Abstract
The effective management of post‐operative wounds is important to prevent potential complications such as surgical‐site infections and wound dehiscence. The purpose of this study was to treat wound dehiscence in elderly patients who were subjected to orthopaedic surgical interventions. The dehisced wounds were treated with autologous micro‐grafts obtained using a promising CE‐certified medical device called Rigeneracons. This instrument is a biological disruptor of human tissues able to specifically select progenitor cells that, as already reported in previous studies, maintain high cell viability but mainly have a high regenerative potential, allowing the repair of damaged tissues. Autologous micro‐grafts obtained by Rigeneracons are ready to use and can be applied alone or in combination with biological scaffolds directly on the injured area. We observed in our patients a complete remission of dehisced wounds, on average, after 30 days from micro‐grafts application and a total wound re‐epithelialisation after 1 year from the surgical intervention. In conclusion, although we reported only three patients, autologous micro‐grafts can be considered a promising approach for the treatment of dehisced wounds, improving the wound‐healing process and in general the patient's quality of life without using other dressings.
Keywords: Autologous, Dehiscence, Micro‐grafts, Tissue regeneration, Wound healing
Introduction
The effective management of post‐operative wounds is important to prevent potential complications such as surgical‐site infections and wound dehiscence. Moreover, this aspect remains today a big challenge for surgeons and patients who often experience high comorbidity correlated to a long and exhausting wound‐healing process 1. The use of post‐operative drains and the type of post‐operative dressing is at the discretion of the surgeon, with no available clinical guidelines. The principal aim of drains is theoretically to decrease the incidence of post‐operative haematoma, while occlusive dressings maintain a sterile barrier for longer time periods post‐operatively 2.
Post‐surgical wound dehiscence can arise as a complication in different type of interventions, such as the transplantation of lung 3 and kidney 4, colon resection 5, the following of a laparotomy procedure with an incidence of about 0.5% 6 and gynaecological procedures, such as Caesarean section and total abdominal hysterectomy with an incidence of 15% 7. Orthopaedic surgical complications can lead to longer hospitalization, increased patient morbidity and high costs for both patients and national health services. Several studies reported the association between wound closure method and wound complications, but the correlation between comorbidities, risk factors and surgical wound dehiscence is not still well defined 8.
All wounds heal for primary, secondary or tertiary intention, and several factors can delay wound healing, including chronic disease, vascular insufficiency, diabetes, neurological defects, nutritional deficiencies, advanced age and local factors such as pressure, infection and oedema. Effective wound care requires an accurate and detailed identification of the specific factors interfering with wound healing in order to auspicate the achievement of an optimal outcome 9. In addition, a preoperative assessment can be appropriate to prevent or limit the risk factors responsible for wound dehiscence incidence 6.
Furthermore, dehisced wounds, similar to chronic wounds, are also becoming a socioeconomic emergency because of the high costs of hospitalisation to care for the patients 10. Actually, re‐operation is the elective approach for dehisced wounds mainly in abdominal interventions, but this approach can lead to a high mortality rate, which was estimated to be between 14% and 50% 11.
Several other strategies can improve the management of these wound complications, including negative pressure wound therapy (NPWT), silver ion‐impregnated dressing or the use of topical growth factors such as platelet‐derived growth factor (PDGF), fibroblast growth factor, transforming growth factor‐beta and epidermal growth factor 12. The benefits of all these strategies were widely reported in the literature and certainly have ameliorated the clinical approach to wound complications, leading to reduction of both costs and number of surgical interventions to close the wound.
Based on these considerations, the aim of this study was to display a new promising clinical protocol, named Rigenera protocol, in the management of post‐surgical dehiscence in patients who underwent primary orthopaedic surgical interventions. This new approach is based on the application of autologous micro‐grafts directly on the site of dehiscence, and the efficacy of this approach was already shown in other clinical fields such as dentistry 13, 14, dermatology 15 and in the management of other post‐operative wounds 16.
Patients and methods
Patients
We analysed in this study three patients enrolled in the Unit of Ortopedics and Traumatoly of Santa Croce Hospital (Turin, Italy) in August 2014 and followed‐up these patients for about 1 year. All patients provided informed consent, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The primary diagnosis and initial operative procedures leading to wound dehiscence are listed in Table 1. We also reported the correlated diseases affecting the patients, which, from a clinical point of view, are all characterised by delays in healing greater than 7 weeks.
Table 1.
Diagnosis and operative procedure of the patients with wound dehiscence
| Patients | Sex | Age | Site of dehiscence | Diagnosis | Correlated diseases | Follow up (year) |
|---|---|---|---|---|---|---|
| 1 | F | 78 | Dehiscence of malleolus with exposed peroneal plaque | Tibial‐tarsal fracture | Hypertension, type 2 diabetes | 1 |
| 2 | F | 61 | Dehiscence of malleolus with exposed peroneal plaque | Tibial‐tarsal fracture | Multiple sclerosis | 1 |
| 3 | M | 78 | Dehiscence of dorsal medial area of foot | Forefoot alignment | Hypertension | 1 |
Methods
All cases reported in this study were treated in ambulatory without the need of a recovery or operating room, and we used the Rigenera protocol on all the patients, which allows the regeneration of damaged human tissues, as already reported in previous studies 16, 17. The Rigenera protocol is based on the use of the Rigenera machine and Rigeneracons (Human Brain Wave, Turin, Italy), a biological disruptor able to disaggregate small pieces of human connective tissues and select a specific cell population that includes progenitor cells, maintaining the capacity to differentiate into several cell types and then regenerate a damaged tissue. These progenitor cells, in association with growth factors derived by starting tissue, create autologous micro‐grafts that are ready to use, which can be applied on the injured area alone or in combination with different biological scaffolds, such as collagen.
The Rigenera protocol consists of four steps: (i) collection of a small piece of skin tissue of 1 cm from a distant donor site with respect to the recipient site, (ii) disaggregation of tissue by Rigeneracons through the addition of 1 ml of sterile saline solution, (iii) collection of autologous micro‐grafts obtained after the disaggregation and (iv) injection of these micro‐grafts alone into the site of injury by perilesional infiltrations or in combination with scaffolds embedded with micro‐grafts.
In our patients, we collected small pieces of tissue by trochanteric region after local anaesthesia, and we disaggregated the tissue for 90–120 seconds and used an equine collagen sponge as a scaffold to apply the micro‐grafts on wound dehiscence. Following the application of micro‐grafts, we performed the first dressing after 7 days and subjected the patients to weekly controls to evaluate the progression of wound healing.
Results
In all three patients, we observed, on average, a good remission of wound dehiscence 1 month after the micro‐grafts application, with a range variable between 15 and 60 days. In general, the micro‐grafts were applied only once, except in patient 1, where the Rigenera protocol was applied twice after 30 days from the first application. In Figure 1, we display the healing of a malleolar dehiscence after 40 days from the first micro‐grafts injection or 10 days after the second micro‐grafts application (Figure 1A–C), and after 1 year, we can observed a complete wound re‐epithelialisation (Figure 1D). In the second patient, we performed the Rigenera protocol only once, observing the healing of dehiscence after 60 days (Figure 1E–G). This difference in the time of dehiscence healing can be attributed to the presence of the peroneal plaque. Finally, in the third patient, we reported a complete healing of a dehiscence in the dorsal and medial region of the foot only 15 days after micro‐grafts application, showing a total regeneration of damaged tissues (Figure 2A–C), and after 1 year, in this case also, we observed a complete wound re‐epithelialisation (Figure 2D). For all our cases, no side effects or complications during the execution of protocol or the following evaluations were reported.
Figure 1.
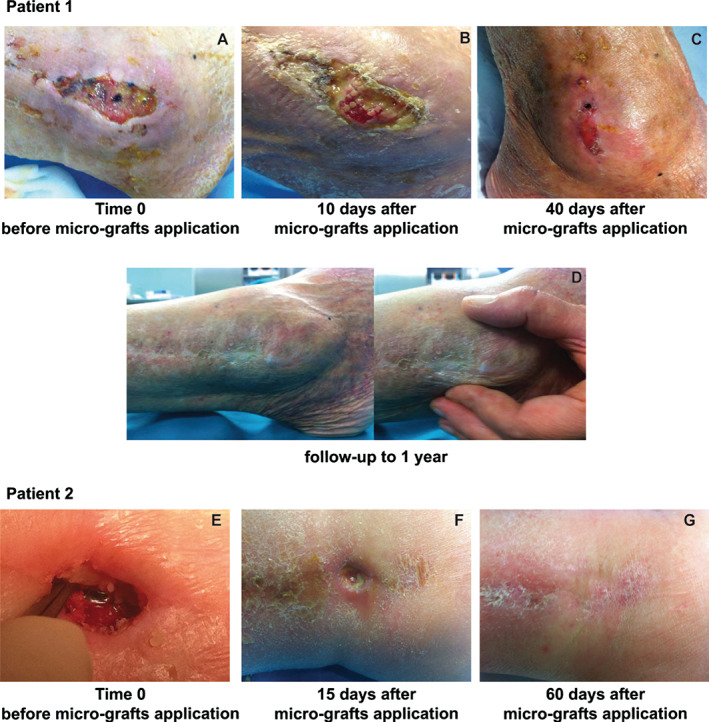
Malleolar dehiscence following osteo‐synthesis with steel peroneal plaque in two different patients. Evaluation of the first patient where the Rigenera protocol was applied twice. (A) Time 0 before micro‐grafts application. (B) Evaluation of dehiscence after 10 days. (C) Progression versus complete healing at 40 days from first application and 10 days after the second micro‐grafts application. (D) Follow‐up at 1 year. Evaluation of the second patient at time 0 before micro‐grafts application (E) and after 15 (F) and 60 days (G).
Figure 2.
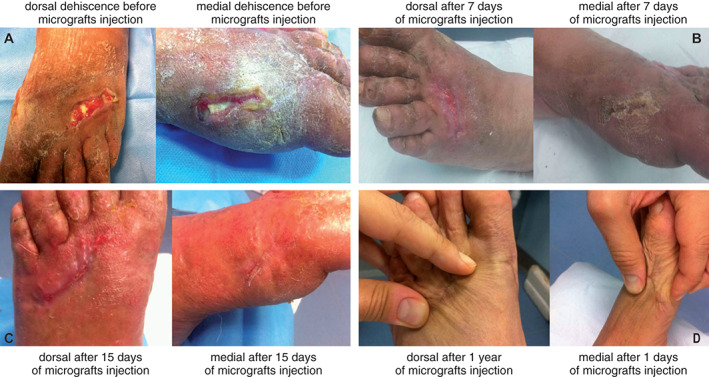
Dehiscence in the dorsal and medial area of the foot caused by forefoot alignment. (A) Before micro‐grafts application. (B–C) After 7 (B) and 15 days (C) from first micro‐grafts application and follow‐up at 1 year (D).
Discussion
In this study, we showed the effectiveness of treatment with autologous micro‐grafts obtained through the Rigenera protocol in ameliorating the healing of post‐surgical orthopaedic dehiscence in patients who previously underwent a primary surgical intervention, such as tibial‐tarsal fracture and forefoot alignment. These results are in accordance with other recent studies where it has been reported that the Rigenera protocol improves the healing of complex wounds that have occurred as post‐operative complications 16. In line with this regenerative potential of the Rigenera protocol, in a previous paper, we demonstrated that micro‐grafts combined with collagen sponges to form a bio‐complex applied on a leg lesion successfully repaired the lesion, promoting the re‐epithelialisation and the softness of the tissue 17. Furthermore, the Rigenera protocol is also successfully applied in the dentistry field where the regeneration of both atrophic maxilla 13 and periodontal tissue 14 was reported and in aesthetic surgery promoting the engraftment of the transplanted hair 15 and the improvement of pathological scars 18. The capacity of these micro‐grafts to improve wound healing was also supported by in vitro results showing that these maintain a high cell viability despite the mechanical disaggregation that is performed to obtain them 17, 19. In addition, these micro‐grafts display a high regenerative potential as indicated by increased positivity to mesenchymal stem cell markers such as CD90, CD73 and CD105 19.
On the basis of this evidence, we believe that the Rigenera protocol can offer an efficient and promising alternative to the already existent approaches to improving wound healing in patients who exhibit wounds hard to heal. Amongst actual approaches, NPWT is certainly one of the most used to treat wound complications, and although NPWT may improve wound healing, the body of evidence available is insufficient to clearly prove an additional clinical benefit of this therapy with respect to advanced dressings 20. In the orthopaedic field, some authors reported that the primary aim of NPWT is to minimise surgical interventions, but further research is required to determine whether NPWT is superior to other management options and also to establish the role of combination therapy, where NPWT is used with instillation of antibiotic solutions or silver‐impregnated sponges 21. According to this evidence, a recent paper described the use of NPWT in orthopaedic oncology, evaluating the management of wounds in sarcoma patients who underwent pelvic and sacral resection. These authors reported the efficacy of NPWT to reduce the wound size, leading to a less invasive surgical procedure, but they outlined the scars compliance of patients because of the management of the device and continuous dressing changes 22. Finally, as previously described, wound management represents a considerable financial burden on health services in terms of manpower requirement, equipment and adjunct therapies, but although NPWT is often perceived to be expensive, there is evidence that its appropriate use leads to faster healing and better quality of life for patients. Further evidence is needed to justify the use of NPWT in chronic wounds in the primary and secondary health care setting 23. For example, it has been reported that comparing the outcomes of open traumatic fractures with 3‐ versus 7‐day intervals between dressing changes, a 7‐day interval between changes of the NPWT is acceptable, reducing the costs related to the NPWT 24. The advantage of the Rigenera protocol with respect to NPWT is the facility of device management, which does not interfere with the life of the patient and does not expect dressing changes at 48 or 72 hours. In fact, the application of micro‐grafts is performed only once without particular precautions or other dressings for the patient.
In line with our results, the research about the grafting material is growing, for example, the use of skin allografts from human deceased donors, and it is very interesting. These allografts in fact promote re‐epithelialisation, but their use is limited to burn injuries for now 25. On the contrary, using the Rigenera protocol, we can create autologous micro‐grafts that are ready to use from the same patient, who, therefore, is both the donor and acceptor of these micro‐grafts.
Usually, post‐operative dehiscences with underlying instrumentations are treated by removing them, but in our case, the premature removal of instrumentations could have caused further interventions accompanied by a longer time of hospitalisation and wound healing. The use of the Rigenera protocol in the malleolar dehiscence with exposure of peroneal plaque allow us to promote tissue regeneration, accelerating the wound healing and allowing the removal of the plaque 1 year after the first intervention following a same incision, without meeting any inconvenience or complication for the patients.
In conclusion, our results, although not yet significant because of the number of treated patients, showed a good and safe clinical effectiveness of the Rigenera protocol in the management of orthopaedic wound dehiscence, but further studies with larger clinical records will be needed to validate the use of this method as an alternative or in combination with traditional wound care therapies.
References
- 1. Yao K, Bae L, Yew WP. Post‐operative wound management. Aust Fam Physician 2013;42:867–70. [PubMed] [Google Scholar]
- 2. Andrew Glennie R, Dea N, Street JT. Dressings and drains in posterior spine surgery and their effect on wound complications. J Clin Neurosci 2015;22:1081–7. DOI: 10.1016/j.jocn.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 3. Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79–93. DOI: 10.1513/pats.200808-094GO. [DOI] [PubMed] [Google Scholar]
- 4. Karam G, Maillet F, Braud G, Battisti S, Hétet JF, Glémain P, Le Normand L, Bouchot O, Rigaud J. Surgical complications in kidney transplantation. Ann Urol (Paris) 2007;41:261–75. [DOI] [PubMed] [Google Scholar]
- 5. Ruggiero R, Sparavigna L, Docimo G, Gubitosi A, Agresti M, Procaccini E, Docimo L. Post‐operative peritonitis due to anastomotic dehiscence after colonic resection. Multicentric experience, retrospective analysis of risk factors and review of the literature. Ann Ital Chir 2011;82:369–75. [PubMed] [Google Scholar]
- 6. Spiliotis J, Tsiveriotis K, Datsis AD, Vaxevanidou A, Zacharis G, Giafis K, Kekelos S, Rogdakis A. Wound dehiscence: is still a problem in the 21th century: a retrospective study. World J Emerg Surg 2009;4:12. DOI: 10.1186/1749-7922-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahana S, Biswas S. An analytical study on wound dehiscence and related factors. Int J Reprod Contracept Obstet Gynecol 2013;2:506–8. [Google Scholar]
- 8. Sandy‐Hodgetts K, Carville K, Leslie GD. Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J 2015;12:265–75. DOI: 10.1111/iwj.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fonder MA, Lazarus GS, Cowan DA, Aronson‐Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206. [DOI] [PubMed] [Google Scholar]
- 10. Hartoch RS, McManus JG, Knapp S, Buettner MF. Emergency management of chronic wounds. Emerg Med Clin North Am 2007;25:203–21. [DOI] [PubMed] [Google Scholar]
- 11. Waqer S, Malik Z, Razzaq A, Abdullah MT, Shaima A, Zahid MA. Frequency and risk factors for wound dehiscence/burst abdomen in midline laparotomies. J Ayub Med Coll Abbottabad 2005;17:70–3. [PubMed] [Google Scholar]
- 12. Stojadinovic A, Carlson JW, Schultz GS, Davis TA, Elster EA. Topical advances in wound care. Gynecol Oncol 2008;111(2 Suppl):S70–80. DOI: 10.1016/j.ygyno.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 13. Brunelli G, Motroni A, Graziano A, D'Aquino R, Zollino I, Carinci F. Sinus lift tissue engineering using autologous pulp micro‐grafts: a case report of bone density evaluation. J Indian Soc Periodontol 2013;17:644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graziano A, Carinci F, Scolaro S, D'Aquino R. Periodontal tissue generation using autologous dental ligament micro‐grafts: case report with 6 months follow‐up. AOMFS 2013;1:20. [Google Scholar]
- 15. Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. Follow‐up of clinical outcome. JCDSA 2014;4:268–74. [Google Scholar]
- 16. Giaccone M, Brunetti M, Camandona M, Trovato L, Graziano A. A new medical device, based on Rigenera protocol, in the management of complex wounds. J Stem Cells Res, Rev & Rep 2014;1:3. [Google Scholar]
- 17. Purpura V, Bondioli E, Graziano A, Trovato L, Melandri D, Ghetti M, Marchesini A, Cusella de Angelis MG, Benedetti L, Ceccarelli G, Riccio M. Tissue characterization after a new disaggregation method for skin micro‐grafts generation. J Vis Exp 2016;109:e53579. doi: 10.3791/53579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svolacchia F, De Francesco F, Trovato L, Graziano A, Ferraro GA. An innovative regenerative treatment of scars with dermal micrografts. J Cosmet Dermatol 2016. DOI: 10.1111/jocd.12212. [DOI] [PubMed] [Google Scholar]
- 19. Trovato L, Monti M, Del Fante C, Cervio M, Lampinen M, Ambrosio L, Redi CA, Perotti C, Kankuri E, Ambrosio G, Rodriguez Y, Baena R, Pirozzi G, Graziano A. A new medical device Rigeneracons allows to obtain viable micro‐grafts from mechanical disaggregation of human tissues. J Cell Physiol 2015;230:2299–303. [DOI] [PubMed] [Google Scholar]
- 20. Gregor S, Maegele M, Sauerland S, Krahn JF, Peinemann F, Lange S. Negative pressure wound therapy: a vacuum of evidence? Arch Surg 2008;143:189–96. DOI: 10.1001/archsurg.2007.54. [DOI] [PubMed] [Google Scholar]
- 21. Streubel PN, Stinner DJ, Obremskey WT. Use of negative‐pressure wound therapy in orthopaedic trauma. J Am Acad Orthop Surg 2012;20:564–74. [DOI] [PubMed] [Google Scholar]
- 22. Siegel HJ. Management of open wounds: lessons from orthopedic oncology. Orthop Clin North Am 2014;45:99–107. [DOI] [PubMed] [Google Scholar]
- 23. Sinha S, Mudge E. The national health‐care agenda in relation to negative pressure wound therapy. Br J Community Nurs 2013;18:(Suppl 9):S6–S13. [PubMed] [Google Scholar]
- 24. Kim YH, Hwang KT, Kim JT, Kim SW. What is the ideal interval between dressing changes during negative pressure wound therapy for open traumatic fractures? J Wound Care 2015;24:536–42. DOI: 10.12968/jowc.2015.24.11.536. [DOI] [PubMed] [Google Scholar]
- 25. Leon‐Villapalos J, Eldardiri M, Dziewulski P. The use of human deceased donor skin allograft in burn care. Cell Tissue Bank 2010;11:99–104. [DOI] [PubMed] [Google Scholar]


