Abstract
Free flaps in combination with arterial reconstruction by means of arteriovenous loops or bypass have, meanwhile, been established as a therapeutic option in defect reconstruction for areas without recipient vessels. Our aim was to analyse the long‐term performance, flap autonomy, and the flap perfusion. Patients receiving this combined reconstruction at a single‐centre institution were included. During follow‐up examination, the patency of arterial reconstruction was investigated by duplex ultrasound. Flap micro‐circulation was assessed by laser Doppler flowmetry and white light tissue spectrometry (O2C) as well as by indocyanine green fluorescence angiography. Twenty‐three patients could be clinically followed up. Duplex ultrasound showed, in four cases, arterial pedicle occlusion in spite of vital flap. Comparison of the O2C perfusion parameters between flaps with occluded pedicles and those with intact inflow showed no significant difference (parameters sO2: P = .82; Flow: P = .31). Similar results were obtained by fluorescence angiography; no significant difference could be detected between both groups (parameters Ingress P = .13; Ingressrate P = .54). Combined vascular reconstruction with free tissue transfer is associated with a good long‐term outcome and wound closure. Even after flap transplantation to areas with critical tissue perfusion, the flap can develop autonomy and thus survive after pedicle occlusion.
Keywords: arteriovenous loop, flap autonomy, flap perfusion, indocyanine green, laser Doppler
1. INTRODUCTION
Transplantation of free flaps on vascular reconstructions (arteriovenous [AV] loops or bypass grafts) has, meanwhile, been established as a feasible therapeutic option for defect reconstruction in body areas without suitable recipient vessels.1, 2, 3 This concept has also been successfully applied for limb salvage in patients with critical limb ischaemia (CLI) and extensive tissue loss as ultima ratio prior to major amputation.4
However, there is few data on the long‐term results and especially the macro‐ and micro‐perfusion of these flaps after transplantation remains unclear. Present research so far postulated the concept of the “nutrient‐flap,” where perfusion of adjacent ischaemic zones is improved by transplantation of well‐vascularized flap tissue (eg, in patients with CLI).5, 6, 7 In addition, also the creation of a new capillary network in neighbouring tissue is suggested.5 However, clinical studies to support this approach have not been performed to this date and would be difficult to realise.
Against this background, our aim was to analyse the perfusion of flaps and their surrounding tissue areas after combined reconstruction by means of arterial revascularization and subsequent free‐flap transfer. Flap micro‐perfusion was evaluated by means of laser‐Doppler flowmetry and perfusion measurements using indocyanine green (ICG)‐based fluorescence angiography. Both methods have been shown feasible for detection of micro‐perfusion changes after revascularization in patients with arterial occlusive disease.8, 9, 10 In this context, the macro‐perfusion of flaps was analysed with duplex ultrasound examination of the arterial reconstruction (AV‐loop or bypass graft including anastomoses and flap pedicles). These data may contribute to deeper comprehension of micro‐circulatory changes as angiogenesis after free‐flap transfer in a clinical setting. This could influence decision‐making as to the appropriate choice of flap entity (muscle vs perforator‐based flaps) and pattern of arterial reconstruction (eg, graft material and loop reconstruction) in future studies.
2. MATERIAL AND METHODS
2.1. Patients
Patients receiving a combination of arterial reconstruction and free‐flap transplantation at a single‐centre institution between the years 2004 and 2015 were included and contacted for follow‐up investigations. The data collection was performed prospectively between February 2015 and August 2016. Study termination date was the 31 August 2016. The median follow‐up time as defined by Allmen et al was 53 months (range 0‐151).11 The study was conducted in accordance with the Declaration of Helsinki and was further approved by the local ethics committee; written informed consent was obtained from all patients. The study results were published in accordance with the guidelines for Strengthening the Reporting of Observational Studies in Epidemiology.12
2.2. Surgical procedure
Surgical procedure has remained unchanged throughout the entire study period. Mandatory is the performance of preoperative digital subtraction angiography (DSA) and phlebopgraphy to exclude upstream arteriel stenosis and compromised venous outflow, for example after deep vein thrombosis. Generally, it was our policy to place the free‐flap anastomosis at a donor artery with the lowest possible load of micro‐vascular arteriosclerotic disease. This was achieved by means of bypass grafts on the lower leg, when upstream macro‐vascular occlusions were present; in the event of heavily calcified (eg, below‐the‐knee arteriosclerotic disease in diabetics) or absent recipient vessels (eg, sternal osteomyelitis in consequence of coronary bypass) in preoperative angiography, AV‐loop reconstruction was performed to allow for free‐flap anastomosis at non‐diseased vessel sections. For both revascularization procedures (AV‐loop and bypass), the reversed greater saphenous vein was used as graft material in all patients. Bypass grafts were tunnelated anatomically below the Sartorius muscle, whereas AV‐loops were placed with their apex in the subcutaneous tissue in order to facilitate access for second‐stage free‐flap connection. This was done by means of a separate auxiliary incision. In this situation, kinking of the AV‐loop limbs must be avoided. Both reconstruction methods, bypass and AV‐loop procedures, were performed by vascular surgeons and intra‐operatively controlled by angiography to exclude anastomotic stenosis or kinking. In case of vascular reconstruction by means of bypass grafts, the flap artery was micro‐surgically anastomosed in an end‐to‐side fashion to the bypass vein, whereas the flap vein was anastomosed end‐to‐end to local deep veins (compare Figure 1). In patients receiving AV‐loop reconstruction, the free‐flap transplantation was performed as a two‐staged procedure, with the free‐flap transplantation scheduled 7‐10 days post‐AV‐loop creation in all the cases. At time of flap transfer, the AV‐loop was dissected at its apex and the flap artery was micro‐surgically connected end‐to‐end to the arterial limb of the former loop. Likewise, flap's vein was anastomosed in an end‐to‐end configuration to the venous limb of the AV‐loop using a coupler system (Flow Coupler Device, Synovis MCA, Birmingham, Great Britain). As, in our experience, full anticoagulation increases only the rates of major post‐operative bleedings but does not contribute to elevated flap survival, it was not applied as routine measure. In general, patients were merely given a prophylactic dosage of heparin (5000 IU twice a day) after AV‐loop creation, whereas therapeutic dosage was applied only in the cases of acute thrombosis of vascular reconstruction following the salvage procedure via thrombectomy. In these cases, it was maintained until the point of free‐flap transplantation (partial thromboplastin time >60 seconds). Prior to free‐flap transfer, patency of vascular graft was controlled via conventional angiography or computertomography‐angio.
Figure 1.
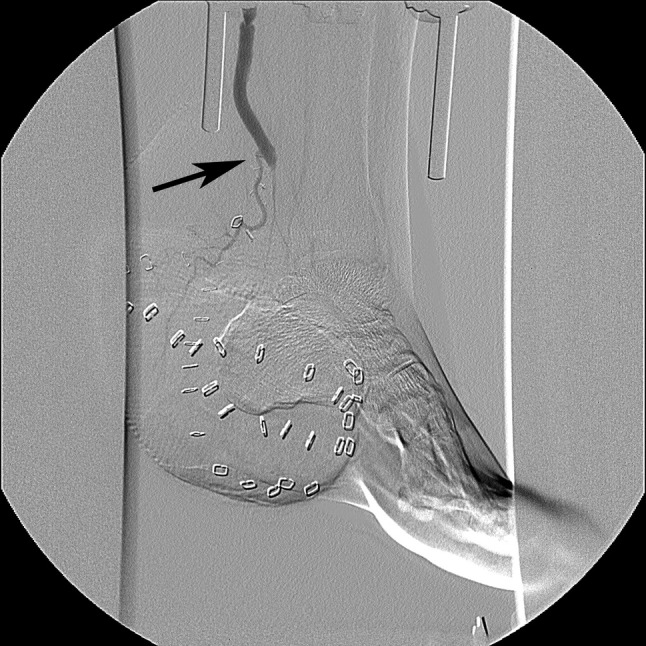
Angiography 7 days after an infra‐malleolar bypass reconstruction on the posterior tibial artery with end‐to‐side connection of the flap pedicle (radial forearm flap) to bypass graft in a patient with a chronic wound on the medial foot due to critical limb ischaemia
2.3. Study design
Macro‐ and micro‐circulations of flaps were evaluated at follow‐up investigation. Macro‐circulation was examined by duplex ultrasound. The patency of the bypass graft, respectively, the AV‐loop pedicles, and the transplanted flap artery itself were analysed. Micro‐circulation was investigated with two different methods: a combined method of laser‐doppler flowmetry and white light tissue spectrometry (O2C, LEA Medizintechnik, Gießen, Germany) and fluorescence angiography with indocyanine green (SPY Elite, NOVADAQ, Ontario, Canada). Measurements were conducted in a darkened room to avoid measurement disturbance by daylight, with room temperature being stable between 21 and 25° Celsius. Prior to measurements, the patient was placed at rest for 15 minutes to ensure physiologic cardiopulmonary rest parameters (medium blood pressure 70‐90 mmHg, heart frequency 60‐80/min). First, micro‐circulation was assessed by laser‐Doppler flowmetry and white light tissue spectrometry, followed by the ICG fluorescence angiography, to avoid confounding of the spectrometry analysis by circulating ICG.
The flap perfusion was assessed by defining regions of interest on the flap. Depending on the location of the incoming pedicle, five zones were defined. Central perfusion was assessed in the “geographic” centre of the transplanted free flap. Perfusion of flap's borders and distal areas (medial, lateral, distal, and proximal of the central zone) was analysed by the remaining four zones of interest, assembled crosswise around the central region of interest (compare Figure 2). In these zones, the micro‐perfusion parameters were calculated for both methods (O2C: sO2, Flow; ICG fluorescence angiography: Ingress, Ingressrate) (compare technical aspects).
Figure 2.
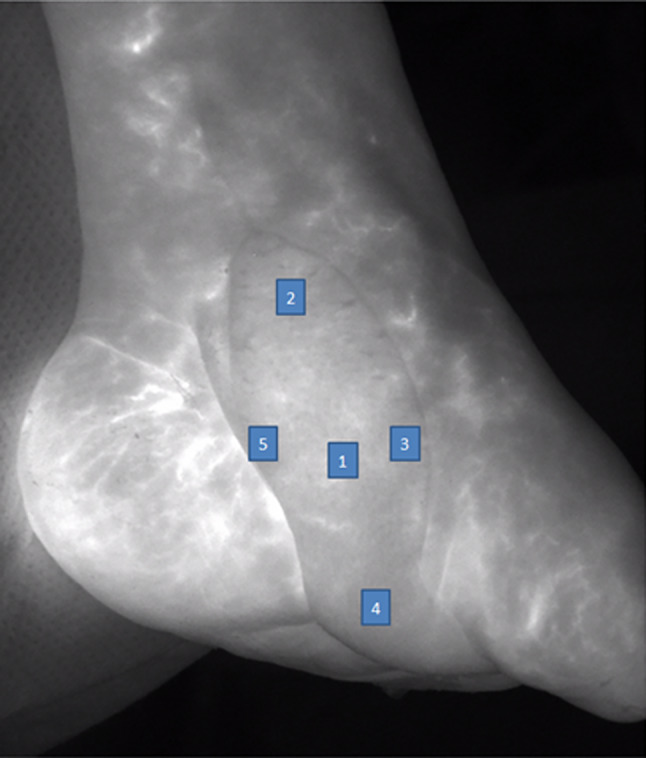
One year postoperative control by indocyanine green fluorescence angiography of a radial forearm flap on the medial foot in case of a critical limb ischaemia patient (digital subtraction angiography compare Figure 1). The arterial pedicle showed an occlusion at the follow‐up investigation. Regions of interest: 1: central flap region; 2: proximal; 3: lateral; 4: distal; 5: medial
For statistical comparison, the patients were divided into two subgroups, depending on the patency of the vascular pedicle. Therefore, the thrombosed arterial pedicles were defined as the OCCLUDED group and the patent pedicles as the OPEN group. Afterwards, a comparison of the micro‐perfusion concerning the O2C and the ICG fluorescence angiography between both groups was conducted.
2.4. Technical aspects
2.4.1. O2C (oxygen‐to‐see)
The O2C device is a combined method of laser‐Doppler flowmetry and tissue spectrometry. Therefore, the parameters sO2 (in %), relative haemoglobin amount (rHb, in arbitrary units [AU]), and relative blood flow (AU) can be measured. It uses white light of 500‐630 nm wave length as well as laser light of 830 nm wave length. Emitted white light registers the haemoglobin oxygen saturation and relative haemoglobin amount (sO2 and rHb). The emitted laser light detects the parameter flow which is caused by a Doppler shift by the erythrocytes on the level of micro‐circulation. The detailed measuring principle has already been reported before.8, 9 As we focused on investigation of the arterial inflow, we analysed the parameters sO2 and flow.
The O2C is available for different types of probes, assessing different tissue depths. In this study, we made use of the probe LFX 32.05 measuring mainly at a tissue depth of 6 mm.
2.4.2. Fluorescence angiography with indocyanine green
The fluorescence angiography is an imaging tool for capturing and viewing fluorescence images of tissue perfusion up to a depth of 5‐7 mm. In this study, we used the SPY Elite fluorescence angiography system. Usage of fluorescence angiography requires intravenous injection of a fluorescence dye (indocyanine green, ICG ICG‐Pulsion, PULSION Medical Systems SE, Feldkrichen, Germany), which is then detected by the integrated near‐infrared camera. The perfusion sequence is then displayed in a video sequence, which can be analysed and quantified afterwards by an integrated analysis software (SPY‐Q). ICG is a water‐soluble tricarbocyanine dye that has been widely used for various medical indications13; it can be safely used in renal insufficient patients as it is metabolised by the hepato‐biliary system.10, 14, 15 In this study, we intravenously applied a standardised dose of 0.1 mg ICG per kg body weight. For the arterial inflow, the two perfusion parameters, namely, Ingress and Ingressrate were calculated (both shown in AU). The Ingress describes the difference of the starting fluorescence intensity to the maximum of fluorescence intensity detected in each video sequence. Ingressrate is calculated out of the increase of fluorescence intensity per second.
2.5. Statistical analysis
SPSS 21 (SPSS Inc., Chicago, Illinois) was used for the statistical analysis. Parameters with a symmetric distribution are shown as mean and SD. Due to slightly skewed distributions of micro‐circulation parameters, descriptive information about central tendency and dispersion of these parameters is provided by the median and the range (minimum‐maximum). The comparisons between independent groups were made by the Mann‐Whitney U test. The statistical significance level was set to P < .05 in all analyses.
3. RESULTS
3.1. Patients and procedure characteristics
A total of 100 patients receiving flap transplantation in combination with an arterial reconstruction due to inadequate recipient vessels were reviewed for the present study. In 79 of the cases, the combined reconstruction method was initially successful, and the flap survived. At follow‐up investigation, which was performed at least 1 year after the reconstruction, 56 patients reaching the combined endpoint with vital flap transplantation and survival remained available for follow‐up investigation. Out of these, 23 patients could be recruited for clinical follow‐up (compare flow chart in Figure 3). In these patients, the arterial reconstruction for subsequent flap transfer has been established in 14 cases by construction of an arterio‐venous loop and in 9 cases by arterial bypass graft on the lower limb. In most of the cases, a latissimus dorsi flap (11) was used for transplantation, followed by a rectus abdominis flap in 5 cases; in 3 cases, a radial forearm flap was used; in 2 cases, a gracilis flap as well and in further 2 cases a vastus lateralis flap. Further description of the included patients is shown in Table 1.
Figure 3.
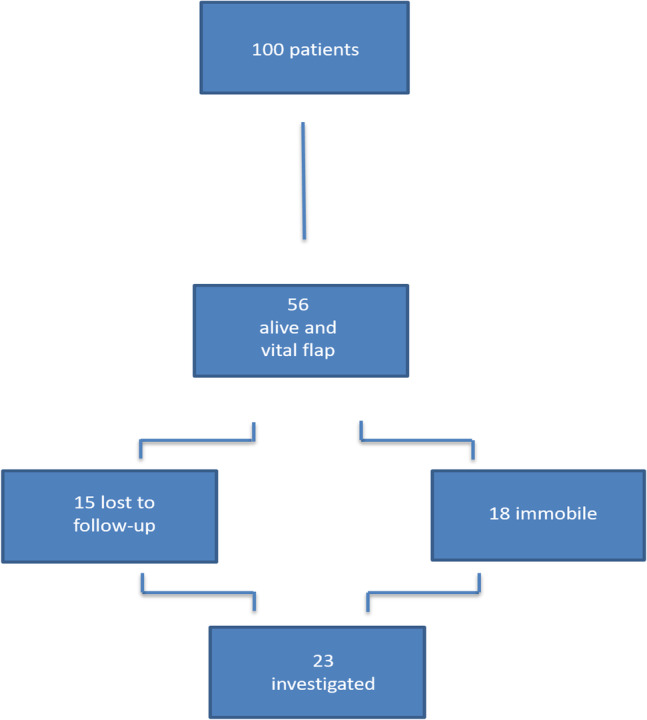
Study flow chart of the patient inclusion
Table 1.
Patients
| Patient | Sex | Age | Flap | Vascular reconstruction | Defect type | Diabetes | Smoker | Arterial pedicle open at follow‐up |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 60 | Latissimus dorsi | Bypass | CLI | No | Yes | Yes |
| 2 | Male | 78 | Latissimus dorsi | Bypass | CLI | Yes | Yes | Yes |
| 3 | Male | 72 | Radial forearm | Bypass | CLI | Yes | No | No |
| 4 | Female | 63 | Latissimus dorsi | AV‐loop | CLI | No | No | Yes |
| 5 | Male | 64 | Rectus abdominis | AV‐loop | CLI | Yes | No | Yes |
| 6 | Female | 46 | Latissimus dorsi | AV‐loop | Rectal carcinoma | No | No | No |
| 7 | Female | 76 | Rectus abdominis | AV‐loop | Sternal ulcus after irradiation | No | No | Yes |
| 8 | Female | 67 | Rectus abdominis | Bypass | CLI | No | No | Yes |
| 9 | Male | 82 | Rectus abdominis | AV‐loop | Sternal osteomyelitis | No | No | Yes |
| 10 | Male | 60 | Latissimus dorsi | Bypass | CLI | No | Yes | Yes |
| 11 | Male | 64 | Radial forearm | Bypass | CLI | Yes | No | Yes |
| 12 | Male | 75 | Gracilis | Bypass | CLI | No | No | Yes |
| 13 | Male | 71 | Rectus abdominis | AV‐loop | CLI | No | No | Yes |
| 14 | Male | 29 | Gracilis | AV‐loop | CLI | No | No | Yes |
| 15 | Female | 58 | Vastus lateralis | AV‐loop | Sternal osteomyelitis | Yes | No | Yes |
| 16 | Female | 76 | Vastus lateralis | AV‐loop | Sternal ulcus after irradiation | Yes | No | Yes |
| 17 | Female | 67 | Latissimus dorsi | AV‐loop | Sternal ulcus after irradiation | No | No | No |
| 18 | Male | 63 | Latissimus dorsi | AV‐loop | Rectal carcinoma (including irradiation) | No | No | Yes |
| 19 | Male | 66 | Latissimus dorsi | AV‐loop | CLI | No | No | Yes |
| 20 | Male | 74 | Latissimus dorsi | Bypass | CLI | No | No | Yes |
| 21 | Male | 63 | Latissimus dorsi | AV‐loop | Sternal ulcus after irradiation | No | Yes | Yes |
| 22 | Male | 69 | Latissimus dorsi | AV‐loop | Rectal carcinoma (including irradiation) | No | No | Yes |
| 23 | Female | 74 | Radial forearm | Bypass | CLI | Yes | Yes | No |
Abbreviation: CLI, critical limb ischaemia.
3.2. Perfusion analysis
To detect the flap's pedicle patency, duplex ultrasound was used. In four cases, the ultrasound showed an occluded arterial flap pedicle; of these, two cases had undergone vascular reconstruction by means of AV‐loop and two cases by means of bypass grafting prior to free‐flap transfer. There was no significant difference with regard to pedicle occlusion, as in all other included cases, the arterial inflow was still patent. In cases of observed vascular pedicles thrombosis, both the arterial limb of the AV loop (respectively, the entire bypass graft) and the flap pedicle were found to be occluded.
In each pre‐defined zone of interest, an O2C perfusion measurement was conducted. Accordingly, the ICG fluorescence angiography measurements were performed. In addition, ICG angiography allows for calculation of an “overall” flap perfusion, which was also done for each flap. This is not feasible for the O2C method, as perfusion can only be detected by probes, placed on the defined regions of interest. For detecting differences between those flaps with an occluded pedicle (OCCLUDED) and those with a still patent arterial inflow (OPEN), the flaps were divided in two groups. First, the median values of the central zones, measured by O2C, were compared between both the groups. Here, no significant difference could be shown for the parameters sO2 and Flow (OCCLUDED: sO2: 52%, range 41%‐63%; Flow: 111 AU, range 26‐117 AU; OPEN: sO2: 47%, range 3%‐70%; Flow: 157 AU, range 38‐337 AU; sO2: P = .82; Flow: P = .31). Further results of the O2C measurements are shown in Table 2.
Table 2.
Results of the O2C perfusion measurement in the different flap zones compared between the OCCLUDED and OPEN group
| OPEN pedicle | OCCLUDED pedicle | |||
|---|---|---|---|---|
| Flap zone | Median (range) | Median (range) | P‐value | |
| sO2 in % | Central | 47.0 (3.0‐70.0) | 52.0 (41.0‐63.0) | .82 |
| Medial | 45.0 (9.0‐92.0) | 62.5 (45.0‐76.0) | .46 | |
| Distal | 46.0 (11.0‐74.0) | 40.0 (33.0‐49.0) | .77 | |
| Lateral | 54.0 (20.0‐79.0) | 54.5 (50.0‐57.0) | 1.00 | |
| Proximal | 46.0 (10.0‐69.0) | 42.0 (29.0‐58.0) | .94 | |
| Flow in A.U. | Central | 157.0 (38.0‐337.0) | 111.0 (26.0‐117.0) | .31 |
| Medial | 187.0 (41.0‐337.0) | 120.0 (41.0‐133.0) | .24 | |
| Distal | 174.0 (51.0‐362.0) | 114.0 (76.0‐171.0) | .34 | |
| Lateral | 154.0 (39.0‐488.0) | 66.0 (38.0‐208.0) | .21 | |
| Proximal | 164.0 (32.0‐359.0) | 68.0 (49.0‐150.0) | .14 |
Second, the results of the ICG fluorescence angiography were compared between the two groups. Even here, no significant difference between the inflow perfusion values of the flap overall perfusion could be detected between both groups (Ingress: OCCLUDED: 104 AU range 63‐157 AU; OPEN: 62.5 AU range 36‐205 AU; P = .13; Ingressrate: OCCLUDED: 3.1 AU range 2.5‐3.7 AU; OPEN: 2.1 AU, range 0.9‐20.7 AU; P = .54). The further values of the investigated different flap zones are displayed in Table 3.
Table 3.
Results of the indocyanine green fluorescence angiography perfusion measurement in the different flap zones compared between the OCCLUDED and OPEN group
| OPEN pedicle | OCCLUDED pedicle | |||
|---|---|---|---|---|
| Flap zone | Median (range) | Median (range) | P‐value | |
| Ingress | Central | 73.0 (48.0‐197.0) | 116.0 (63.0‐147.0) | .07 |
| Medial | 56.5 (15.0‐242.0) | 124.5 (81.0‐150.0) | .047 | |
| Distal | 60.5 (11.0‐207.0) | 76.0 (47.0‐104.0) | .85 | |
| Lateral | 58.0 (32.0‐218.0) | 112.0 (73.0‐179.0) | .014 | |
| Proximal | 50.5 (25.0‐206.0) | 99.0 (70.0‐146.0) | .023 | |
| Ingressrate | Central | 3.6 (0.8‐18.4) | 4.2 (3.6‐5.0) | .67 |
| Medial | 2.4 (0.9‐29.1) | 4.3 (3.3‐4.6) | .3 | |
| Distal | 2.9 (0.6‐20.9) | 2.4 (1.6‐3.1) | .64 | |
| Lateral | 2.1 (1.0‐22.5) | 3.9 (3.4‐4.6) | .17 | |
| Proximal | 1.4 (0.6‐19.6) | 3.4 (2.4‐5.5) | .17 |
An additional perfusion analysis was performed comparing muscle and myocutaneous flaps. In the OPEN group, 16 muscle and 3 myocutaneous flaps were included. For the O2C method, the central zones were evaluated as described previously. Comparing the two flap categories, muscle and myocutaneous as to the parameters sO2 and Flow, no significance could be detected (Muscle: sO2: 49% range 8‐70% Flow: 14 AU range 2‐43 AU, Myocutaneous: sO2: 44% range 3%‐54%, Flow: 27 AU range 1‐34 AU; sO2 P = .39; Flow P = .41). Accordingly, the testing was performed for the ICG fluorescence angiography by comparing the overall flap perfusion in both the groups. Even here, for the parameters Ingress and Ingressrate, no statistical significances could be observed (Ingress: Muscle: 57.5 AU range 36‐205 AU, Myocutaneous: 91.5 range 75‐108 AU; P = .34; Ingressrate: Muscle: 2.1 AU range 0.9‐20.7 AU, Myocutaneous: 6.3 AU range 1.9‐10.6 AU; P = .47).
An additional subgroup analysis was performed comparing the flap perfusion in patients after AV loop and bypass for the patients with open vascular pedicles at the time point of follow‐up. For the O2C method, the central zones of the flaps were compared and yielded no significant differences (sO2: Bypass: 43.5% range 34%‐54%, AV‐loop: 51.0% range 3%‐70%, P = .360, Flow: Bypass: 16 AU range 4‐20 AU, AV‐loop: 23 range 6‐43, P = .104). Similarly, results showed the comparison of the ICG fluorescence parameter (Ingress: Bypass: 83 AU range 36‐171 AU, AV‐loop 59 range 46‐205 AU P = .874, Ingressrate: Bypass 5 AU range 1‐5 AU, AV‐loop 1.9 AU range 1‐21 AU, P = .266) (compare Figures 4 and 5).
Figure 4.
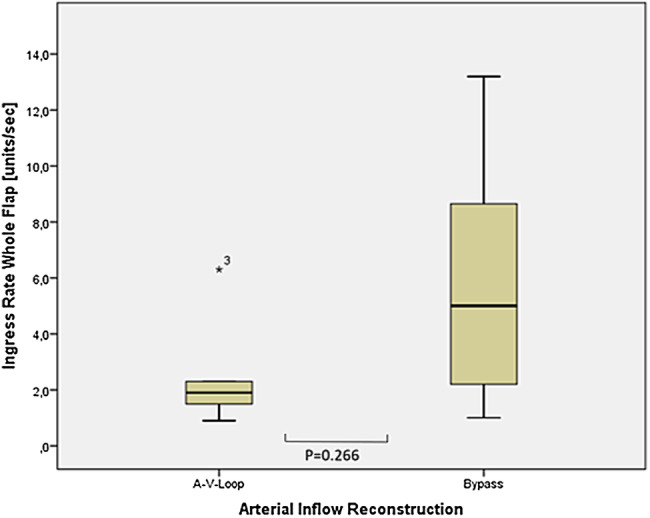
Comparison of the median values of the indocyanine green fluorescence angiography analysis between AV‐loop and bypass patients (ingress rate units/s)
Figure 5.
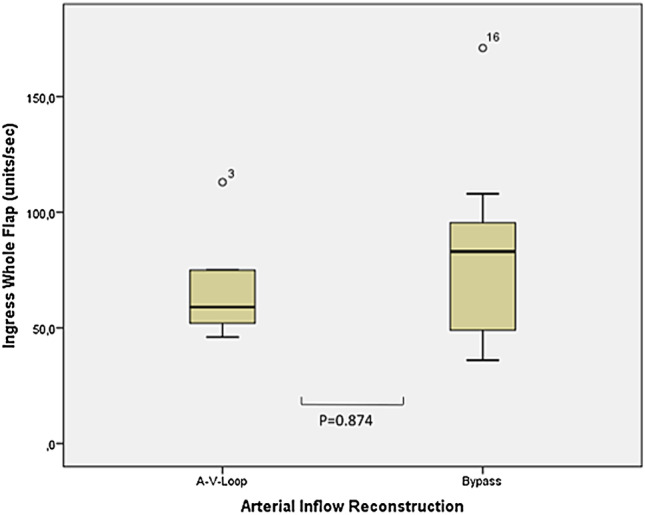
Comparison of the median values of the indocyanine green fluorescence angiography analysis between AV‐loop and bypass patients (ingress units/s)
4. DISCUSSION
The combined method of vascular reconstruction and free‐flap transplantation in patients with large tissue defects has been established as a promising therapeutic option to avoid amputation or ensure defect healing.1, 2, 3, 4, 16 However, due to higher complication rates compared with single free‐flap transplantation, it is still awaiting wider acceptance.17 Several studies reported varying complication rates, mainly depending on the type of tissue defect and the defect's location.4, 16, 18, 19, 20 These published results are mainly in accordance with our findings, as 56% of the patients reached the combined endpoint of overall and flap survival after a median follow‐up time of 53 months (range 0‐151) in our study. In comparison, patency rates of femoro to distal (below the knee) vein bypasses are reported to be 75.3% after 12 months and 51.7% after 5 years in patients with CLI.21
In our experience, failure of vascular reconstruction is based on acute thrombosis in the majority of cases; development of intimal hyperplasia especially at site of the venous loop anastomosis must be avoided by limiting the interval to flap transfer to a maximum of 10 days. This phenomenon was previously described in patients with AV loop fistulas for haemodialysis access.22 Stenosis in the area of venous anastomosis is considered as main cause for graft thrombosis. Animal models as well as clinical studies indicate that significant intimal hyperplasia takes its origin at 28th day after fistula creation; unphysiological shear stress and altered flow pattern in AV loops might be causative; for this reason, it is our policy to perform free‐flap transfer within the first 10 days after AV‐loop creation.23, 24 Of note, stenosis of arterial or venous anastomosis must be excluded during fistula creation and documented by intra‐operative imaging. In all performed salvage thrombectomies of occluded grafts, particular patency of anastomoses could be confirmed; thus, the main cause of flap failure might be based on the relatively high vascular resistance of free flap; therefore, mismatch between AV‐loop (saphenous vein) size and flap pedicle should be kept as low as possible. In the case of varicose or dilated saphenous vein, harvesting of upper extremity veins as cephalic or basilic vein might be an option.
As there have been reports published on flap survival in spite of an occluded vascular pedicle, we reinvestigated our patient cohort to detect the rate of occluded AV loops or bypass grafts. Among the 23 patients who could be followed by duplex ultrasound, four patients presented with an occluded arterial vascular pedicle (two AV‐loops and two bypass grafts)—however, these flaps were clinically inconspicuous. In this study, the duplex ultrasound was chosen for investigation, as the patients were followed up in an ambulatory setting. Therefore, DSA which would have been more detailed was not possible.
Nevertheless, investigation of micro‐circulation showed no statistically significant difference between the OPEN and OCCLUDED groups. In these cases, flap autonomy may have occurred, although the flaps were transferred into regions of mal‐perfusion. Against this background, current findings support the hypothesis that slow, graduated occlusion of flaps pedicle, for example, caused by intimal hyperplasia is well tolerated by myocutaneuos flaps, whereas acute ischaemia by thrombosis of arterial inflow often leads to complete flap loss, particularly in the early postoperative phase.
AV‐loop reconstructions are generally performed as two‐staged approach on our clinic. While there is some evidence that results between one‐ and two‐staged approaches may be similar for the whole collection of patients, we feel that especially loops for reconstruction of limb defects in compromised arterial perfusion might be better performed as stage procedure due to its increased safety. Especially older and frailer patients might benefit from a staged procedure in terms of reduced operation time and complexity.4, 23
The phenomenon of flap autonomy has already been described in the literature. Granzow et al for example reported three cases of early arterial thrombosis after free‐flap transfer during the first 2 weeks after surgery.25 Although this pedicle obstruction occurred very early, these three flaps survived. They concluded that pedicle loss during the first 10 days after operation may not necessarily lead to complete flap loss. Similar results have been demonstrated in an animal model and in vivo study by Mucke et al.26, 27 They investigated the perfusion of flaps based on the superficial inferior epigastric artery in rats after ligating the vascular pedicle. In this study, they demonstrated neovascularization on the level of micro‐circulation, initiated 3 days after surgery with a completed major flap autonomization after 10 days. Similar results could be demonstrated by Bradshaw et al, who analysed the outcome of muscle flaps after pedicle ligation in the rat animal model.28 They found pedicle autonomy initiating at 21 days even in muscle flaps. During investigation of the muscle perfusion by laser Doppler, Bradshaw et al could show that the perfusion decreased until the 35th postoperative day after pedicle ligation, with a rise of perfusion from that day on. This indicates a certain, still poorly understood dynamic in micro‐perfusion changes subsequent to pedicle occlusion. However, this group pointed out that the skin covering of the muscle flap does play a relevant role in the development of flap autonomy, as reorganisation of micro‐perfusion may have its origin as well as it seems to be the recipient of the new formed vessels at the inset side even in muscle flaps. This phenomenon has already been described by Theile et al for cutaneous flaps. Bradshaw et al found similar results for the muscle flaps, as they pointed out the relevance of the skin at the point of flap inset.28, 29
In our series, two of the occluded flaps were fasciocutaneous radial forearm flaps and two latissimus dorsi muscle flaps in one case with persistent monitor skin island and the other case with split thickness skin grafted muscle flap. This finding may speak in favour of the thesis that the skin, in general, could play an important role for flap neovascularization and subsequent autonomy. Of note, the effects of fasciocutaneous flaps, respectively, skin covering on neovascularization cannot be further specified by the present study.
However, the perfusion analysis of these four flaps showed no altered flow pattern. In all the cases, the flaps were homogenously perfused during ICG angiography; hereby, we could not detect a predominant point of origin; on the contrary, the flap perfusion was found to be synchronous to the surrounding skin.
Similar results were found by comparing the perfusion patterns in muscle and mycutaneous flaps; even here, no significant differences were recognised.
A further interesting topic is that in most of our cases, the free‐flap transplantation was performed to regions with impaired tissue perfusion, due to preoperative radiation or diabetic micro‐angiopathy. Therefore, following the concept of the nutrient flap, the perfusion of adjacent ischaemic zones might be improved by the transplantation of well‐vascularized tissue. This concept seems especially attractive in the cases of tissue defects due to CLI, as it suggests neovascularization by flap transplantation in the surrounding tissue; however, it cannot be differentiated in the present design, if the flap is supported by its bed or, on the contrary, if it even acts as origin of neovascularization in surrounding tissue. Further studies are required to solve this issue.
Finally, there are some limitations of this study. Major limitation is the comparatively small number of cases; nevertheless, it is still among the largest series reported. Accordingly, the included sample of transplanted flaps is heterogeneous by the nature of this study, and defect origin also varied between included patients. However, this may be attributed to the highly specific and complex defect reconstruction by means of combined vascular reconstruction and free tissue transfer. A further limitation is the instance that we could not evaluate the exact time point of pedicle occlusion in these specific four patients in our retrospective approach. Taking these limitations into account, the present analysis is intended as a pilot study for the evaluation of long‐term flap perfusion after transplantation; its results and influence on decision making have to be proven in future larger studies.
Decision making in defect reconstruction without suitable recipient vessels is highly influenced by defect localization and angiographic findings. In case of trunk defects (eg, sternal osteomyelitis), revascularization is, after our experience, best performed by means of AV‐loops, connected to the sub‐clavian or axillary vessels; limb defects (CLI, chronic osteomyelitis) generally require angiography prior to defect reconstruction; in case of long‐segment in‐flow occlusions of femoral vessels, these should first be treated by means of bypass grafts. Free‐flap transplantation is then possible directly to the bypass graft in appropriate cases; given poor quality of lower leg vessels and distal defect localization, an AV‐loop connected to the popliteal vessels can be created as preparation for subsequent flap transfer.
However, the combined vascular reconstruction with free tissue transfer is a feasible option for defects without suitable recipient vessels. Even after flap transplantation to areas with critical tissue perfusion, such as the CLI, the flap can develop autonomy and thus survive after pedicle occlusion. Therefore, present results may speak in favour of the nutrient flap theory.
5. CONCLUSION
Despite its complexity, combined vascular reconstruction with free tissue transfer is a feasible option for defects without suitable recipient vessels. Vascular complications represent the main causes of flap failure. Two thirds of these occur within the first month after transplantation. Even after flap transplantation to areas with critical tissue perfusion, such as the CLI, the flap can develop autonomy and thus survive after pedicle occlusion. The analysis of micro‐vascular perfusion pattern showed no difference between flaps with occluded and patent pedicle during follow‐up.
Rother U, Müller‐Mohnssen H, Lang W, et al. Wound closure by means of free flap and arteriovenous loop: Development of flap autonomy in the long‐term follow‐up. Int Wound J. 2020;17:107–116. 10.1111/iwj.13239
REFERENCES
- 1. Meyer A, Horch RE, Schoengart E, et al. Results of combined vascular reconstruction by means of AV loops and free flap transfer in patients with soft tissue defects. J Plast Reconstr Aesthet Surg. 2016;69(4):545‐553. [DOI] [PubMed] [Google Scholar]
- 2. Cavadas PC. Arteriovenous vascular loops in free flap reconstruction of the extremities. Plast Reconstr Surg. 2008;121(2):514‐520. [DOI] [PubMed] [Google Scholar]
- 3. Vogt PM, Steinau HU, Spies M, et al. Outcome of simultaneous and staged microvascular free tissue transfer connected to arteriovenous loops in areas lacking recipient vessels. Plast Reconstr Surg. 2007;120(6):1568‐1575. [DOI] [PubMed] [Google Scholar]
- 4. Meyer A, Goller K, Horch RE, et al. Results of combined vascular reconstruction and free flap transfer for limb salvage in patients with critical limb ischemia. J Vasc Surg. 2015;61(5):1239‐1248. [DOI] [PubMed] [Google Scholar]
- 5. Mimoun M, Hilligot P, Baux S. The nutrient flap: a new concept of the role of the flap and application to the salvage of arteriosclerotic lower limbs. Plast Reconstr Surg. 1989;84(3):458‐467. [PubMed] [Google Scholar]
- 6. Horch RE, Horbach T, Lang W. The nutrient omentum free flap: revascularization with vein bypasses and greater omentum flap in severe arterial ulcers. J Vasc Surg. 2007;45(4):837‐840. [DOI] [PubMed] [Google Scholar]
- 7. Horch RE, Lang W, Arkudas A, et al. Nutrient free flaps with vascular bypasses for extremity salvage in patients with chronic limb ischemia. J Cardiovasc Surg (Torino). 2014;55(2 suppl 1):265‐272. [PubMed] [Google Scholar]
- 8. Rother U, Krenz K, Lang W, et al. Immediate changes of angiosome perfusion during tibial angioplasty. J Vasc Surg. 2017;65(2):422‐430. [DOI] [PubMed] [Google Scholar]
- 9. Rother U, Kapust J, Lang W, Horch RE, Gefeller O, Meyer A. The angiosome concept evaluated on the basis of microperfusion in critical limb ischemia patients‐an oxygen to see guided study. Microcirculation. 2015;22(8):737‐743. [DOI] [PubMed] [Google Scholar]
- 10. Rother U, Lang W, Horch RE, Ludolph I, Meyer A, Regus S. Microcirculation evaluated by intraoperative fluorescence angiography after tibial bypass surgery. Ann Vasc Surg. 2017;40:190‐197. [DOI] [PubMed] [Google Scholar]
- 11. von Allmen RS, Weiss S, Tevaearai HT, et al. Completeness of follow‐up determines validity of study findings: results of a prospective repeated measures cohort study. PLoS One. 2015;10(10):e0140817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495‐1499. [DOI] [PubMed] [Google Scholar]
- 13. Rother UL, Lang W. Noninvasive measurements of tissue perfusion in critical limb ischemia. Gefässchirurgie. 2018;23(suppl 1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rother U, Gerken ALH, Karampinis I, et al. Dosing of indocyanine green for intraoperative laser fluorescence angiography in kidney transplantation. Microcirculation. 2017;24(8). [DOI] [PubMed] [Google Scholar]
- 15. Rother U, Lang W, Horch RE, et al. Pilot assessment of the angiosome concept by intra‐operative fluorescence angiography after tibial bypass surgery. Eur J Vasc Endovasc Surg. 2018;55(2):215‐221. [DOI] [PubMed] [Google Scholar]
- 16. Arkudas A, Horch RE, Regus S, et al. Retrospective cohort study of combined approach for trunk reconstruction using arteriovenous loops and free flaps. J Plast Reconstr Aesthet Surg. 2018;71(3):394‐401. [DOI] [PubMed] [Google Scholar]
- 17. Randon C, Jacobs B, De Ryck F, Van Landuyt K, Vermassen F. A 15‐year experience with combined vascular reconstruction and free flap transfer for limb‐salvage. Eur J Vasc Endovasc Surg. 2009;38(3):338‐345. [DOI] [PubMed] [Google Scholar]
- 18. Tukiainen E, Kallio M, Lepantalo M. Advanced leg salvage of the critically ischemic leg with major tissue loss by vascular and plastic surgeon teamwork: long‐term outcome. Ann Surg. 2006;244(6):949‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludolph I, Lehnhardt M, Arkudas A, et al. Plastic reconstructive microsurgery in the elderly patient ‐ consensus statement of the German Speaking Working Group for microsurgery of the peripheral nerves and vessels. Handchir Mikrochir Plast Chir. 2018;50(2):118‐125. [DOI] [PubMed] [Google Scholar]
- 20. Gunter G. Complication management is risk management is reduction of loss ratio: a personal stance. Handchir Mikrochir Plast Chir. 2017;49(4):229‐233. [DOI] [PubMed] [Google Scholar]
- 21. Uhl C, Grosch C, Hock C, Topel I, Steinbauer M. Comparison of long‐term outcomes of heparin bonded polytetrafluoroethylene and autologous vein below knee femoropopliteal bypasses in patients with critical limb ischaemia. Eur J Vasc Endovasc Surg. 2017;54(2):203‐211. [DOI] [PubMed] [Google Scholar]
- 22. Kelly BS, Heffelfinger SC, Whiting JF, et al. Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int. 2002;62(6):2272‐2280. [DOI] [PubMed] [Google Scholar]
- 23. Henn D, Wahmann MST, Horsch M, et al. One‐stage versus two‐stage arteriovenous loop reconstructions: an experience on 103 cases from a single center. Plast Reconstr Surg. 2019;143(3):912‐924. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka Y, Koghure T, Ueno M, et al. Effects of flow patterns and hemodynamic force on vascular endothelium in the temporary arteriovenous shunt loop in rabbits. J Reconstr Microsurg. 2013;29(5):331‐340. [DOI] [PubMed] [Google Scholar]
- 25. Granzow J, Li AI, Caton A, Boyd JB. Free flap survival following failure of the vascular pedicle. Ann Plast Surg. 2015;75(1):44‐48. [DOI] [PubMed] [Google Scholar]
- 26. Mucke T, Borgmann A, Wagenpfeil S, et al. Autonomization of epigastric flaps in rats. Microsurgery. 2011;31(6):472‐478. [DOI] [PubMed] [Google Scholar]
- 27. Mucke T, Wolff KD, Rau A, Kehl V, Mitchell DA, Steiner T. Autonomization of free flaps in the oral cavity: a prospective clinical study. Microsurgery. 2012;32(3):201‐206. [DOI] [PubMed] [Google Scholar]
- 28. Bradshaw K, Wagels M. Perfusion of muscle flaps independent of the anatomical vascular pedicle: pedicle autonomy. J Plast Reconstr Aesthet Surg. 2017;70(11):1547‐1555. [DOI] [PubMed] [Google Scholar]
- 29. Theile DR, Kane AJ, Romeo R, et al. A model of bridging angiogenesis in the rat. Br J Plast Surg. 1998;51(3):243‐249. [DOI] [PubMed] [Google Scholar]


