Abstract
GPR18 is a G protein-coupled receptor that belongs to the orphan class A family. Even though it shares low sequence homology with the cannabinoid receptors, CB1R and CB2R, a growing body of research suggests its relationship with the endocannabinoid system, not only because it is able to recognize cannabinoid ligands, but also because of its expression and ability to heteromerize with CBRs. In this perspective, we aim to analyze the biological relevance, reported modulators and structural features of GPR18. In order to guide future drug design in this field, highlights from molecular modeling of GPR18 will be provided.
Keywords: GPR18, Agonist, Antagonist, Cannabinoid, Modeling, GPCR, NAGly
Graphical Abstract
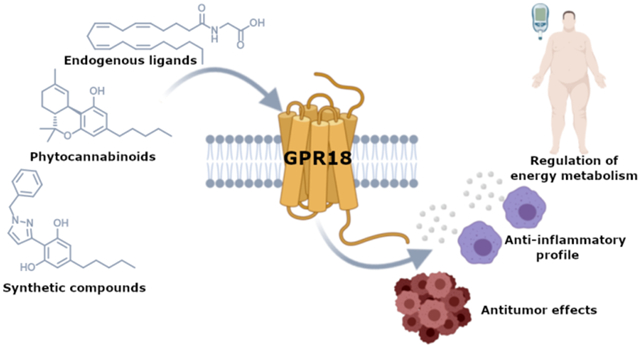
INTRODUCTION
The class A orphan G protein-coupled receptor (GPCR) GPR18 was first cloned by Gantz and coworkers in 1997.1 This receptor, which is 331 amino acids long, was initially isolated from canine gastric mucosa and human colon cancer cells, and subsequently located to human chromosome 13q32.1 GPR18 shares low sequence homology with the cannabinoid receptors CB1R and CB2R (~13% and 8%) and moderate identity with the putative cannabinoid receptor GPR55 (21%). Nonetheless, it has been related to the endocannabinoid system (ECS) since a range of endogenous, phytogenic and synthetic cannabinoids have shown to modulate GPR18.2-4
GPR18 human tissue profiling reveals high expression in spleen, thymus, peripheral blood leukocytes, lymph node, cerebellum, lung and testis among others.1,5 This receptor has been shown to signal through Gαi/o and Gαq/11,3,6,7 while no coupling was detected through Gαz or Gα15.8 One study has reported coupling through Gαs.9 Different studies indicate that this orphan receptor exhibits intrinsic constitutive activity.10,11 However, further studies are needed to fully unravel GPR18 signaling pathways due to the current lack of selective pharmacological tools and its challenging heterologous expression.
There is growing evidence demonstrating the therapeutic potential of GPR18. Modulation of GPR18 has been associated with physiopathological processes including pain, sperm physiology, immunomodulation, intraocular pressure, metabolism or cancer.11-15
Two different endogenous compounds have been proposed as putative ligands of GPR18: a carboxylic metabolite of the endocannabinoid anandamide (AEA), N-arachidonoylglycine6,14,16 (NAGly, Figure 1), and the endogenous polyunsaturated fatty acid metabolite, resolvin D29 (RvD2, Figure 1). However, due to the lack of sufficient in vivo data, GPR18 is still categorized as an orphan receptor according to the International Union of Basic and Clinical Pharmacology (IUPHAR).17
Figure 1.
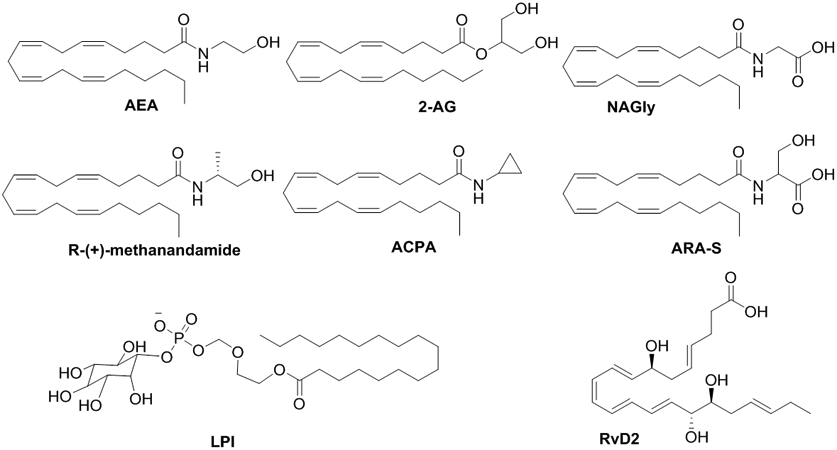
Chemical structures of lipid-like compounds.
Despite its low structural homology with the cannabinoid receptors, its ability to recognize a variety of cannabinoid chemotypes3,4 or form oligomers with the cannabinoid receptors18 makes GPR18 a very attractive target. Due to the current worldwide interest on cannabinoid-based medicines, understanding GPR18-mediated cannabinoid effects is crucial to unravel the role of this orphan receptor in the physiopathology of the ECS.
Elucidating the interactions between GPR18 and cannabinoid ligands is critical to determine the molecular basis of their activity and to help in the design of novel GPR18 selective compounds. For that purpose, we will analyze not only the structure of this orphan GPCR but also selected reported scaffolds. Molecules targeting GPR18 will be selected depending on their activity at other receptors including the related proteins CB1R, CB2R and GPR55. With this, we aim to provide insights for further drug design and therapeutic validation of GPR18.
BIOLOGICAL RELEVANCE
GPR18 is abundantly expressed in several immune cell types where it is involved in different biological functions. GPR18 regulates BV2 microglial cell migration and proliferation,14,15 neutrophil infiltration,9,19,20 CD8αα γδTCR positive lymphocytes maturation21 and macrophage differentiation and efferocytosis.7,9,19,22 GPR18 also regulates cell migration and proliferation in other cell types, mainly endothelial cells,23,24 but also in tumoral cells,11 HEC-1B endometrial cells4 and human spermatozoa.13
GPR18 is especially relevant during the resolution phase of an inflammatory response. During this phase there is a shift from pro-inflammatory to anti-inflammatory factors, which in turn induces a pro-resolution phenotype on macrophages characterized by an increased clearance of the harmful agent and dead polymorphonuclear cells (PMNs). Pro-resolving lipid mediators, such as the GPR18 endogenous ligand RvD2, are the ones coordinating this shift.25 The pro-resolution role of GPR18 has been shown in an E. coli-induced peritonitis animal model, where a single RvD2 treatment induced macrophage efferocytosis, reduced PMN numbers, and lowered counts of E. coli colonies in a GPR18-dependent manner.9 The pro-resolution profile of GPR18 activation has been also reproduced in a periodontitis model where RvD2 reduced inflammation and cell accumulation, while upregulating GPR18 expression.20 Moreover, GPR18 was shown to be indispensable for the pro-resolving effects and increased survival under RvD2 treatment in a mouse model of polymicrobial systemic sepsis.22 In fact, low GPR18 expression levels on PMNs of septic patients have been shown to correlate with increased severity and poorer prognosis, suggesting that GPR18 expression could be considered as a marker for sepsis outcome.26
GPR18 is also involved in resolution of sterile inflammation, such as ischemia/reperfusion (I/R) injury.27 During I/R injury high levels of reactive oxygen species (ROS) and an exacerbated inflammatory response further extend tissue damage. After cerebral I/R injury producing endogenous RvD2 and GPR18 downregulation, exogenous RvD2 reduced pro-inflammatory cytokines, decreased edema and infarct area size, and recovered GPR18 levels in neurons and endothelial cells.27 The protective effect of RvD2 treatment was also observed in a model of lung I/R injury, where GPR18-WT mice, but not KO mice, showed lower PMN infiltration and protection from organ reflow injury.9 The decrease of pro-inflammatory cytokine levels by RvD2 was also observed in an animal hind limb ischemia model, where RvD2 enhanced endothelial cell migration and increased arteriogenesis. These effects were reversed by the GPR18 antagonist O-1918 described later in the text.24
Interestingly, RvD2 was also able to reverse defective reperfusion in obese mice.24 Indeed, metabolic disorders such as obesity or type 2 diabetes causes the adipose tissue to release pro-inflammatory adipocytokines, generating a systemic chronic low-grade inflammation status which, in turn, becomes a risk factor for cardiovascular diseases, strokes, or cancer.28 This chronic inflammation affects hypothalamic neurons that oversee caloric intake which, overtime, become unresponsive to stimulus. Both RvD2 and its precursor docosahexaenoic acid (DHA) reduced hypothalamic pro-inflammatory cytokines, caloric intake and glucose tolerance while upregulating GPR18 expression.29 Furthermore, abnormal cannabidiol (Abn-CBD), a full agonist at GPR18, reduced oxidative stress, restored NO and adiponectin levels and upregulated GPR18 in diabetic rats.30 These cardioprotective effects were blocked when co-administrating O-1918. Studies on vasoactive effects of GPR18 have been published.31-34 The GPR18 agonists NAGly and Abn-CBD showed vasodilation effects on murine retinal arterioles,31 as well as, hypotensive and antioxidant effects in the murine rostral ventrolateral medulla.32 The mechanism behind the decrease on blood pressure appeared to involve the eNOS-NO-cGMP pathway33,34 and it was reversed by O-1918.31-34 In addition, GPR18 activation by NAGly35 or (−)Δ9-tetrahydrocannabinol (Δ9-THC)36 has been shown to lower intraocular pressure in male mice during the day, providing a new potential therapeutic target for glaucoma. It is worth mentioning that Δ9-THC exhibits this effect through a combined action at CB1R and GPR18.36
Chronic inflammation may promote tumor initiation and growth, and vice versa the tumor microenvironment promotes further pro-tumorigenic inflammation, while becoming resistant to anti-tumorigenic actions of the immune system.37 However, the role that GPR18 might play in cancer is yet unclear. Bioinformatic analysis using The Cancer Genome Atlas (TCGA) and/or Gene Expression Omnibus (GEO) public database, has recently shown that GPR18 may be a potential protective factor in hepatocellular carcinoma38 and breast cancer.39 In contrast, GPR18 was found to be overexpressed in human melanoma metastases and blockade of GPR18 expression with siRNA enhanced apoptosis.11 Another relevant role of GPR18 for cancer patients might be in pain reduction. Treatment with RvD2 reduced mechanical allodynia and hyperalgesia in a xenograft mouse model of oral squamous carcinoma, while also reducing pro-inflammatory cytokines and stimulating macrophage efferocytosis.19 In fact, both of the GPR18 endogenous ligands, NAGly and RvD2, have been shown to decrease inflammatory hyperalgesia, neuropathic pain and mechanical allodynia.40-42
Systemic chronic inflammation can also induce pathological changes in the central nervous system that may lead to the development of neurodegenerative diseases. The defensive function of microglia is central to maintain neuroprotection. However, it may exacerbate neuronal damage during neurodegenerative disorders such as Alzheimer’s disease or multiple sclerosis.43 GPR18 is expressed in BV-2 microglial cells, where nanomolar concentrations of NAGly stimulate cell migration, proliferation, morphology and cytokine production.14,15,44 NAGly reduced NMDA-induced excitotoxic damage in organotypic hippocampal cultures in a GPR18-dependent manner.45 Confirmatory studies are still required to ascertain the potential role of GPR18 on pathologies affecting the central nervous system.
Additional roles have also been ascribed to GPR18. Activation of GPR18 expressed in the midpiece of human spermatozoa by NAGly promoted the acrosome reaction, a pivotal step towards egg cell fertilization.13 Moreover, GPR18 was also found on human endometrial cell cultures, where activation by both NAGly and Δ9-THC induced endometrial cell migration,4 a key hallmark in endometriosis.46
MOLECULES TARGETING GPR18
Endogenous ligands
NAGly is an endogenous signaling lipid with a wide variety of biological activity, however, until recently, GPR18’s mechanism of signaling was poorly understood. By measuring intracellular Ca2+ mobilization of a library of 198 lipid compounds using GPR18-expressing L929 cells, NAGly was identified as the first endogenous GPR18 agonist.6 NAGly also induces an increase in intracellular Ca2+ concentration in GPR18-expressing HEK293 cells3 and a decrease in the accumulation of cAMP in GPR18-CHO cells.6 The fact that these properties are abolished by pre-treatment with PTX6 and that NAGly does not have an effect on β–arrestin recruitment3,47 supports the hypothesis that NAGly is a biased-Gαi/o coupled GPR18 ligand that signals via G-protein only. NAGly induces another downstream effector of Gαi/o coupled receptor, P44/42 MAPK.4 NAGly also mediates microglia migration through a PTX-sensitive pathway in different cell types.4,7,14 However, unexpected negative outcomes were obtained for NAGly in SCG neurons suggesting that NAGly could not activate GPR18 signaling via canonical pathways.8 Since activation of Gαi coupled GPCRs is generally associated with a decrease in calcium current via N-channel modulation, yet an increase in calcium current following exposure to NAGly and AbnCBD was observed, these investigators reported that these ligands did not activate GPR18.8 In contrast, Console-Bram et al3 examined the properties of GPR18 in HEK293 cells that are known to be devoid of N-type voltage gated calcium channels,48 and ryanodine receptors49. Consequently, any change in calcium mobilization would be a consequence of GPR18 activation, not channel activity. Their findings demonstrated that GPR18 is activated by NAGly resulting in increased calcium mobilization. Finlay et al 10 reported that GPR18 was unresponsive to NAGly in several different cell types (with heterologous and endogenous GPR18 expression), however calcium mobilization was not assessed in any of the lines. Thus, functional discrepancies between readouts evidence the complex pharmacology of GPR18 (Table 1). It is interesting to highlight that NAGly has recently been proposed as an endogenous GPR55 modulator.50 GPCR-independent modulation of ion channels and transporters has also been demonstrated by Bondarenko and colleagues.51-53
Table 1.
Activity at GPR18 of lipid-like, natural or synthetic compounds in reported pharmacological assays.
| Compd | Ca2+ mobilization | cAMP | BRET/ cAMP |
β-Arrestin recruitment | Cellular migration Cellular viability |
p44/42 MAPK |
ERK ½ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPR18- K562 and GPR18- CHO |
GPR18- L929 |
GPR18- HEK – CAMYEL |
GPR18- HEK293 |
GPR18-CHO | GPR18- HEK – CAMYEL |
GPR18- HEK293- BAEA |
GPR18- CHO-K1 |
BV-2 | GPR18 -HEK293 |
HEC-1B | RAW264.7 | GPR18- HEK293 |
GPR18- HEK293 |
|
| Lipid-like compounds | ||||||||||||||
| NAGly | Agonist6 | Weak agonist6 | n.e.8 | Agonist 3 | Potent inhibition6 | n.e.8,10 | n.e.47 | n.e.3,56 | Potent agonist14 | Agonist4 | Agonist7 | Full potent agonist4 | Agonist3 n.e.10 |
|
| RvD2 | Potent stimulationt9 | Potent agonist9 | ||||||||||||
| AEA | n.e.10 | n.e.47 | n.e.3 | Potent agonist14 | Agonist4 | Full agonist4 | n.e.10 | |||||||
| 2-AG | n.e.47 | Potent agonist14 | ||||||||||||
| ARA-S | n.e.3 | Potent antagonist14 | Antagonist14 | |||||||||||
| ACPA | Weak full agonist4 | |||||||||||||
| R1-metAEA | n.e4 | |||||||||||||
| LPI | n.e.56 | |||||||||||||
| Phytocannabinoids and derivatives | ||||||||||||||
| O-1602 | n.e.8 | Agonist 3 | n.e.10 | n.e.3 | n.e.3 | Potent agonist14 | Potent full agonist4 n.e.10 |
Agonist3 n.e.10 |
||||||
| O-1918 | Agonist3 | n.e.3 | Antagonist14 | Antagonist14 | Agonist3 | |||||||||
| Abn-CBD | n.e.8 | Agonist 3 | n.e.3 | Potent agonist14 | Full agonist4 | Agonist3 n.e.10 |
||||||||
| CBD | Very weak agonist3 | Antagonist14,44 | Weak partial agonist /antagonist4 | |||||||||||
| Δ9-THC | Agonist3 | Agonist3,58 | Full agonist4 | Agonist3 | ||||||||||
| CP55,940 | Antagonist58 | n.e4 | ||||||||||||
| JWH-133 | n.e4 | |||||||||||||
| Aminoalkylindoles | ||||||||||||||
| WIN 55212-2 | n.e4 | |||||||||||||
| JWH-015 | n.e4 | |||||||||||||
| Arylpyrazoles | ||||||||||||||
| SR141716A | Weak antagonist58 | n.e4 | ||||||||||||
| AM251 | n.e.10 | Antagonist4 | Partial Agonist/ Antagonist 4 | |||||||||||
| SR144528 | n.e4 | |||||||||||||
| Fungal metabolites | ||||||||||||||
| Amauromine | Antagonist60 | |||||||||||||
| 27-O-methylasporyzin C | Weak antagonist61 | |||||||||||||
| Coumarins | ||||||||||||||
| PSB-18337 | Weak antagonist63 | |||||||||||||
| Imidazothiazinones | ||||||||||||||
| PSB-CB5 | Potent antagonist56 | |||||||||||||
| PSB-CB27 | Potent antagonist62 | |||||||||||||
| Pyrazolylbenzene-1,3-diols | ||||||||||||||
| S4 | Antagonist64 | Inverse agonist64 | ||||||||||||
| S5 | Agonist64 | Partial agonist64 | ||||||||||||
| S9 | Partial agonist64 | Inverse agonist64 | ||||||||||||
| Tricyclic xanthines | ||||||||||||||
| PSB-KD107 | Potent agonist65 | |||||||||||||
n.e.: no effect
N-Arachidonoyl-L-serine (ARA-S; Figure 1), an endocannabinoid-like lipid initially isolated from bovine brain and characterized by very weak activity at CBRs, TRPV-1, and agonism at GPR55,54 was considered the first selective endogenous ligand of GPR18.55 Activation of this receptor by ARA-S results in phosphorylation of p44/42MAPK and protein kinase B/Akt in human umbilical vein endothelial cells.55 However, it has also been reported that ARA-S inhibits NAGly-induced directed migration of HEK293-GPR18 cell with the same potency as the putative GPR18 antagonist O-1918.14 Inhibition of MAPK activity induced by different GPR18 agonists supports ARA-S as an antagonist.3
As aforementioned, the lipid, RvD2, has been proposed as an endogenous ligand of GPR18 based on enhancement of phosphorylation of CREB, ERK1/2, and STAT3 by RvD2 in WT but not in DRV2/GPR18-KO macrophages.22 RvD2 also enhances cAMP levels by a DRV2/GPR18-dependent mechanism.22 Despite evidence for RvD2 to bind recombinant-GPR18 using radiolabeled-RvD binding assay,9 RvD2 as a GPR18 ligand has not been deeply explored probably in part due to stability issues.
Considering GPR55 and GPR18 promiscuity, in general terms, it is worthy to note here that the GPR55 endogenous ligand LPI does not significantly recruit β-arrestin at GPR18.56 To the best of our knowledge, LPI GPR18 activity through other signaling pathways has not been yet reported.
Cannabinoid-related GPR18 ligands
Endocannabinoid-related GPR18 ligands
Investigations have raised the prospect of AEA (Figure 1) biological activities are in part via non-CB1R and non-CB2R mechanisms among them GPR18.4 Metabolic studies support evidence that AEA metabolizes into NAGly, thus non-CBR effects of AEA could be due to the action of NAGly at GPR18.57 So far, signaling of AEA and other endocannabinoids at GPR18 has been poorly studied. The agonist profile of AEA and 2-AG (Figure 1) has been proposed on the basis of their capacity to elicit microglial migration albeit with less potency than NAGly.4 AEA and its synthetic analogs arachidonylcyclopropylamide (ACPA; Figure 1), and R1-methanandamide (R1-methAEA; Figure 1) induce P44/42 MAPK phosphorylation,4 but AEA did not have any significant effect on β-arrestin recruitment, suggesting possible signaling bias at GPR18.3,47
Phytocannabinoid-related GPR18 ligands
Cannabinoids Δ9-THC, Abn-CBD and CBD (Figure 2) have been assessed for biological activity at GPR18 as outlined in this review. However, their signaling at GPR18 has been poorly characterized pharmacologically. As shown in Table 1, evidence supports Δ9-THC as agonist with significant potency in Ca2+ mobilization,3 β-arrestin recruitment,3,58 and P44/42 MAPK4 and ERK1/23 phosphorylation assays. CBD displays low efficacy as an agonist in β-arrestin recruitment,3 whereas it efficiently antagonizes the effects of NAGly and Δ9-THC on microglia migration and morphology, and cytokine signaling plasticity in BV-2 microglia.4,14,44 This agonist/antagonist functionality difference has been suggested to depend on receptor expression-levels.44 The regioisomer of CBD, Abn-CBD, played a major role in the discovery of GPR18 that was first called Abn-CBD sensitive endothelial receptor.59 Abn-CBD is described as full agonist at GPR18 in several pharmacological assays.3,4,14 A negative β-arrestin recruitment outcome3 could suggest a possible G-protein biased-agonism.
Figure 2.
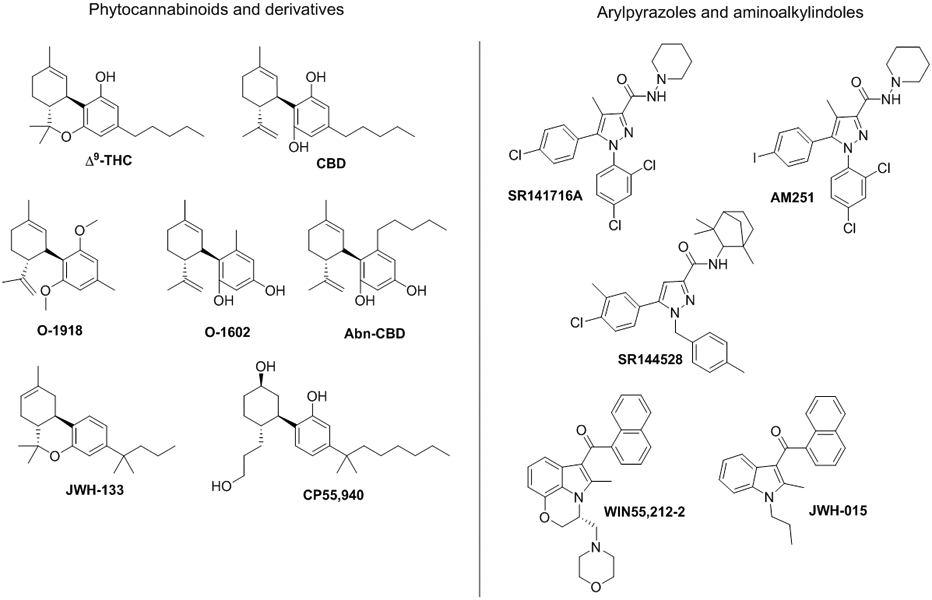
Chemical structures of cannabinoid-related compounds.
O-1602 and O-1918 (Figure 2) are two synthetic analogs of CBD and Abn-CBD respectively. Lacking activity at CB1R and CB2R, both are used a pharmacological tools for GPR55 and GPR18 activity.4 There is evidence that O-1602 has significant agonist effects at GPR18.3,4 O-1602 induces receptor trafficking through GPR18 by stimulating Ca2+ influx, and by inducing MAPK (p44/424; ERK1/23) phosphorylation, both in HEK293 cells (Table 1). Bias-functionality has been suggested due to its inability to recruit β–arrestin in a CHO-K1 GPR18 cell line.3 Interestingly, O-1602 drives cellular migration in BV-2 microglia with the same potency as NAGly.14 The Abn-CBD analog O-1918 has been used in different biological assays as a GPR18 antagonist, as previously commented in the biological relevance of GPR18 section. It has also been shown to attenuate NAGly-, Abn-CBD- and O-1602-induced migration in BV-2 or HEK293-GPR18 cells.14 However, O-1918 showed similar agonist properties to O-1602 in ERK1/2 phosphorylation and Ca2+ assays with a lack of activity in β–arrestin recruitment assays.3 Activity differences between assays highlight the need of further characterize the pharmacology of this putative antagonist in vitro and in vivo. GPR18 knockdown assays may clarify the functionality of this antagonist.
Amauromine (Figure 3) has a natural origin but does not come from Cannabis sativa. This alkaloid extracted from the marine sponge-derived fungus Auxarthron reticulatum is known for its vasodilating and antibiotic properties. Recently, it has been described as a potent GPR18 antagonist and CB1R antagonist without effect on GPR55.60 27-O-methylasporyzin C, another natural product, is the first reported selective GPR18 ligand of fungal origin that significantly inhibits LPI-mediated β-arrestin recruitment.61
Figure 3.
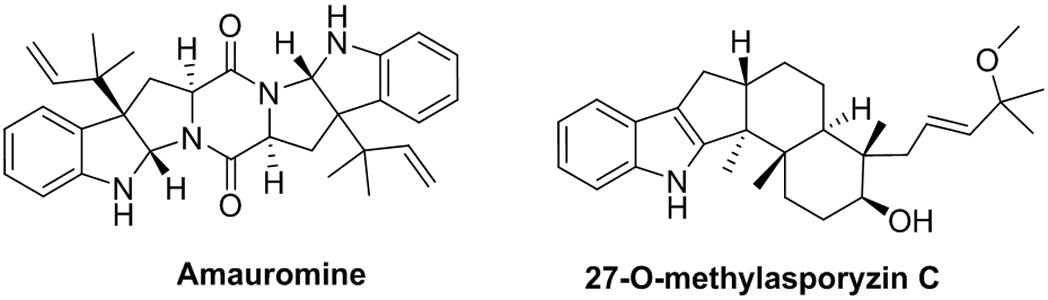
Chemical structure of the natural products amauromine and 27-O-methylasporyzin.
Synthetic cannabinoid-related GPR18 ligands
Despite being a large family of structurally diverse compounds capable of exerting a broad range of pharmacologic effects, only standard synthetic cannabinoids have been tested and reported as GPR18 ligands. In β-arrestin recruitment assays, CP 55,940 and SR141716 (Figure 2) showed the same weak antagonistic properties at GPR18, while they differed in their cannabinoid and GPR55 activity (CP 55,940: CB1R/CB2R agonist and GPR55 antagonist; SR141716: CB1R antagonist and GPR55 agonist).58 WIN55212-2, CP55,940, SR144528, SR141716, JWH-133 and JWH-015 (Figure 2) have no effect on MAPK phosphorylation assays performed in HEK293 cells overexpressing GPR18, whereas AM251 is able to antagonize NAGly and Δ9-THC.4
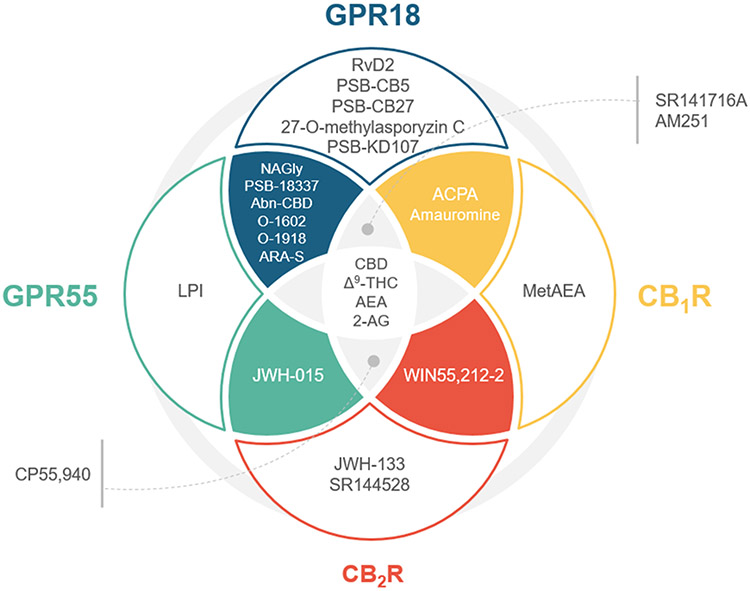
Efforts have been devoted to the pharmacology of endogenous compounds and structurally related cannabinoids that raise issues in terms of selectivity vs GPR55, CB1R and CB2R. The Venn diagram shown in figure 4 illustrates the selectivity profile among cannabinoid related GPCRs of compounds tested at GPR18. Table 1 summarizes reported functionality of these compounds at GPR18.
Figure 4.
Venn diagram classification of compounds tested at GPR18 regarding their activity at the related GPCRs CB1R, CB2R, GPR55 and GPR18. Compounds S4, S5 and S9 have not been included in the diagram since their activity at these other receptors has been reported yet.
Non-CBR related GPR18 compounds
Very few studies focused on the discovery of selective synthetic GPR18 ligands. The only synthetic non-cannabinoid GPR18 ligands reported so far are based on imidazothiazinone and coumarin scaffolds.56,58,62 The first coumarins acting on GPR18 were discovered among a series of chromen-4-one-2-carboxylic acids designed as GPR55 ligands.58 Using β–arrestin recruitment assays on CHO cells stably expressing GPR18, some of these GPR55 ligands antagonized the effects of Δ9-THC at GPR18. The most potent compound of this series at GPR18 is PSB-18337 (Figure 5). This compound exhibits very high potency, but low efficacy at GPR55.63 Screening a compound library focusing on lipophilic structures, Müller and co-workers56 identified imidazothiazinone as a GPR18 scaffold using β–arrestin recruitment assays. Further systematic structural modification on this scaffold led to PSB-CB556 and PSB-CB2762 (Figure 5). PSB-CB5 was identified as the first potent GPR18 antagonist with good selectivity vs related GPCRs (over 36-fold selective vs GPR55 and CB1R, and 14-fold vs CB2R).56 Subsequent optimization efforts led these authors to identify PSB-CB27, which displays improved blockade of THC-induced GPR18 activation and increased selectivity.62
Figure 5.
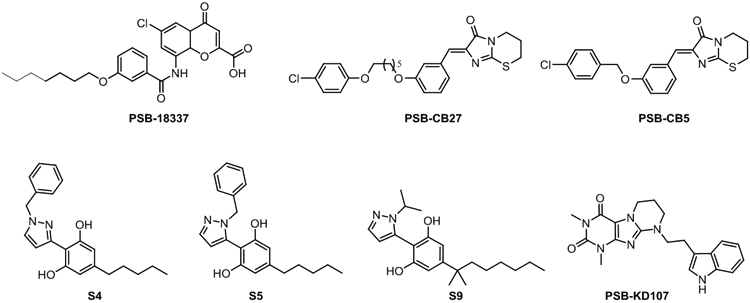
Chemical structures of selected synthetic GPR18 ligands.56,58,62
Very recently, a series of pyrazolylbenzene-1,3-diols have been disclosed in a patent.64 Two regioisomers S4 and S5 present different pharmacological profiles (Figure 5). S5 acts as a GPR18 agonist in intracellular Ca2+ measurements and β–arrestin recruitment assays, whereas S4 antagonizes the effect of NAGly in Ca2+ assays and behaves as an inverse agonist in β–arrestin pathway.64 From this series, S9 (Figure 5), an inverse agonist at β–arrestin recruitment and partial agonist at Ca2+ assay, also display antagonism in TPRV1-expressing cells.
Moreover, a just released article describes the identification of a family of tricyclic xanthines that activate GPR18 in β–arrestin recruitment assays.65 PSB-KD107 (Figure 5), one of the most potent and efficacious agonists of this series, shows full selectivity vs GPR55, CB1R and CB2R.
STRUCTURAL HIGHLIGHTS FROM MOLECULAR MODELING
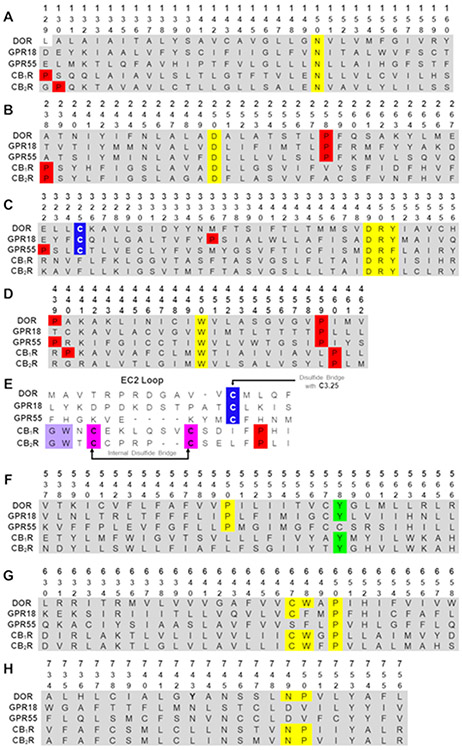
Even though in the last five years the amount of protein structures solved through NMR, crystallographic and cryo-EM studies has grown exponentially, very little is still known about orphan class A GPCRs. The lack of structural knowledge on GPR18 led us to recently develop a human GPR18 homology model based on the δ opioid receptor (DOR, PDB-ID 4N6H).66 The high resolution crystal of the inactive state DOR was used as a template, not only because of its relatively high sequence homology (26%), but also because of its similarities at key structural features including crucial cysteines in the extracellular loop 2 (EC-2 loop) and transmembrane helix 3 (TMH3) C3.25 that form a disulfide bridge, or the conserved proline at position 2.58 (the Ballesteros and Weinstein numbering system67 has been used throughout this article). GPR18 shares key conserved residues or motifs not only with the DOR, but also with GPR55, whereas the CBRs differ from them resulting in significant structural divergences (Figure 6 shows the sequence alignment of human GPR18 with the DOR and the cannabinoid related receptors CB1R, CB2R and GPR55).
Figure 6.
Human sequence alignments of GPR18, DOR, GPR55, CB1R and CB2R. (A) Transmembrane helix 1 (TMH1), (B) TMH2, (C) TMH3, (D) TMH4, (E) EC2 loop, (F) TMH5, (G) TMH6, and (H) TMH7. Color code: red: prolines; yellow: highly conserved residues across Class A GPCRs; magenta: cysteines in an internal disulfide bridge; blue: cysteines in TMH3 to EC2 disulfide bridge; lavender: GW motif.
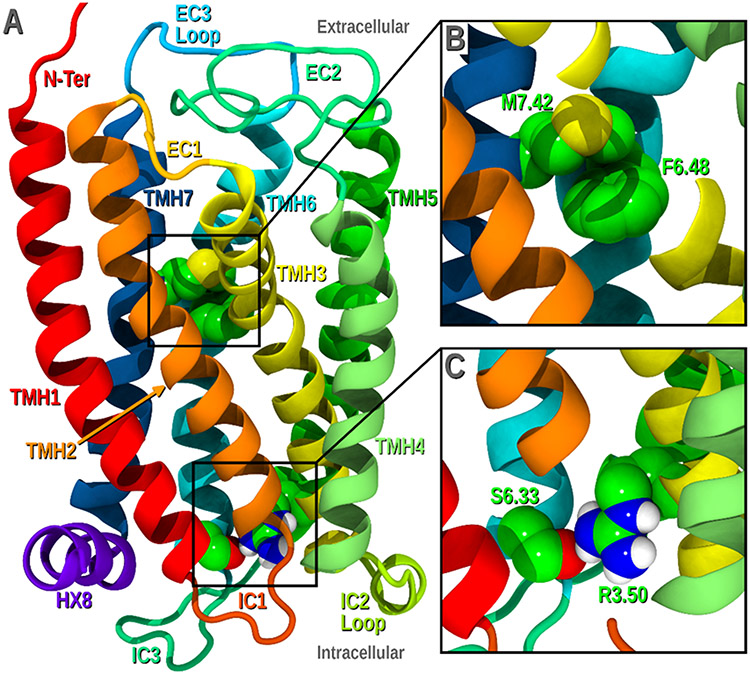
Despite the sequence similarities, specific residue differences between GPR18 and DOR (Figure 6) were identified and studied due to their potential to dictate conformational deviations from the initial template. These include the presence of a proline at position 3.36 in the GPR18 sequence (absent in the DOR), and a significant change in the NPXXY motif in TMH7 (DVILY in GPR18, vs. NPXXY in DOR). This motif displays two striking features that characterize GPR18: it lacks the highly conserved proline at 7.50, and it has a D7.49 instead of an N7.49, which may have a significant effect on the sodium binding pocket in GPR18.66 These differences have the ability to impact the overall bundle and therefore, they were explored using the Conformational Memories program.66 Figure 7A shows the GPR18 inactive state bundle obtained upon conformational examination and subsequent minimization of the receptor.
Figure 7.
GPR18 inactive state model. A) Lipid view of the bundle. B) Zoomed in view of the toggle switch residues M7.42 and F6.48. C) Zoomed in view of the “ionic lock” formed by R3.50 and S6.33. This figure has been done for this perspective using our previously reported model.66
As widely demonstrated among class A GPCRs, G protein activation is characterized by two main conformational rearrangements: at the “ionic lock” and the toggle switch.68,69 The “ionic lock” consists of a strong interaction formed by residues at the intracellular (IC) end of TMH3 and TMH6 (typically a salt bridge between R3.50 and D/E6.30) that hold the receptor in its inactive state. Breakage of this interaction generates an opening that enables Gα coupling. GPR18 lacks a negatively charged residue at the end of TMH6 (Figure 6), consequently, this “ionic lock” is formed by a hydrogen bond between S6.33 and R3.50 (Figure 7C). MD simulations with our homology models suggest that GPR18 constitutive activity could be due to the weakness of this “ionic lock”.66
The so-called toggle switch refers to binding pocket residues which change their conformational state upon receptor activation. Generally, in the inactive state, W6.48 is held in its χ1=g+ conformation by another binding pocket residue transitioning to χ1=trans when an agonist binds. As illustrated in Figure 7B F6.48 and M7.42 form the GPR18 toggle switch.
Given the therapeutic interest in GPR18, other groups have also recently reported GPR18 homology models.70,71 Neumann et al70 averaged the inactive and active template coordinates in their method using Modeller, while Kuder and coworkers71 discarded the chosen active state template and only included the inactive template. None of these models address activation structural features or the development of active state bundles.
Binding pocket
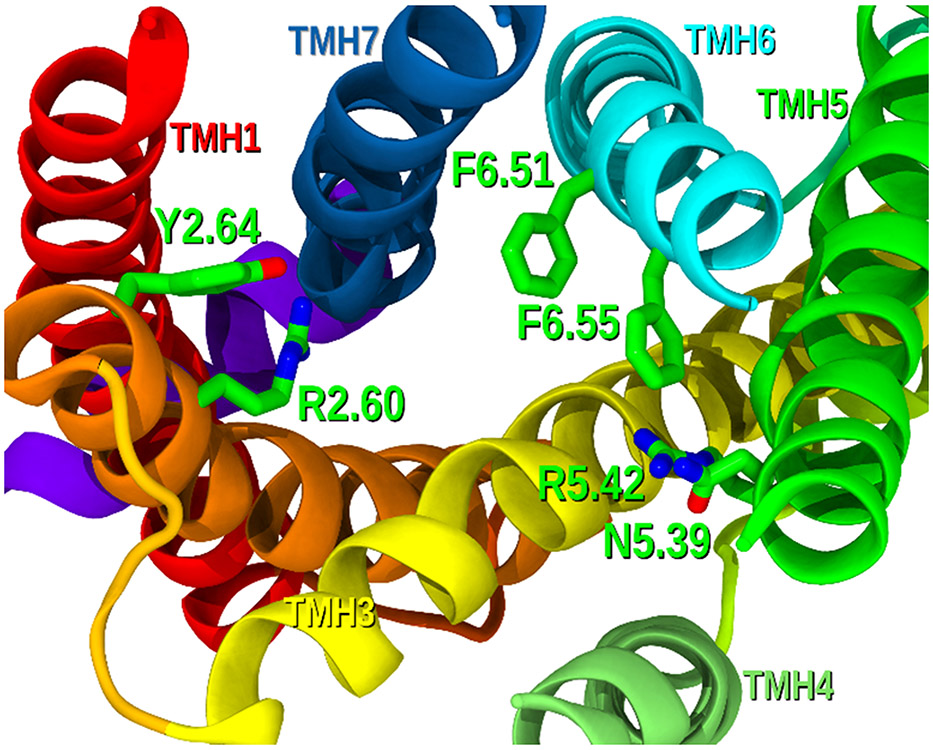
GPR18 contains diverse positively charged amino acids that face the binding crevice and could therefore be crucial spots for ligand docking. These residues include arginines R2.60 and R5.42 (Figure 8), which could establish strong interactions with orthosteric GPR18 modulators. Phenylalanines at positions 6.55 and 6.51, as well as residues Y2.64 and N5.39 are also accessible for ligand binding and could be crucial interaction sites for specific chemotypes (Figure 8). Specific residues in the EC-2 loop, including K(161), K(174) or Y(180), can point towards the binding crevice and may also be critical at stabilizing ligands at the GPR18 orthosteric site. Nonetheless, direct site mutagenesis is required to determine the structural relevance of these residues for agonist and antagonist binding.
Figure 8.
GPR18 binding pocket (EC view, loops have been removed for clarity). Potential interacting residues are displayed in green tubes.
Reported docking studies with the imidazothiazinone antagonist PSB-CB5 and phytocannabinoid GPR18 agonists CBD and THC suggest similar interacting sites to what we have observed in our model.70,71 However, discrepancies in binding site interactions have been found between these two reports. While Kuder at al71 proposes that PSB-CB5 directly interacts with arginines R2.60 and R5.42, according to Neumann and coworkers,70 this imidazothiazinone is mainly stabilized by interactions with K(161), Y2.64 and C6.54. Similarly, the first article proposes both arginines as H-bonding residues with the hydroxyl groups of CBD, whereas in the second report THC complexes with GPR18 engaging with R5.42, K(161) and N5.39.
This structural knowledge may help understanding this orphan GPCR and guide future drug design of compounds with tailored activities at GPR18.
CONCLUSIONS
To date, the therapeutic potential of GPR18 has been demonstrated through in vitro and animal model studies. Due to the lack of selective modulators and its challenging biological validation, little research has been translated into humans. So far, the role of GPR18 in human pathophysiology has only been explored from a predictive standpoint, where this GPCR could be used as a biomarker in patients with sepsis,26 several cancer types,38,39,72 and sarcoidosis.73
Due to its relationship with the ECS, the interest of this target has exponentially increased in the last years. A wide range of cannabinoids have been tested at this receptor and even though some of them present intriguing functional profiles their activity at GPR18 has been proved to mediate some of their therapeutic effects. The putative GPR18 endogenous signaling lipids NAGly and RvD2 produce also a wide variety of biological activity whose signaling at GPR18 is still poorly understood.
As previously indicated, GPR18 shares sequence commonalities with GPR55 at crucial positions while clearly diverges from the cannabinoid receptors at specific structural features. Similarities between these two ECS-related orphan receptors is also demonstrated by their ability to recognize similar ligands that do not bind at CB1R or CB2R as illustrated in the Venn diagram (Figure 4). However, some endocannabinoids and phytocannabinoids are able to target these four lipidic GPCRs probably due to their hydrophobic nature.
Crystal and cryo-EM structures of CB1R74-79 and CB2R74,80,81 in their active and inactive states have been recently reported shedding light to cannabinoid pharmacology. Nevertheless, as aforementioned, due to their low sequence homology, these structures do not serve as a basis to understand GPR18. In this context, our recently reported GPR18 homology model provides insights into the activation mechanism of this orphan GPCR.66 Identification of key binding site residues may guide future design of selective GPR18 compounds to fully unravel this promising GPCR. Selective GPR18 ligands are yet emerging and their pharmacology at this complex receptor is not still fully unraveled. Due to the intricate signaling responses of GPR18, characterization at different pathways is needed. In fact, identification of potential GPR18 G-protein biased agonists could be useful for the treatment of GPR18-related chronic diseases such as diabetes, autoimmune or neurodegenerative disorders with reduced tolerance as already shown for other GPCRs such as opioids.82
ACKNOWLEDGMENT
PM, ALF, and NJ are supported by the Ministry of Science, Innovation, and Universities, Spain (MCIU)/FEDER grant RTI2018-095544-B-I00, the Spanish National Research Council (CSIC) grant PIE-201580E033. MEA, PHR, and NJ are supported by National Institutes of Health grant R01 DA0455698-01. P Morales acknowledges the Comunidad de Madrid (CM) programme ‘Atraccion de Talento’ number 2018-T2/BMD-10819.
ABBREVIATIONS
- Abn-CBD
abnormal cannabidiol
- ACPA
arachidonylcyclopropylamide
- AEA
anandamide
- CBRs
cannabinoid receptors
- ARA-S
N-arachidonoyl-L-serine
- CB1R
CB1 cannabinoid receptor
- CB2R
CB2 cannabinoid receptor
- cAMP
cyclic adenosine monophosphate
- CBD
cannabidiol
- DOR
δ-opioid receptor
- EC-2 loop
extracellular-2 loop
- ECS
endocannabinoid system
- E. coli
Escherichia coli
- cryo-EM
cryogenic electron microscopy
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- GPCR
G protein-coupled receptor; I/R, ischemia/reperfusion
- KO mice
knock-out mice
- LPI
L-α-lysophosphatidylinositol
- MAPK
mitogen-activated protein kinases
- NAGly
N-arachidonoylglycine
- NO
nitric oxide
- PTX
pertussis toxin
- RvD2
resolvin D2
- SCG
superior cervical ganglion
- Δ9-THC
(−)Δ9-tetrahydrocannabinol
- TMH
transmembrane helix
- TRPV
transient receptor potential cation channel
- WT
mice, wild-type mice
Biography
Paula Morales obtained her PhD in Medicinal Chemistry in 2015 from the Autonomous University of Madrid upon completion of her thesis project on the development of novel cannabinoid ligands at the Instituto de Química Médica (IQM-CSIC). After that, she moved to a postdoctoral position on computational chemistry at the University of North Carolina at Greensboro (2016-2018), and then to the protein NMR group at the Instituto de Química Física Rocasolano (2018-2019). She is currently a postdoctoral fellow at IQM-CSIC and her research interests include the identification of novel molecules targeting orphan receptors involved in the endocannabinoid system.
Ana Lago-Fernandez obtained her bachelor’s degree in Biochemistry and Molecular Biology in 2015 from the University Rovira i Virgili (Spain) and her master’s degree in Mental Health Research in 2019 from the University of Cádiz (Spain). Her M.A. thesis explored GPCR activity and biased signaling. Currently, she is a first-year PhD student at the Instituto de Química Médica (IQM-CSIC), where she is developing novel ligands for cannabinoid-related receptors through a multidisciplinary approach.
Dow P. Hurst is Senior Research Scientist in the research group of Dr. Patricia Reggio at UNC Greensboro. Mr. Hurst received his B.S. degree in Chemistry from Kennesaw State University in 1996. He has been part of the Reggio group for 20years. Todate,Mr. Hurst has co-authored 45peer-reviewed papers in top-tier international journals. His first author paper on the lipid pathway for ligand binding in the cannabinoid CB2 receptor (PMID: 20220143) identified the TMH6-TMH7 portal through which the CB2 endogenous ligand, 2-AGenters CB2. This paper was a Faculty of 1000 Medicine Selection and has been highly cited in subsequent X-ray crystal structure papers of lipid type receptors (e.g. S1P1 receptor) in which such lipid portals are clearly visible.
Noori Sotudeh obtained his Ph.D. from the University of North Carolina at Greensboro where he focused on the construction and validation of GPR18 in silico model. He continued modeling of GPCRs as a Postdoctoral associate in Dr. Auerbach’s lab, University at Buffalo, and succeeded to develop a model for muscarinic receptors and performed simulations against electrophysiology experimental data. Currently, he is working as Research fellow in Dana Farber Cancer Institute in Boston to help advance understanding of precision Cancer medicine, drug resistance, and immunotherapy.
Eugen Brailoiu obtained his MD at the University of Medicine and Pharmacy, Iasi (Romania) in 1990, and subsequently was awarded a faculty position there. In 1998, he did post-doctoral studies at East Tennessee State University (USA), and remained there as an Assistant Professor until 2003, when he joined Temple University (USA). He is currently an Associate Professor in the Center for Substance Abuse Research. He has made ground-breaking discoveries in cell signaling, by characterizing and identifying new Ca2+ mobilizing second messengers.
Patricia H. Reggio, PhD. is Marie Foscue Rourk Professor of Chemistry and Biochemistry at the University of North Carolina, Greensboro, NC (2004-present). Dr. Reggio has focused her entire research career on the cannabinoids and the receptors through which cannabinoids signal. This includes the Class A G-Protein Coupled Receptors (GPCRs), the cannabinoid CB1 and CB2 receptors; orphan Class A GPCRs, such as GPR18, GPR55, GPR3, GPR6 and GPR12; and, the ionotropic cannabinoid receptor, the TRP-V1 channel. Dr. Reggio was the recipient of the 2010 Mechoulam Award from the International Cannabinoid Research Society for outstanding contributions to the cannabinoid field. Her work on the cannabinoids has been funded by the National Institutes of Health for over 30 years.
Mary E. Abood completed her Ph.D. degree at the University of California San Francisco (USA) in 1986. She did post-doctoral studies at Stanford University (USA). In 1990, she joined Virginia Commonwealth University as an Assistant Professor in Pharmacology and Toxicology, and tenured in 1996. In 1999, she joined California Pacific Medical Center Research Institute (USA), and continued her research program there until 2008, when she moved to Temple University (USA); she is currently a Professor in the Center for Substance Abuse Research and Department of Anatomy and Cell Biology. She has studied cannabinoid receptors and related GPCRs since 1991, and was honored to receive the Mechoulam Award for Outstanding Contributions to Cannabinoid Research by the International Cannabinoid Research Society in 2015.
Nadine Jagerovic completed her Ph.D. degree at the University of Burgundy (France) in 1990 before carrying out a postdoctoral position at the University of California Davis (USA). In 1993, she joined the Medicinal Chemistry Institute (Madrid, Spain), with a Marie Curie Fellowship in the program “Human Capital and Mobility”, where she was later appointed with a permanent position in 1999. Over the last 15 years, her scientific interests are focused on the discovery of modulators of G protein-coupled receptors related to the endocannabinoid system.
REFERENCES
- (1).Gantz I; Muraoka A; Yang YK; Samuelson LC; Zimmerman EM; Cook H; Yamada T Cloning and Chromosomal Localization of a Gene (GPR18) Encoding a Novel Seven Transmembrane Receptor Highly Expressed in Spleen and Testis. Genomics 1997, 42 (3), 462–466. [DOI] [PubMed] [Google Scholar]
- (2).Pertwee RG; Howlett AC; Abood ME; Alexander SPH; Di Marzo V; Elphick MR; Greasley PJ; Hansen HS; Kunos G International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev 2010, 62 (4), 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Console-Bram L; Brailoiu E; Brailoiu GC; Sharir H; Abood ME Activation of GPR18 by Cannabinoid Compounds: A Tale of Biased Agonism. Br. J. Pharmacol 2014, 171 (16), 3908–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).McHugh D; Page J; Dunn E; Bradshaw HB Δ9-Tetrahydrocannabinol and N-Arachidonyl Glycine Are Full Agonists at GPR18 Receptors and Induce Migration in Human Endometrial HEC-1B Cells. Br. J. Pharmacol 2012, 165 (8), 2414–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Vassilatis DK; Hohmann JG; Zeng H; Li F; Ranchalis JE; Mortrud MT; Brown A; Rodriguez SS; Weller JR; Wright AC; Bergmann JE; Gaitanaris GA The G Protein-Coupled Receptor Repertoires of Human and Mouse. Proc. Natl. Acad. Sci. U. S. A 2003, 100 (8), 4903–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kohno M; Hasegawa H; Inoue A; Muraoka M; Miyazaki T; Oka K; Yasukawa M Identification of N-Arachidonylglycine as the Endogenous Ligand for Orphan G-Protein-Coupled Receptor GPR18. Biochem. Biophys. Res. Commun 2006, 347 (3), 827–832. [DOI] [PubMed] [Google Scholar]
- (7).Takenouchi R; Inoue K; Kambe Y; Miyata A N-Arachidonoyl Glycine Induces Macrophage Apoptosis via GPR18. Biochem. Biophys. Res. Commun 2012, 418 (2), 366–371. [DOI] [PubMed] [Google Scholar]
- (8).Lu VB; Puhl HL; Ikeda SR N-Arachidonyl Glycine Does Not Activate G Protein-Coupled Receptor 18 Signaling via Canonical Pathways. Mol. Pharmacol 2013, 83 (1), 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chiang N; Dalli J; Colas RA; Serhan CN Identification of Resolvin D2 Receptor Mediating Resolution of Infections and Organ Protection. J. Exp. Med 2015, 212 (8), 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Finlay DB; Joseph WR; Grimsey NL; Glass M GPR18 Undergoes a High Degree of Constitutive Trafficking but Is Unresponsive to N-Arachidonoyl Glycine. PeerJ 2016, 4, e1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Qin Y; Verdegaal EME; Siderius M; Bebelman JP; Smit MJ; Leurs R; Willemze R; Tensen CP; Osanto S Quantitative Expression Profiling of G-Protein-Coupled Receptors (GPCRs) in Metastatic Melanoma: The Constitutively Active Orphan GPCR GPR18 as Novel Drug Target. Pigment Cell Melanoma Res. 2011, 24 (1), 207–218. [DOI] [PubMed] [Google Scholar]
- (12).Ramírez-Orozco RE; García-Ruiz R; Morales P; Villalón CM; Villafán-Bernal JR; Marichal-Cancino BA Potential Metabolic and Behavioural Roles of the Putative Endocannabinoid Receptors GPR18, GPR55 and GPR119 in Feeding. Curr. Neuropharmacol 2019, 17 (June), 947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Flegel C; Vogel F; Hofreuter A; Wojcik S; Schoeder C; Kieć-Kononowicz K; Brockmeyer NH; Müller CE; Becker C; Altmüller J; Hatt H; Gisselmann G Characterization of Non-Olfactory GPCRs in Human Sperm with a Focus on GPR18. Sci. Rep 2016, 6 (August 2015), 32255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).McHugh D; Hu SSJ; Rimmerman N; Juknat A; Vogel Z; Walker JM; Bradshaw HB N-Arachidonoyl Glycine, an Abundant Endogenous Lipid, Potently Drives Directed Cellular Migration through GPR18, the Putative Abnormal Cannabidiol Receptor. BMC Neurosci. 2010, 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).McHugh D; Wager-Miller J; Page J; Bradshaw HB SiRNA Knockdown of GPR18 Receptors in BV-2 Microglia Attenuates N-Arachidonoyl Glycine-Induced Cell Migration. J. Mol. Signal 2012, 7 (0), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bradshaw HB; Lee SH; McHugh D Orphan Endogenous Lipids and Orphan GPCRs: A Good Match. Prostaglandins Other Lipid Mediat. 2009, 89 (3–4), 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Alexander SPH; Battey J; Benson HE; Benya RV; Bonner TI; Anthony P; Eguchi S; Harmar A; Holliday N; Jensen RT; Karnik S; Kostenis E; Liew WC; Monaghan AE; Mpamhanga C; Neubig C; Pawson AJ; Pin J; Sharman JL; Spedding M; Spindel E; Stoddart L; Storjohann L; Thomas WG; Tirupula K; Vanderheyden P Class A Orphans (Version 2019.5) in the IUPHAR/BPS Guide to Pharmacology Database. Br. J. Pharmacol 2019, 1–52. [Google Scholar]
- (18).Reyes-resina I; Navarro G; Aguinaga D; Canela EI; Schoeder CT; Zaluski M; Kiec-kononowicz K; Saura CA; Müller CE; Franco R Molecular and Functional Interaction between GPR18 and Cannabinoid CB2 G-Protein-Coupled Receptors. Relevance in Neurodegenerative Diseases. Biochem. Pharmacol 2018, 157, 169–179. [DOI] [PubMed] [Google Scholar]
- (19).Ye Y; Scheff NN; Bernabé D; Salvo E; Ono K; Liu C; Veeramachaneni R; Viet CT; Viet DT; Dolan JC; Schmidt BL Anti-Cancer and Analgesic Effects of Resolvin D2 in Oral Squamous Cell Carcinoma. Neuropharmacology 2018, 139, 182–193. [DOI] [PubMed] [Google Scholar]
- (20).Siddiqui YD; Omori K; Ito T; Yamashiro K; Nakamura S; Okamoto K; Ono M; Yamamoto T; Van Dyke TE; Takashiba S Resolvin D2 Induces Resolution of Periapical Inflammation and Promotes Healing of Periapical Lesions in Rat Periapical Periodontitis. Front. Immunol 2019, 10 (February), 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wang X; Sumida H; Cyster JG GPR18 Is Required for a Normal CD8αα Intestinal Intraepithelial Lymphocyte Compartment. J. Exp. Med 2014, 211 (12), 2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Chiang N; de la Rosa X; Libreros S; Serhan CN Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol 2017, 198 (2), 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Murataeva N; Daily L; Taylor X; Dhopeshwarkar A; Hu SSJ; Miller S; McHugh D; Oehler O; Li S; Bonanno JA; Mackie K; Straiker A Evidence for a GPR18 Role in Chemotaxis, Proliferation, and the Course of Wound Closure in the Cornea. Cornea 2019, 38 (7), 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang MJ; Sansbury BE; Hellmann J; Baker JF; Guo L; Parmer CM; Prenner JC; Conklin DJ; Bhatnagar A; Creager MA; Spite M Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation. Circulation 2016, 134 (9), 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Park J; Langmead CJ; Riddy DM New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery. ACS Pharmacol. Transl. Sci 2020, 3 (1), 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang L; Qiu C; Yang L; Zhang Z; Zhang Q; Wang B; Wang X GPR18 Expression on PMNs as Biomarker for Outcome in Patient with Sepsis. Life Sci. 2019, 217 (November 2018), 49–56. [DOI] [PubMed] [Google Scholar]
- (27).Zuo G; Zhang D; Mu R; Shen H; Li X; Wang Z; Li H; Chen G Resolvin D2 Protects against Cerebral Ischemia/Reperfusion Injury in Rats. Mol. Brain 2018, 11 (9), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Castro AM; Macedo-de la Concha LE; Pantoja-Meléndez CA Low-Grade Inflammation and Its Relation to Obesity and Chronic Degenerative Diseases. Rev. Médica del Hosp. Gen. México 2017, 80 (2), 101–105. [Google Scholar]
- (29).Pascoal LB; Bombassaro B; Ramalho AF; Coope A; Moura RF; Correa-da-Silva F; Ignacio-Souza L; Razolli D; de Oliveira D; Catharino R; Velloso LA Resolvin RvD2 Reduces Hypothalamic Inflammation and Rescues Mice from Diet-Induced Obesity. J. Neuroinflammation 2017, 14 (5), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Matouk AI; Taye A; El-Moselhy MA; Heeba GH; Abdel-Rahman AA Abnormal Cannabidiol Confers Cardioprotection in Diabetic Rats Independent of Glycemic Control. Eur. J. Pharmacol 2018, 820, 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Macintyre J; Dong A; Straiker A; Zhu J; Howlett SE; Bagher A; Denovan-Wright E; Yu DY; Kelly MEM Cannabinoid and Lipid-Mediated Vasorelaxation in Retinal Microvasculature. Eur. J. Pharmacol 2014, 735 (1), 105–114. [DOI] [PubMed] [Google Scholar]
- (32).Penumarti A; Abdel-Rahman AA The Novel Endocannabinoid Receptor GPR18 Is Expressed in the Rostral Ventrolateral Medulla and Exerts Tonic Restraining Influence on Blood Pressure. J. Pharmacol. Exp. Ther 2014, 349 (4), 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Al Suleimani YM; Al Mahruqi AS The Endogenous Lipid N-Arachidonoyl Glycine Is Hypotensive and Nitric Oxide-CGMP-Dependent Vasorelaxant. Eur. J. Pharmacol 2017, 794, 209–215. [DOI] [PubMed] [Google Scholar]
- (34).Matouk AI; Taye A; El-Moselhy MA; Heeba GH; Abdel-Rahman AA The Effect of Chronic Activation of the Novel Endocannabinoid Receptor GPR18 on Myocardial Function and Blood Pressure in Conscious Rats. J. Cardiovasc. Pharmacol 2017, 69 (1), 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Miller S; Leishman E; Oehler O; Daily L; Murataeva N; Wager-Miller J; Bradshaw H; Straiker A Evidence for a GPR18 Role in Diurnal Regulation of Intraocular Pressure. Investig. Ophthalmol. Vis. Sci 2016, 57 (14), 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Miller S; Daily L; Leishman E; Bradshaw H; Straiker A Δ 9 -Tetrahydrocannabinol and Cannabidiol Differentially Regulate Intraocular Pressure. Investig. Ophthalmol. Vis. Sci 2018, 59 (15), 5904–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Greten FR; Grivennikov SI Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51 (1), 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Qiao G-J; Chen L; Wu J-C; Li Z-R Identification of an Eight-Gene Signature for Survival Prediction for Patients with Hepatocellular Carcinoma Based on Integrated Bioinformatics Analysis. PeerJ 2019, 7, e6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ren H; Hu D; Mao Y; Su X Identification of Genes with Prognostic Value in the Breast Cancer Microenvironment Using Bioinformatics Analysis. Med. Sci. Monit 2020, 26, e920212-1–e920212-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Nourbakhsh F; Atabaki R; Roohbakhsh A The Role of Orphan G Protein-Coupled Receptors in the Modulation of Pain: A Review. Life Sci. 2018, 212 (April), 59–69. [DOI] [PubMed] [Google Scholar]
- (41).Zhang L. yu; Liu Z. hua; Zhu Q; Wen S; Yang C. xian; Fu Z. jian; Sun T Resolvin D2 Relieving Radicular Pain Is Associated with Regulation of Inflammatory Mediators, Akt/GSK-3β Signal Pathway and GPR18. Neurochem. Res 2018, 43 (12), 2384–2392. [DOI] [PubMed] [Google Scholar]
- (42).Guerrero-Alba R; Barragán-Iglesias P; González-Hernández A; Valdez-Moráles EE; Granados-Soto V; Condés-Lara M; Rodríguez MG; Marichal-Cancino BA Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol 2019, 9, 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hickman S; Izzy S; Sen P; Morsett L; El Khoury J Microglia in Neurodegeneration. Nat. Neurosci 2018, 21 (10), 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).McHugh D; Roskowski D; Xie S; Bradshaw HB Δ9-THC and N-Arachidonoyl Glycine Regulate BV-2 Microglial Morphology and Cytokine Release Plasticity: Implications for Signaling at GPR18. Front. Pharmacol 2014, 4, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Grabiec U; Hohmann T; Ghadban C; Rothgänger C; Wong D; Antonietti A; Groth T; Mackie K; Dehghani F Protective Effect of N-Arachidonoyl Glycine-GPR18 Signaling after Excitotoxical Lesion in Murine Organotypic Hippocampal Slice Cultures. Int. J. Mol. Sci 2019, 20 (6), 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Weimar CHE; Macklon NS; Post Uiterweer ED; Brosens JJ; Gellersen B The Motile and Invasive Capacity of Human Endometrial Stromal Cells: Implications for Normal and Impaired Reproductive Function. Hum. Reprod. Update 2013, 19 (5), 542–557. [DOI] [PubMed] [Google Scholar]
- (47).Yin H; Chu A; Li W; Wang B; Shelton F; Otero F; Nguyen DG; Caldwell JS; Chen YA Lipid G Protein-Coupled Receptor Ligand Identification Using Beta-Arrestin PathHunter Assay. J. Biol. Chem 2009, 284 (18), 12328–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Berjukow S; Döring F; Froschmayr M; Grabner M; Glossmann H; Hering S Endogenous Calcium Channels in Human Embryonic Kidney (HEK293) Cells. Br. J. Pharmacol 1996, 118 (3), 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Aoyama M; Yamada A; Wang J; Ohya S; Furuzono S; Goto T; Hotta S; Ito Y; Matsubara T; Shimokata K; Chen W; Imaizumi Y Requirement of Ryanodine Receptors for Pacemaker Ca2+ Activity in ICC and HEK293 Cells. J. Cell Sci. 2004, 117 (13), 2813–2825. [DOI] [PubMed] [Google Scholar]
- (50).Console-Bram L; Ciuciu SM; Zhao P; Zipkin RE; Brailoiu E; Abood ME N-Arachidonoyl Glycine, Another Endogenous Agonist of GPR55. Biochem. Biophys. Res. Commun. 2017, 490 (4), 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Deak AT; Groschner LN; Alam MR; Seles E; Bondarenko AI; Graier WF; Malli R The Endocannabinoid N-Arachidonoyl Glycine (NAGly) Inhibits Store-Operated Ca2+ Entry by Preventing STIM1-Orai1 Interaction. J. Cell Sci 2013, 126 (4), 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Bondarenko AI; Panasiuk O; Drachuk K; Montecucco F; Brandt KJ; Mach F The Quest for Endothelial Atypical Cannabinoid Receptor: BKCa Channels Act as Cellular Sensors for Cannabinoids in in Vitro and in Situ Endothelial Cells. Vascul. Pharmacol 2018, 102 (January), 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Bondarenko AI; Drachuk K; Panasiuk O; Sagach V; Deak AT; Malli R; Graier WF N-Arachidonoyl Glycine Suppresses Na+ / Ca2+ Exchanger-Mediated Ca2+ Entry into Endothelial Cells and Activates BKCa Channels Independently of GPCRs. Br. J. Pharmacol 2013, 169 (4), 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Zhang X; Maor Y; Wang JF; Kunos G; Groopman JE Endocannabinoid-like N-Arachidonoyl Serine Is a Novel pro-Angiogenic Mediator. Br. J. Pharmacol 2010, 160 (7), 1583–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Milman G; Maor Y; Abu-lafi S; Horowitz M; Gallily R; Batkai S; Mo F; Offertaler L; Pacher P; Kunos G; Mechoulam R Brain Constituent with Vasodilatory Properties. Pnas 2006, 103 (7), 2428–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Rempel V; Atzler K; Behrenswerth A; Karcz T; Schoeder C; Hinz S; Kaleta M; Thimm D; Kiec-Kononowicz K; Müller CE Bicyclic Imidazole-4-One Derivatives: A New Class of Antagonists for the Orphan G Protein-Coupled Receptors GPR18 and GPR55. Medchemcomm 2014, 5 (5), 632–649. [Google Scholar]
- (57).Bradshaw HB; Rimmerman N; Hu SJ; Benton VM; Stuart JM; Masuda K; Cravatt BF; O’Dell DK; Walker JM The Endocannabinoid Anandamide Is a Precursor for the Signaling Lipid N-Arachidonoyl Glycine by Two Distinct Pathways. BMC Biochem. 2009, 10 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Rempel V; Volz N; Gläser F; Nieger M; Bräse S; Müller CE Antagonists for the Orphan G-Protein-Coupled Receptor GPR55 Based on a Coumarin Scaffold. J. Med. Chem 2013, 56 (11), 4798–4810. [DOI] [PubMed] [Google Scholar]
- (59).Franklin A; Stella N Arachidonylcyclopropylamide Increases Microglial Cell Migration through Cannabinoid CB2 and Abnormal-Cannabidiol-Sensitive Receptors. Eur. J. Pharmacol 2003, 474 (2–3), 195–198. [DOI] [PubMed] [Google Scholar]
- (60).Nazir M; Harms H; Loef I; Kehraus S; El Maddah F; Arslan I; Rempel V; Müller CE; König GM GPR18 Inhibiting Amauromine and the Novel Triterpene Glycoside Auxarthonoside from the Sponge-Derived Fungus Auxarthron Reticulatum. Planta Med. 2015, 81 (12–13), 1141–1145. [DOI] [PubMed] [Google Scholar]
- (61).Harms H; Rempel V; Kehraus S; Kaiser M; Hufendiek P; Müller CE; König GM Indoloditerpenes from a Marine-Derived Fungal Strain of Dichotomomyces Cejpii with Antagonistic Activity at GPR18 and Cannabinoid Receptors. J. Nat. Prod 2014, 77 (3), 673–677. [DOI] [PubMed] [Google Scholar]
- (62).Schoeder CT; Kaleta M; Mahardhika AB; Olejarz-Maciej A; Łażewska D; Kieć-kononowicz K; Müller CE Structure-Activity Relationships of Imidazothiazinones and Analogs as Antagonists of the Cannabinoid-Activated Orphan G Protein-Coupled Receptor GPR18. Eur. J. Med. Chem 2018, 155, 381–397. [DOI] [PubMed] [Google Scholar]
- (63).Schoeder CT; Meyer A; Mahardhika AB; Thimm D; Blaschke T; Funke M; Müller CE Development of Chromen-4-One Derivatives as (Ant)Agonists for the Lipid-Activated G Protein-Coupled Receptor GPR55 with Tunable Efficacy. ACS Omega 2019, 4 (2), 4276–4295. [Google Scholar]
- (64).Jagerovic N; Lago-Fernandez A; Morales P; Abood ME; Brailoiu E; Leo LM; Zhao P; Reggio PH; Hurst DP; Chafi N Pyrazolylbenzene-1,3-Diols for Diseases Associated with G Protein-Coupled Receptor 18 and in Combination with Transient Receptor Potential Vanilloid 1. European Patent Application EP20382324. April 22, 2020. [Google Scholar]
- (65).Schoeder CT; Mahardhika AB; Drabczyńska A; Kieć-Kononowicz K; Müller CE Discovery of Tricyclic Xanthines as Agonists of the Cannabinoid-Activated Orphan G-Protein-Coupled Receptor GPR18. ACS Med. Chem. Lett 2020, acsmedchemlett.0c00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Sotudeh N; Morales P; Hurst DP; Lynch DL; Reggio PH Towards A Molecular Understanding of The Cannabinoid Related Orphan Receptor GPR18 : A Focus on Its Constitutive Activity. Int. J. Mol. Sci 2019, 20, 2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ballesteros J; Weinstein H Integrated Methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G Protein-Coupled Receptors. In Methods in Neurosciences; Stuart CS, Ed.; San Diego, CA, 1995; Vol. 25, pp 366–428. [Google Scholar]
- (68).Zhou Q; Yang D; Wu M; Guo Y; Guo W; Zhong L; Cai X; Dai A; Jang W; Shakhnovich E; Liu Z; Stevens R; Lambert NA; Babu MM; Wang M; Zhao S Common Activation Mechanism of Class a GPCRs. Elife 2019, 8, e50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Weis WI; Kobilka BK The Molecular Basis of G Protein–Coupled Receptor Activation. Annu. Rev. Biochem 2018, 87 (1), 897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Neumann A; Engel V; Mahardhika AB; Schoeder CT; Namasivayam V; Kieć-Kononowicz K; Müller CE Computational Investigations on the Binding Mode of Ligands for the Cannabinoid-Activated G Protein-Coupled Receptor GPR18. Biomolecules 2020, 10 (5), 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Kuder KJ; Karcz T; Kaleta M; Kiec-Kononowicz K Molecular Modeling of an Orphan GPR18 Receptor. Lett. Drug Des. Discov 2019, 16 (10), 1167–1174. [Google Scholar]
- (72).Liu Y; Wang L; Lo K-W; Lui VWY Omics-Wide Quantitative B-Cell Infiltration Analyses Identify GPR18 for Human Cancer Prognosis with Superiority over CD20. Commun. Biol 2020, 3 (1), 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Zhao M; Di X; Jin X; Tian C; Cong S; Liu J; Wang K Identification of Biomarkers for Sarcoidosis and Tuberculosis of the Lung Using Systematic and Integrated Analysis. Med. Sci. Monit 2020, 26, e925438-1–e925438-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Hua T; Li X; Wu L; Iliopoulos-Tsoutsouvas C; Wang Y; Wu M; Shen L; Johnston CA; Nikas SP; Song F; Song X; Yuan S; Sun Q; Wu Y; Jiang S; Grim T; Benchama O; Stahl EL; Zvonok N; Zhao S; Bohn LM; Makriyannis A; Liu Z Activation and Signaling Mechanism Revealed by Cannabinoid Receptor-Gi Complex Structures. Cell 2020, 180 (4), 655–665.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Shao Z; Yan W; Chapman K; Ramesh K; Ferrell AJ; Yin J; Wang X; Xu Q; Rosenbaum DM Structure of an Allosteric Modulator Bound to the CB1 Cannabinoid Receptor. Nat. Chem. Biol 2019, 15 (12), 1199–1205. [DOI] [PubMed] [Google Scholar]
- (76).Krishna Kumar K; Shalev-Benami M; Robertson MJ; Hu H; Banister SD; Hollingsworth SA; Latorraca NR; Kato HE; Hilger D; Maeda S; Weis WI; Farrens DL; Dror R; Malhotra SV; Kobilka B; Skiniotis G Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176 (3), 448–458.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Shao Z; Yin J; Chapman K; Grzemska M; Clark L; Wang J; Rosenbaum DM High-Resolution Crystal Structure of the Human CB1 Cannabinoid Receptor. Nature 2016, 540 (7634), 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Hua T; Vemuri K; Nikas SP; Laprairie RB; Wu Y; Qu L; Pu M; Korde A; Jiang S; Ho J-H; Han GW; Ding K; Li X; Liu H; Hanson MH; Zhao S; Bohn LM; Makriyannis A; Stevens R; Liu Z-J Crystal Structures of Agonist-Bound Human Cannabinoid Receptor CB1. Nature 2017, 547 (7664), 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Hua T; Vemuri K; Pu M; Makriyannis A; Stevens RC; Liu Z Crystal Structure of the Human Cannabinoid CB 1. Cell 2016, 167, 750–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Li X; Hua T; Vemuri K; Ho JH; Wu Y; Wu L; Popov P; Benchama O; Zvonok N; Locke K; Qu L; Han GW; Iyer MR; Cinar R; Coffey NJ; Wang J; Wu M; Katritch V; Zhao S; Kunos G; Bohn LM; Makriyannis A; Stevens R; Liu Z-J. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176 (3), 459–467.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Xing C; Zhuang Y; Xing C; Zhuang Y; Xu T; Feng Z; Zhou XE; Chen M; Wang L Cryo-EM Structure of the Human Cannabinoid Receptor CB2-G i Signaling Complex. Cell 2020, 180, 645–654.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Fernandez TJ; De Maria M; Lobingier BT A Cellular Perspective of Bias at G Protein-Coupled Receptors. Protein Sci. 2020, No. February, 1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]