Abstract
Nearly 80% of all burns include the hands of affected individuals. Skin grafting is the gold standard in burns treatment, but in the case of the burn wound bed, it may require the necessity of utilising skin substitutes to facilitate closure. The aim of this study is to assess the impact of a porcine‐derived wound dressing (Oasis™) for application to hand burns compared to a synthetic dressing (Suprathel™). Comparative assessments were made, including the time to heal, quality of healing and pain intensity. A retrospective, unblinded, matching pair case‐control of hand burns was performed. A control group of 24 patients was treated with Suprathel dressing, and a study group of six patients underwent application of the Oasis dressing. The wound healing process was evaluated by taking histopathological specimens and also utilising the Bates‐Jensen Wound Assessment Tool. A 10‐cm Visual Analogue Scale (VAS) was used for pain assessment. Other parameters measured included dressing loss because of infection and the need of rehabilitation. The progress of wound healing on the fourth day in the study group was 30%. A decrease in the level of pain was recorded on the fourth day after surgery. There was a decrease of 5% in the risk of rehabilitation in the treatment group.
Keywords: Biological dressing, Burns, Hydrosurgery, Infections, Wound closure
Introduction
The surface of the hand represents 3% of the total body surface area (TBSA). Over half of patients with minor burns (mean TBSA of 15%) sustain burns to the hand and upper extremities 1. Nearly 80% of all burns also include burns on the hands 2. Burn deformities do not occur when appropriate treatment is provided in the acute situation. The hand is one of the most frequent sites of burns scar contracture deformities, which affect functionality of the hands. The presence of contracting scars on hands greatly impacts the quality of life in burn survivors 3. The depth of the burn is also crucial in the organisation and implementation of the treatment plan 2, 4. A key step in the treatment of patients with burns is the removal of necrotic tissue followed by quick and definitive cover of the burn 5, 6, 7. The execution of debridement in burn patients removes the source of devitalised tissues and affects the humoral immune response affecting the concentration of endotoxins in the patient's blood 5, 6. Hydrosurgical debridement with VERSAJET™ (Smith & Nephew, plc. Hull, UK) allows for the precise removal of necrotic tissue with biofilm 8, 9. Following a wound prepared in this manner, a cover with a three‐dimensional structure containing natural extracellular matrix that facilitates the cell's migration should be applied. Such a dressing will fill the wound bed and stimulate the regenerative processes 10, 11.
Dressing the hand with appropriate wound covers should minimise the level of pain, prevent the formation of scar contractures and guarantee the largest possible functionality. Conventional wound dressings such as paraffin gauze do not meet the above criteria for the treatment 12. It is believed that skin grafting of partial‐thickness wounds offer positive clinical effects in 97% of patients with superficial wounds of the hands and in 81% of patients with deep wounds. Although only 9% of burned patients with injuries including extensor muscles, the joint capsule or bones manage to restore functional capacity, 90% afflicted in this group of patients are able to perform independent everyday life activities 13. Harvesting autologous skin in sufficient amounts for covering burned body surfaces with massive burn wounds (>70% of body surface) is not possible. When this is combined with the possibility of infection developing and skin grafting, this implies the necessity of utilising appropriate skin substitutes 10, 11, 14, 15, 16, 17. When a hand is burned, choosing the proper dressing will provide the maximum benefit to the patient. The hand is always exposed, and aesthetically acceptable outcomes are required 3. In many publications, autologous skin is used for hand burn coverage 2, 3, 18, but hands are especially vulnerable to infections and subsequent loss of the graft. This implies the need to search for alternative dressings. OASIS® Wound Matrix (Smith & Nephew, plc) is a wound dressing fulfilling the proper coverage assumptions highlighted previously. The main component of extracellular matrix is derived from porcine small intestinal submucosa. This results in a bioresorbable dressing containing collagen, elastin, glycosaminoglycans, glycoproteins and proteoglycans, which stimulate the regenerative process in patients with burns and other acute wounds. In randomised clinical trials, the application of the OASIS wound dressing in the treatment of chronic venous ulcers resulted in a heal rate of 55% compared to 34% in the control group (P = 0·0196). No recurrence within a 6‐month observation period was seen. In randomised clinical trials, the application of the OASIS wound dressing to difficult‐to‐heal wounds resulted in an 80% heal rate within 8 weeks. Furthermore, a greater number of observations of the presence of the granulation tissue in wounds in the group treated with OASIS wound dressing were reported (P < 0·05) 19, 20.
Objective
The aim of this study is to assess the impact of the OASIS dressing for treating hand burns in hospitalised patients in the Centre for Burn Treatment in Siemianowice Śląskie. Parameters to be measured included the time and quality of healing and pain intensity among the patients whose hand burns treatment were performed with the OASIS dressing compared to that shown by Suprathel® (Polymedics Innovations GmbH, Denkendorf, Deutschland) dressing. Results of outcomes associated with the use of OASIS will be presented, including whether it improves on drawbacks such as undesirable features associated with scar quality, pain, infection, wound healing and length of stay.
Materials and Methods
All patients gave their consent for the treatment as well as the harvest of histopathological specimens.
An unblinded, matched pair retrospective case–control study of hand burns was performed. Six patients underwent the two‐step treatment that involved the removal of necrotic tissue with the VERSAJET device and the application of OASIS wound dressing in one surgical procedure (Table 1). For this treatment group, the method of 1:4 pairs of hospitalised patients with hand burns were identified and evaluated in the Centre for Burn Treatment between 2009 and 2015. The chosen control group of 24 patients was treated with our standard therapy (Suprathel dressing; Table 1). Subjects for inclusion in the control group were chosen by selection of the parameters minimising the differences between the case and control groups (pair matching for age, gender, type of burn, burn of both hands, % TBSA and time from injury to surgery).
Table 1.
Preparation of wound prior to surgery and skin substitute application
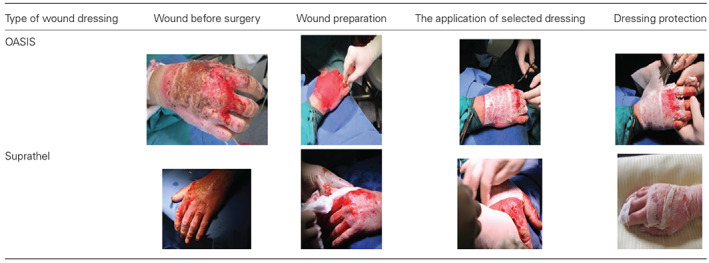
The average percentage of burns in the treatment group of patients was 21% of the TBSA and 23·8% in the control group. In the case of other parameters identified, no clinically significant differences were seen (Table 2).
Table 2.
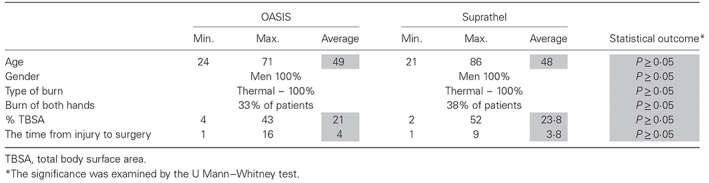
Summary of demographics and wound description
On the 14th and 21st day after surgery, histopathological specimens were harvested from patients. The specimens were subjected to histopathological examination. The wound‐healing progress was evaluated by the Bates‐Jensen Wound Assessment Tool (BWAT). This wound assessment tool consists of 13 parameters, including wound size, depth, edges, undermining, necrotic tissue type, amount of necrotic, granulation and epithelialisation tissue, type and amount of exudate, surrounding skin colour, oedema and induration. Overall, 13 assessment parameters are measured on a scale of 1–5. The allocated points are summed and equated to a standard in which 13 points equate to a healed wound. The level of pain intensity was assessed by the patients before surgery and on the 4th, 17th and 20th day after surgery. A 10‐cm Visual Analogue Scale (VAS) was used for pain assessment. In addition, the absolute risk reduction (ARR) 21 associated with the loss of a dressing because of infection and the need of rehabilitation was calculated according to the following formula:
where:
nc – the total number of patients in the treatment group.
nk – the total number of patients in the control group.
zc – the event rate in the treatment group (the number of patients who achieved an outcome variable).
zk – the event rate in the control group (the number of patients who achieved an outcome variable).
Results
The VERSAJET device was seen to be suitable for wound preparation prior to the application of the OASIS dressing. Following wetting, the elasticity and conformability ensured easy application to the patient's hands (Table 3).
Table 3.
Application challenges of dressings to the burned body surfaces
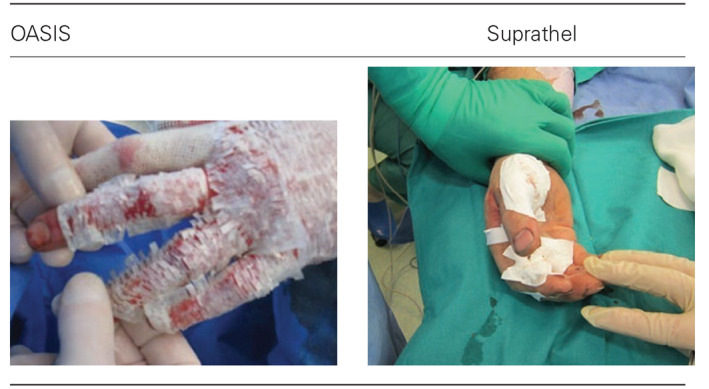
Both the OASIS wound dressing and the Suprathel wound dressing are designed for the treatment of first‐degree and second‐degree burns. Patients with deeper burns cannot be qualified for this therapy. However, the efficacy and safety of OASIS were examined in a randomised clinical trial for the treatment of full‐thickness venous leg ulcers 22.
Surgeons involved in this evaluation believed that the OASIS dressing adheres better to the wound surface of the hands than the Suprathel dressing. Throughout the period of hospitalisation, wound dressings were sterile. Wound healing is clearly visible between the 12th and 17th day. Additionally, matrix engraftment was observed (Table 4). ‘Carmelization’ of the wound is a normal observation associated with the use of Oasis dressing.
Table 4.
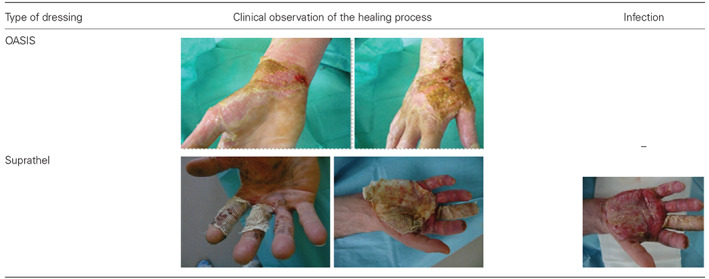
Profile of wound healing in the treatment and control group
Following observations of the histopathological preparations, the process of epithelialisation was observed. An increase of the mature epidermis composed of multiple cell layers was observed within 3 weeks, and inflammatory reactions were not observed (Table 5). This histopatological image corresponded with the clinical state. Complete epithelialisation is an essential component of wound healing, and a wound cannot be considered healed in the absence of epithelium. Moreover, faster epithelialisation enables the earlier introduction of rehabilitation if needed.
Table 5.
The wound‐healing process that was confirmed by the results of histopathological examination on the 14th (photos tagged no. 1) and 21st day (photos tagged no. 2)
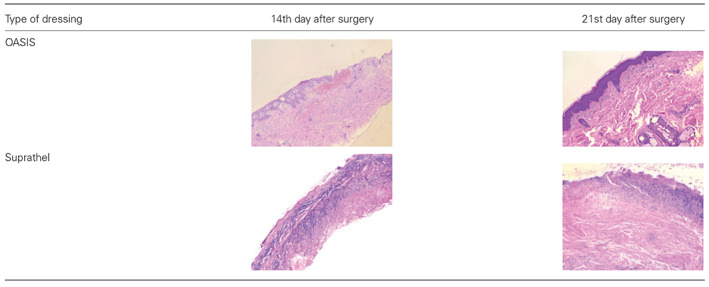
Histopathological examinations resulted in observations of the wound‐healing process through time in the treatment group of patients (Figure 1). On the 17th day after surgery, the wound was covered by epidermis in 75% of patients. The progress of wound healing on the fourth day was 30% closure.
Figure 1.
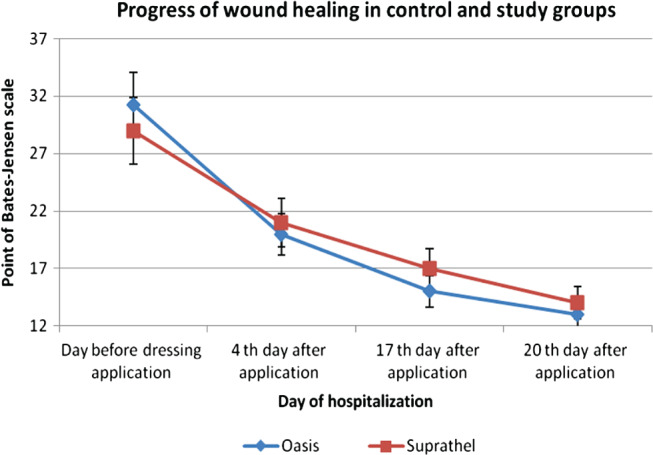
A comparison of the rate of wound healing when treated with OASIS and Suprathel.
A significant decrease in the level of patient's pain intensity was recorded on the fourth day after surgery. The decrease in the level of pain was greater in the case of hand burn wounds, which were treated with the OASIS dressing (Figure 2).
Figure 2.
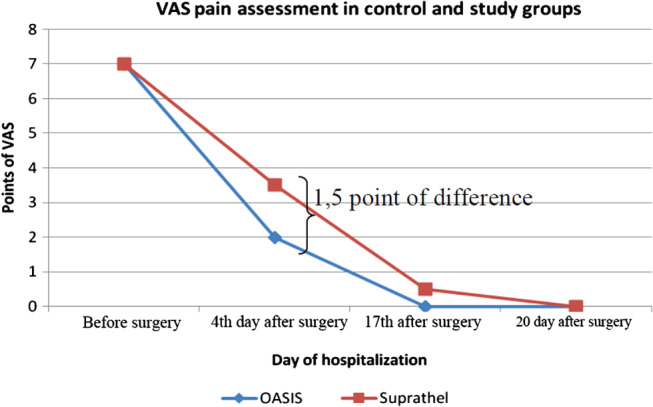
A comparison of experienced pain in the analysed groups.
In summary, the combined therapy of VERSAJET/OASIS is particularly useful in treating wounds localised in areas of the body, such as hands, that are difficult to dress in terms of wound management materials. The application of OASIS wound dressing on partial‐thickness burns results in positive wound‐healing outcomes. The OASIS dressing also minimises the level of pain in patients with burns. There is a 5% decrease in the risk of rehabilitation in the treatment group with the OASIS dressing compared to the patients treated with the Suprathel dressing (ARR). The application of the Oasis wound matrix results in better effects of the early rehabilitation of the hand in a shorter time (better hand grip and range of motion). Improved results of rehabilitation could be achieved by decreased pain sensation in the wake of Oasis utilisation. A slight third‐degree burn limits hand function less than an extensive second‐degree burn. The third‐degree burn is equal to decreased pain sensation and better hand range of motion 23. During the hospitalisation period, no statistically significance differences were observed (Figure 3).
Figure 3.
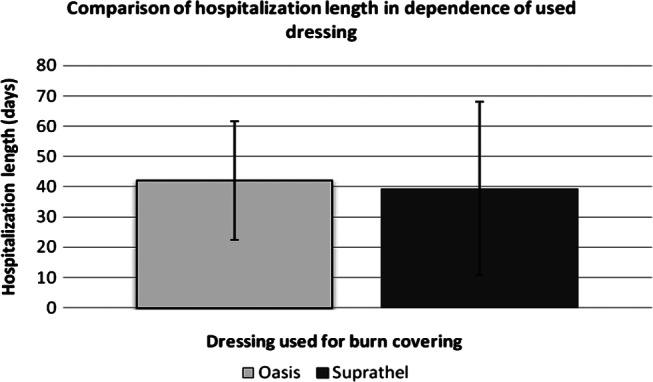
The relationship between the length of hospitalisation (days) and the kind of wound dressing used.
Discussion
In our specialised Centre for Burn Treatment, patients with hand burns were treated with Suprathel and Biobrane dressing during the years 2009–2015. Suprathel is a synthetic wound dressing containing DL‐lactide copolymer and methyl carbonate and ϵ‐caprolactone and is a substitute of the epidermis. It has claimed to stimulate the wound‐healing process in partial‐thickness burns 24. Suprathel is a copolymer‐based wound dressing mainly consisting of DL‐lactide, trimethylenecarbonate and e‐caprolactone. It is a fully synthetic porous membrane that shows a large plasticity and imitates the properties of natural epithelium. It adapts instantly to the wound surface at body temperature. Because of this special property, it can also be used in critically and functionally important regions like fingers. It adheres to the wound ground, and because of its water permeability, it prevents an accumulation of wound exudates and wound infection and ensures a moist wound environment. This is a favourable precondition for excellent wound healing and fast reepithelialisation. Changing of Suprathel is not necessary because it autonomously peels away in line with reepithelialisation. This dressing is transparent on the wound, and therefore, an evaluation of the wound ground without removing Suprathel is feasible 25, 26.
Biobrane (Smith & Nephew, plc. Hull, England, UK) is a universal biosynthetic dressing 27 that is not expensive, is easy to store and is reliable when used in accordance with the prescriptions 28. In spite of many advantages, Lal et al. suggest that the dressing should be applied in partial‐thickness burns not exceeding 25% of the TBSA on the grounds of infection risk 29. Hubik and his collaborations confirm that the use of Biobrane is associated with the risk of infection in 37·8% of patients 30. These studies indicate the need for seeking other kinds of dressings that would minimise the risk of infection and could be used in the treatment of partial‐thickness hand burns with small parts of full‐thickness burns. Nowadays, none of specialised dressings fulfil all characteristics of the ideal wound dressing. According to the philosophy of Tissue, Infection, Moisture and wound Edge (TIME), the dressing should be dedicated to a specific phase of wound healing. Burn wound protection along with the application of specialised wound dressing containing silver can decrease the risk of infection; however, in comparison with synthetic skin substitutes, it does not significantly affect the acceleration of the skin formation processes.
Another product that has gained widespread use in the clinical treatment of deep partial‐thickness and full‐thickness burn wounds is Integra®. It is a dermal regeneration template consisting of bovine collagen, chondroitin‐6‐sulphate and a silastic membrane. This artificial skin allows temporary coverage after burn excision, transformation of matrix in the neo dermis and definitive engraftment. The bovine collagen dermal analogue integrates with the patient's own cells, and the temporary epidermal silicone is peeled away as the dermis regenerates. Infections are the most common complication of this technique 31, 32. In recent years, the level of knowledge about wound healing has increased. Synthesis and depositions of extracellular matrix (ECM) is crucial to wound healing, which are characterised by the loss of natural matrix of the dermis. The interaction between extracellular matrix, growth factors and cells is crucial in the process of tissue regeneration. The phases of wound healing include the inflammatory, proliferation and maturation phases. During the inflammatory phase, fibronectin and other parts of the extracellular matrix located in the area of the wound play the role of chemotactic agents for the monocytes that bind to extracellular matrix protein. This association stimulates phagocytosis, leading to the removal of bacteria, necrotic tissue and other impurities in the wound. Adherence of monocytes to extracellular matrix proteins induces the expression of growth factors that influences the bioactivity of cells (e.g. increased proteoglycan synthesis by fibroblasts). Interactions between growth factors and ECM can take a direct form, such as the direct binding of growth factors by ECM components, or indirect forms involving the impaired response of cells that are not connected with the cell matrix on the particular cellular signals 33, 34.OASIS Wound Matrix dressing is composed of extracellular matrix derived from porcine small intestinal submucosa. This dressing has components similar to those found in human skin and includes collagens (type I, III, IV, VI), glycosaminoglycans, glycoproteins and proteoglycans. OASIS is integrated into the wound during the wound‐healing process 35. The overall structure of this wound dressing allows for the migration of the autologous cells, which accelerates the healing process 33. Because of the relatively small sample size, the differences between speed and quality of healing measured by the Bates‐Jensen scale did not demonstrate statistically significant differences at any of the specified time points, but in the case of OASIS, earlier granulation tissue was observed. Additional research using the OASIS dressing on larger study populations may demonstrate statistical significance. Reduction of pain on the 4th day after surgery is definitely both a clinical and patient advantage. Burns are the cause of intense and long‐lasting pain that may lead to mental disorders 36, 37, 38, regardless of the depth and extent of the wounds 39. The painful stimulation of nerve endings caused by peripheral simulation and central mechanisms results in an intensification of the level of pain and development of chronic pain syndromes 36. A better understanding of the pain treatment process is a crocs‐disciplinary approach to the provision of burn management in a specialised unit 39. In patients with preserved consciousness, the assessment of experienced pain level should start from the quantification of the level of pain 37, and in our study, the VAS scale was used. In clinical practice, opioids are applied despite the side effects; however, painkillers are increasingly recommended 36. Apart from the primary source of pain, changing the dressing is an additional factor and can significantly increase the level of pain in patients with burns 37, 38. Advanced wound dressings should be definitive in wound closure and easily bioabsorb to the wound 40. Application of synthetic skin substitutes are particularly significant in difficult‐to‐dress areas, such as hands. Decreasing the pain in the affected hand reduces the likelihood of destruction of the dressing by the patient and improves their attitude to the proposed treatment. Following the OASIS wound dressing application, on the fourth day after surgery, a decrease in the level of pain in 70% of patients was reported in the treatment group. The aesthetic and functional aspects of the healing hands are also important. Application of the OASIS wound dressing prevented the formation of scar contractures and resulted in a good aesthetic outcome. Despite the advantages, the differences between the hospitalisation time in the treatment and control group were not observed. We should take into account the impact of the accompanying diseases and the patient's general condition on this parameter as a confounding factor in addition to low numbers of the treatment group.
References
- 1. Tredget EE. Management of the acutely burned upper extremity. Hand Clin 2000;16:187–203. [PubMed] [Google Scholar]
- 2. Richards WT, Vergara E, Dalaly DG, Coady‐Fariborzian L, Mozingo DW. Acute surgical management of hand burns. J Hand Surg [Am] 2014;39:2075–85. [DOI] [PubMed] [Google Scholar]
- 3. Sabapathy SR, Bajantri B, Bharathi RR. Management of post burn hand deformities. Indian J Plast Surg 2010;43:S72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nuchtern JG, Engrav LH, Nakamura DY, Dutcher KA, Heimbach DM, Vedder NB. Treatment of fourth‐degree hand burns. J Burn Care Rehabil 1995;16:36–42. [DOI] [PubMed] [Google Scholar]
- 5. Kawecki M, Hoff‐Lenczewska D, Klama‐Baryła A, Glik J, Łabuś W, Nowak M. Oparzenia. Przegląd piśmiennictwa chirurgicznego. Tom XX. Pod redakcją Adama Dzikiego.
- 6. Lloyd EC, Michener M, Williams MS. Outpatient burns: prevention and care. Am Fam Physician 2012;85:25–32. [PubMed] [Google Scholar]
- 7. Jian‐Hui Z, Jian‐Sheng D, Wen‐Sen X, Yong P, Yan H. Clinical application of full‐face, whole, full‐thickness skin grafting: a case report. J Plast Reconstr Aesthet Surg 2012;65(11):1576–1579 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8. Rennekampff HO, Schaller HE, Wisser D, Tenenhaus M. Debridement of burn wounds with a water jet surgical tool. Burns 2006;32:64–9. [DOI] [PubMed] [Google Scholar]
- 9. Kawecki M, Mikuś‐Zagórska K, Glik J, Nowak M. The efficiency of burn wounds debridement with use of hydrosurgery‐our experiences. Pol Przegl Chir 2015;87:1–5. [DOI] [PubMed] [Google Scholar]
- 10. Selig HF, Lumenta DB, Giretzlehner M, Jeschke MG, Upton D, Kamolz LP. The properties of an "ideal" burn wound dressing ‐ What do we need in daily clinical practice? Results of a worldwide online survey among burn care specialists. Burns 2012; 38(7):960–966 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11. Philandrianos C, Andrac‐Meyer L, Mordon S, Feuerstein JM, Sabatier F, Veran J, Magalon G, Casanova D. Comparison of five dermal substitutes in full‐thickness skin wound healing in a porcine model. Burns 2012;38:820–9. [DOI] [PubMed] [Google Scholar]
- 12. Edwards J, Mason S. Hand burn management: minimizing pain and trauma at dressing change. Br J Nurs 2013;22(S46):S48–50. [DOI] [PubMed] [Google Scholar]
- 13. Sheridan RL, Hurley J, Smith MA, Ryan CM, Bondoc CC, Quinby WC Jr, Tompkins RG, Burke JF. The acutely burned hand: management and outcome based on a ten‐year experience with 1047 acute hand burns. J Trauma 1995;38:406–11. [DOI] [PubMed] [Google Scholar]
- 14. Sorg H, Betzler C, Rennekampff HO, Vogt PM. Burns. Unfallchirurg 2012;115:635–48. [DOI] [PubMed] [Google Scholar]
- 15. Devgan L, Bhat S, Aylward S, Spence RJ. Modalities for the assessment of burn wound depth. J Burns Wounds 2006;5:e2. [PMC free article] [PubMed] [Google Scholar]
- 16. Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plast Surg 2007;59:109–15. [DOI] [PubMed] [Google Scholar]
- 17. Chen B, Hu DH, Jia CY, Ding GB, Yao QJ, Liu YL. Management of a patient with massive and deep burns: early care and reconstruction after convalescence. Zhonghua Shao Shang Za Zhi 2007;23:112–6. [PubMed] [Google Scholar]
- 18. Bhattacharya S. Avoiding unfavorable results in postburn contracture hand. Indian J Plast Surg 2013;46:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41:837–43. [DOI] [PubMed] [Google Scholar]
- 20. Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult‐to‐heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care 2010;23:34–8. [DOI] [PubMed] [Google Scholar]
- 21. Schechtman E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat‐which of these should we use? Value Health 2002;5:431–6. [DOI] [PubMed] [Google Scholar]
- 22. Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41:856–62. [DOI] [PubMed] [Google Scholar]
- 23. Kamolz LP, Kitzinger HB, Karle B, Frey M. The treatment of hand burns. Burns 2009;3:327–37. [DOI] [PubMed] [Google Scholar]
- 24. Schwarze H, Küntscher M, Uhlig C, Hierlemann H, Prantl L, Ottomann C, Hartmann B. Suprathel, a new skin substitute, in the management of partial‐thickness burn wounds: results of a clinical study. Ann Plast Surg 2008;60:181–5. [DOI] [PubMed] [Google Scholar]
- 25. Keck M, Selig HF, Lumenta DB, Kamolz LP, Mittlböck M, Frey M. The use of Suprathel(®) in deep dermal burns: first results of a prospective study. Burns 2012;38:388–95. [DOI] [PubMed] [Google Scholar]
- 26. Uhlig C, Rapp M, Hartmann B, Hierlemann H, Planck H, Dittel KK. Suprathel‐an innovative, resorbable skin substitute for the treatment of burn victims. Burns 2007;33:221–9. [DOI] [PubMed] [Google Scholar]
- 27. Whitaker IS, Prowse S, Potokar TS. A critical evaluation of the use of Biobrane as a biologic skin substitute: a versatile tool for the plastic and reconstructive surgeon. Ann Plast Surg 2008;60:333–7. [DOI] [PubMed] [Google Scholar]
- 28. Greenwood JE, Clausen J, Kavanagh S. Experience with biobrane: uses and caveats for success. Eplasty 2009;9:e25. [PMC free article] [PubMed] [Google Scholar]
- 29. Lal S, Barrow RE, Wolf SE, Chinkes DL, Hart DW, Heggers JP, Herndon DN. Biobrane improves wound healing in burned children without increased risk of infection. Shock 2000;14:314–8; discussion 318–9. [DOI] [PubMed] [Google Scholar]
- 30. Hubik DJ, Wasiak J, Paul E, Cleland H. Biobrane: a retrospective analysis of outcomes at a specialist adult burns centre. Burns 2011;37:594–600. [DOI] [PubMed] [Google Scholar]
- 31. Peck MD, Kessler M, Meyer AA, Bonham Morris PA. A trial of the effectiveness of artificial dermis in the treatment of patients with burns greater than 45% total body surface area. J Trauma 2002;52:971–8. [DOI] [PubMed] [Google Scholar]
- 32. Bargues L, Boyer S, Leclerc T, Duhamel P, Bey E. Incidence and microbiology of infectious complications with the use of artificial skin Integra in burns. Ann Chir Plast Esthet 2009;54:533–9. [DOI] [PubMed] [Google Scholar]
- 33. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–62. [DOI] [PubMed] [Google Scholar]
- 34. Marie Brown‐Etris RN, CWOCN , Cutshall WD, Hiles MC. A new biomaterial derived from small intestine submucosa and developed into a wound matrix device. Wounds 2002;14:1–7. [Google Scholar]
- 35. OASIS Wound Matrix . Healthpoint Biotherapeutics. [internet] 1 p. URL http://www.OASISwoundmatrix.com/home [accessed on 3 November 2011].
- 36. Gallagher G, Rae CP, Kinsella J. Treatment of pain in severe burns. Am J Clin Dermatol 2000;1:329–35. [DOI] [PubMed] [Google Scholar]
- 37. Orhan ME, Sencan A. Pain treatment in burn patients. Agri 2004;16:9–16. [PubMed] [Google Scholar]
- 38. Connor‐Ballard PA. Understanding and managing burn pain: part 2. Am J Nurs 2009;109:54–62. [DOI] [PubMed] [Google Scholar]
- 39. Connor‐Ballard PA. Understanding and managing burn pain: part 1. Am J Nurs 2009;109:48–56. [DOI] [PubMed] [Google Scholar]
- 40. Demling RH. Burns: what are the pharmacological treatment options? Expert Opin Pharmacother 2008;9:1895–908. [DOI] [PubMed] [Google Scholar]


