overdue our beautiful baby was born into the world, the best day of our lives.
Having a baby during the COVID-19 pandemic was incredibly worrying at times. Luckily my boyfriend could be there for the birth but had to go home shortly after. Once I gave birth I fell ill and had to stay in hospital for 5 days. Due to the COVID-19 visiting rules my boyfriend was not allowed to visit us, this was incredibly hard. During this time my boyfriend and I experienced every parent’s worst nightmare of our beautiful baby falling ill and being cared for in NICU. We have never experienced something so hard and heart-breaking. Initially the doctors did not know the reason behind my baby’s high temperatures and heart rate. When we found out it was HSV we were in total shock. We have both never had a single symptom of any kind of Herpes.
My baby’s time in NICU was an extremely worrying and stressful 23 days for us. When we visited our baby in NICU we were not allowed to go in together, due to the COVID-19 rules. This was incredibly hard because we could not comfort each other while in NICU and we could not see and enjoy our baby together. As a new mother my fairy tale dream of having my baby and being home enjoying parenthood and being a family unit had been taken away from me. I am an extremely strong-minded person and I feel I dealt with this awful situation as best as any new mother could. However, it was very emotionally draining. Leaving NICU every time without my baby was so difficult, I would cry under my mask every time I left. I found it very unnatural and a massive stress not to be with my baby. I lost count the amount of times I cried and prayed for my baby’s health to be ok.
The day we took our baby home was just surreal and the moment we had been dreaming of. Now home, we have loved every single second of being with our baby. My baby is the most wonderful little human and the best thing that has ever happened to me. I thank the nurses and doctors that made my baby boy healthy and well.
Learning points.
Neonatal sepsis is not a straightforward diagnosis—unusual features should lead to a search for unusual causes and fever does not always equate to sepsis.
Thorough physical examination, including the skin and scalp, is vital to diagnosis as neonatal herpes can have an initial subtle presentation and negative serology does not rule out herpes infection.
Hyperferritinaemia in feverish neonates can point to herpes simplex virus (HSV) infection even in the absence of suggestive maternal history.
Empiric therapy with acyclovir in neonates with unexplained fever and elevated transaminases, while awaiting confirmatory HSV investigations, can mitigate the severe consequences of HSV hepatitis if left untreated.
COVID-19 inflammatory biomarkers should be interpreted with caution and on a case-by-case basis in paediatric and neonatal population.
Acknowledgments
Dr David Inwald, consultant in paediatric intensive care at Cambridge University Hospital Foundation Trust for his contribution and valuable suggestions for the final manuscript.
Footnotes
Contributors: MKB contributed to the patient’s care, contributed to the literature search and designed the manuscript and the revisions. SH contributed to the patient’s care and designed the manuscript and the revisions. CB contributed to the patient’s care and designed the manuscript and revisions. HH contributed to the patient’s care and designed the manuscript, revisions and the graph.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Patient/guardian consent was obtained for publication.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.British Paediatric Surveillance Unit . BPSU annual report, 2019-2020. London: British Paediatric Surveillance Unit/Royal College of Paediatrics and Child Health, 2020. [Google Scholar]
- 2.James SH, Kimberlin DW. Neonatal herpes simplex virus infection: epidemiology and treatment. Clin Perinatol 2015;42:47–59. 10.1016/j.clp.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med 2009;361:1376–85. 10.1056/NEJMra0807633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catlin EA, Warren HS, Shailam R, et al. Case records of the Massachusetts General Hospital. Case 19-2012. A premature newborn boy with respiratory distress. N Engl J Med 2012;366:2409–19. 10.1056/NEJMcpc1109276 [DOI] [PubMed] [Google Scholar]
- 5.Liew JW, Jones BL, Hunter AJ. Disseminated herpes simplex masquerading as hemophagocytic lymphohistiocytosis: a case report. Perm J 2019;23:18–202. 10.7812/TPP/18-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laptook AR, Bell EF, Shankaran S, et al. Admission temperature and associated mortality and morbidity among moderately and extremely preterm infants. J Pediatr 2018;192:53–9. 10.1016/j.jpeds.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med 1993;329:1437–41. 10.1056/NEJM199311113292001 [DOI] [PubMed] [Google Scholar]
- 8.Baraff LJ, Oslund SA, Schriger DL, et al. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J 1992;11:257–65. 10.1097/00006454-199204000-00001 [DOI] [PubMed] [Google Scholar]
- 9.Pinninti SG, Kimberlin DW. Management of neonatal herpes simplex virus infection and exposure. Arch Dis Child Fetal Neonatal Ed 2014;99:F240–4. 10.1136/archdischild-2013-303762 [DOI] [PubMed] [Google Scholar]
- 10.Drumm CM, Caufield MC, DeKlotz CM, et al. Intrauterine herpes simplex virus infection presenting as a Zosteriform eruption in a newborn. AJP Rep 2018;8:e33–6. 10.1055/s-0038-1635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 2001;108:230–8. 10.1542/peds.108.2.230 [DOI] [PubMed] [Google Scholar]
- 12.Kotzbauer D, Frank G, Dong W, et al. Clinical and laboratory characteristics of disseminated herpes simplex virus infection in neonates. Hosp Pediatr 2014;4:167–71. 10.1542/hpeds.2013-0086 [DOI] [PubMed] [Google Scholar]
- 13.Soliman M, Akanbi O, Almagdub I, et al. A rare case of herpes simplex virus-2 hepatitis with negative serology. Case Reports Hepatol 2019;2019:1–4. 10.1155/2019/4808143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imashuku S, Ueda I, Teramura T, et al. Occurrence of haemophagocytic lymphohistiocytosis at less than 1 year of age: analysis of 96 patients. Eur J Pediatr 2005;164:315–9. 10.1007/s00431-005-1636-9 [DOI] [PubMed] [Google Scholar]
- 15.Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Yamamoto Y, Uchiyama A, et al. Successful treatment of neonatal herpes simplex-type 1 infection complicated by hemophagocytic lymphohistiocytosis and acute liver failure. Tohoku J Exp Med 2008;214:1–5. 10.1620/tjem.214.1 [DOI] [PubMed] [Google Scholar]
- 17.Vladescu IA, Browning WL, Thomsen IP. Massive ferritin elevation in neonatal herpes simplex virus infection: hemophagocytic lymphohistiocytosis or herpes simplex virus alone? J Pediatric Infect Dis Soc 2015;4:e48–52. 10.1093/jpids/piv005 [DOI] [PubMed] [Google Scholar]
- 18.Halstead ES, Rajasekaran S, Fitzgerald JC, et al. Hyperferritinemic sepsis: an opportunity for earlier diagnosis and intervention? Front Pediatr 2016;4:77. 10.3389/fped.2016.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takehara H, Hirohata K, Mutoh H, et al. Critically severe case of neonatal herpes with high viral load and hemophagocytic syndrome. Tohoku J Exp Med 2019;247:149–52. 10.1620/tjem.247.149 [DOI] [PubMed] [Google Scholar]
- 20.Takehara H, Hirohata K, Mutoh H, Averiit G, Al-Rahawan M, Levent F, et al. Critically severe case of neonatal herpes with high viral load and hemophagocytic syndrome. Tohoku J Exp Med 2019;247:149-152. 10.1620/tjem.247.149 [DOI] [PubMed] [Google Scholar]