Abstract
The inflammatory phase of wound healing cascade is an important determinant of the fate of the wound. Acute inflammation is necessary to initiate proper wound healing, while chronic inflammation abrogates wound healing. Different endosomal members of toll‐like receptor (TLR) family initiate inflammatory signalling via a range of different inflammatory mediators such as interferons, internal tissue damaged‐associated molecular patterns (DAMPs) and hyperactive effector T cells. Sustained signalling of TLR9 and TLR7 contributes to chronic inflammation by activating the plasmacytoid dendritic cells. Diabetic wounds are also characterised by sustained inflammatory phase. The objective of this study was to analyse the differential expression of endosomal TLRs in human diabetic wounds compared with control wounds. We analysed the differential expression of TLR7 and TLR9 both at transcriptional and translational levels in wounds of 84 patients with type 2 diabetes mellitus (T2DM) and 6 control subjects without diabetes using quantitative real‐time polymerase chain reaction (RT‐PCR), western blot and immunohistochemistry. TLR7 and TLR9 were significantly up‐regulated in wounds of the patients with T2DM compared with the controls and were dependent on the infection status of the diabetic wounds, and wounds with microbial infection exhibited lower expression levels of endosomal TLRs. Altered endosomal TLR expression in T2DM subjects might be associated with wound healing impairment.
Keywords: Diabetic foot ulcer, T2DM, TLR7, TLR9, Toll‐like receptors, Wound healing impairment
Introduction
Wound healing is a dynamic process in which cells, tissues and structure damaged by any injury are restored. A wound healing cascade requires a perfect orchestrated system for the proper healing of wounds 1. Normal wound healing process is divided into four overlapping stages starting from (i) clot formation, via (ii) acute inflammation, (iii) proliferation stage and finally (iv) remodelling of the newly laid matrix 2. Each of these steps also requires a well‐coordinated interaction of growth factors, cytokines, proinflammatory molecules, vasculature‐promoting agents and components of extracellular matrix 3.
The inflammatory phase of wound healing is a vital phase in which pathogenic microbes are encountered along with repairing the tissue. But this inflammatory phase should be short, self‐resolving and perfectly controlled. Prolonged inflammatory stage may lead to permanent residence of neutrophils and macrophages in the wound microenvironment, thereby abrogating the normal healing and finally transforming the wound into non‐healing chronic ulcers 4.
The involvement of the immune mechanisms also plays an important part in the process of wound healing. The members of innate immune system by their proinflammatory roles serve to eliminate infectious agents in the wound microenvironment 5. The effectors of the innate immune system identify the unique structures on microbes by their Pattern Recognising Receptors (PRRs). Toll‐like receptors (TLRs) are one of these PRRs that recognise the pathogen‐associated molecular patterns on the microbes and hence help in the activation of the immune system 6. All of the 12 members of TLR family known in mice contribute in initiating antimicrobial defence system. The TLRs generally mount an inflammatory response, which is protective in nature. However, over‐expression of TLRs can result in inflammatory diseases such as systemic lupus erythematosus (SLE), atherosclerosis, type 1 diabetes mellitus (T1DM), multiple sclerosis and rheumatoid arthritis 7. The TLRs are classified into two distinct groups on the basis of their cellular distribution and ligand repertoire. External TLRs 1, 2, 4, 5 and 6 are located on the cell surface and recognise ligands of mainly bacterial and fungal origin. Internal members of TLR family (TLR3, 7, 8 and 9) are localised to endosomes where their primary function is to sense microbial and host‐derived nucleic acids. Recently, endosomal TLRs have been shown to be potential contributors to the inflammation in different human studies and rodent models 8.
TLR9 resides in the endosomal compartment of the cell and it recognises unmethylated CpG residues in the DNA taken up via the process of endocytosis 9. Then, in the endolysosome, the ecto domain of TLR9 is cleaved to produce a functional receptor, which allows the recruitment of their adaptor molecules and the activation of NF‐κB or interferon (IFN) 10. This proteolytic cleavage of TLR9 is a prerequisite for its activation, as after cleavage, one of its fragments binds with Myd88 to induce downstream signalling in a wide variety of cells. TLR9 is expressed primarily by subsets of B cells and dendritic cells in humans and is involved in systemic as well as local inflammatory responses 11. It is also shown to be a negative regulator of wound healing. The stimulation of TLR9 may result in triggering extensive neurotoxicity, thereby damaging the neurons 12. It also serves as a sensor for endogenous ligands released during the process of tissue necrosis. TLR9 molecule is also shown to increase liver ischaemia‐reperfusion injury 13. TLR7 is homologous to TLR9 and takes part in the discrimination of nucleic acid–like structures in microorganisms. TLR7 recognises guanosine‐ or uridine‐rich single‐stranded RNA (ssRNA) from viruses and helps the host in dealing with viral infection. Human plasmacytoid dendritic cells possess high levels of TLR7 along with TLR9, which on activation release a wave of type I and type III IFNs, tumour necrosis factor‐α (TNF‐α) and interleukin‐6, and, hence promote inflammation by Th1 polarisation 11.
Type 2 diabetes mellitus (T2DM) has its role to play in each and every step of wound healing as persistent hyperglycaemic condition generally increases the inflammation through activation of TLRs and results in the spillage of the inflammatory phase molecules into the proliferation phase 14, 15. Our group has recently shown that wounds of T2DM patients exhibit differential expression of TLR4 along with other genetic alterations leading to impairment in the wound healing cascade 16, 17, 18. Recent studies have shown the pivotal role of endosomal TLRs in the development of secondary complications of T2DM because of their proinflammatory nature 19. Taking all these facts together, we hypothesised that altered expression levels of these endosomal members of the TLR family may be one of the causes of persistent inflammatory phase in chronic wounds like diabetic foot ulcer (DFU) and may contribute to the impaired wound healing in T2DM patients.
Materials and methods
Subjects
A total of 90 lower extremity wounds of different grades were analysed in which 84 were DFU cases and 6 were controls without having T2DM. Samples were collected from the outpatient department clinics and the operation theatres of the Department of Endocrinology and Metabolism and the Department of Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Patients were advised to undergo a standardised clinical and laboratory evaluation (Table 1). Family history, habits (smoking, alcoholism, etc.) and disease status of each patient were recorded through a questionnaire. All the DFU patients included were diabetic patients who had non‐healing wounds of >4 weeks duration, thus qualifying as diabetic wounds. Majority of the patients had lower extremity wounds, 90% of which were located on the foot alone and the remaining 10% on the foot + lower leg. Both the plantar and the dorsal aspects of the foot were involved in majority of the cases. The samples were collected at the time of their (the patients) first visit to the diabetic foot clinic. Samples were taken from the wound margins during the debridement process and histological analysis was performed to determine the cell types. Tissue samples were collected in RNAlater solution (P/N AM7020, Ambion, Inc., Austin, TX) and phosphate‐buffered saline (PBS) for isolation of RNA and DNA, respectively, and kept frozen at −80°C until use. For immunohistochemical staining, the samples were collected in Bouin's fixative solution and kept at room temperature. Biochemical markers, such as serum creatinine and cholesterol levels, were measured using biochemical Autoanalyzer (Beckman Coulter) at the clinical laboratory of the Department of Endocrinology and Metabolism. T2DM patients were diagnosed on the basis of the World Health Organization (WHO) criteria, that is fasting plasma glucose ≥126 mg/dl and 2 hour plasma glucose ≥200 mg/dl. Poor glycaemic control was assessed by measuring the glycated hemoglobin A1c (HbA1C) levels. The mean HbA1c levels of DFU subjects were 10·6% (range 8·7–13%). Screening for neuropathy was performed by taking a history of sensory loss and other symptoms such as burning sensation or paraesthesias. The clinical neurological examination included assessment of the vibratory perception threshold using a 128 Hz tuning fork and assessment of pain and fine touch with a pin and 10 g monofilament, respectively. The tendon reflexes and muscle power were measured in patients with sensory neuropathy. Screening for vascular involvement included detailed history of vascular insufficiency, clinical examination for signs of chronic ischaemia and assessment of all lower limb pulses. A bedside hand‐held Doppler study was carried out in all the clinically suspicious cases and ABPI (ankle brachial pressure index) of <0·9 was considered indicative of peripheral vascular disease. Classification of wounds was performed on the basis of the Wagner Grading System 20. The infection status of the wounds was also taken into account. The study was approved by the Institutional Human Ethics Committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Informed written consent was obtained from every participant of each group.
Table 1.
Biochemical and demographic parameters of DFU patients (N = 84)*
| Parameters | DFU (N = 84) |
|---|---|
| Average age | 53·62 ± 9·20 years |
| Average BMI (kg/m2) | 21·54 ± 2·81 |
| Average duration of type 2 diabetes in years | 10·26 ± 4·5 |
| Male | 59 (70·23%) |
| Female | 25 (29·76%) |
| HbA1c levels (%) (mean, range) | 10·6 (8·7–13) |
| Family history present (N) (%) | 12 (14·2) |
| Nephropathy present (serum creatinine >1·4 mg/dl) (N) (%) | 25 (29·76) |
| Neuropathy present (by 10 g monofilament test) (N) (%) | 50 (59·53) |
| Hypertension present (systolic BP > 140 mm of Hg) (N) (%) | 27 (32·14) |
| Retinopathy present (N) (%) | 13 (15·47) |
| Dyslipidaemia present (serum cholesterol and triglyceride levels > 200 mg/dl) (N) (%) | 16 (19·04) |
| Infection present (wound culture positive for microbes) (N) (%) | 45 (53·57) |
| Bone involvement (osteomyelitis) (N) (%) | 29 (34·52) |
BMI, body mass index; DFU, diabetic foot ulcer.
Data are presented as mean ± SD or as number (percentage).
Quantitative real‐time polymerase chain reaction
Total RNA was isolated from wound biopsies using TRIzol reagent followed by DNase treatment. cDNA was synthesised and quantitative real‐time polymerase chain reaction (RT‐PCR) analysis of TLR9 and TLR7 was performed in 84 DFU cases and 6 controls according to the manufacturer's protocol (Applied Biosystems, Carlsbad, CA) using previously described primers for TLR9, TLR7 and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) 21. For TLR9 expression, PCR was performed with forward primer 5′‐GGACCTCTGGTACTGCTTCCA‐3′ and the reverse primer 5′‐AAGCTCGTTGTACACCCAGTCT‐3′, resulting in 151 bp fragment. For TLR7 expression, PCR was performed with forward primer 5′‐TCTCATGCTCTGCTCTCTTCAAC‐3′ and the reverse primer 5′‐TTGTCTCTTCAGTGTCCACATTGGAAA‐3′, resulting in 92 bp fragment. Briefly, 20 µl total reaction volume containing 10 µl SYBR Green, 0·1 µl each of the forward and reverse primers (10 pm/µl) and 2 µl cDNA was used in PCR using ABI 7500 instrument (Applied Biosystems). PCR was performed with an initial incubation at 50°C for 2 minutes, then followed by 10 minutes denaturation at 95°C and 40 cycles at 95°C for 15 seconds, 60°C for 1 minute and 72°C for 1 second. Gene expression data of these TLRs were normalised to the mRNA levels of housekeeping gene GAPDH. Primers used for GAPDH were GAPDH forward 5′‐CATGAGAAGTATGACAACAGC‐3′ and GAPDH reverse 5′‐AGTCCTTCCACGATACCAAAG‐3′.
Immunohistochemical staining
Wound tissue samples obtained during debridement process were fixed in Bouin's solution, embedded in paraffin and sectioned into 3 µm thick sections. Five DFU wounds and five control wounds were randomly selected for the study. Anti‐TLR9 antibody (clone 26C593, Catalog No. ab12121, Abcam, Inc., Cambridge, MA; 1:75 in PBS) and anti‐TLR7 antibody (Catalog No. IMG‐6070A, Imgenex Corp., San Diego, CA; 1:75 in PBS) were applied separately to the deparaffinised sections and incubated in a wet chamber at 4°C for 12 hours. Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) was used for immunohistochemical staining. Slides were counterstained with haematoxylin (Himedia, Mumbai, India). Cells having brown‐stained cytoplasm were regarded as positive. Similar staining time and procedure were adopted for all the tissue samples. Expression patterns of TLR7 and TLR9 in control and diabetic wounds were observed under the microscope (Nikon, New York, NY) using different magnifications (4, 10, 20 and 40). Documentation of the acquired images was performed using a calibrated digital camera system (Nikon eclipse 80i) together with the software evaluation package (NIS Elements software, Nikon). The expression densities of TLR7 and TLR9 in wound biopsies were computed according to a previous study by Souil et al. 22
Western blot
Western blot analysis was performed for TLR9 and TLR7 on whole tissue extracts of wound biopsies (43 DFU cases and 6 controls) to verify the results of immunohistochemistry (IHC). About 50 µg of protein was loaded on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) gel, transferred to nitrocellulose membrane and then blocked with 5% of skimmed milk in Tris‐buffered saline. For TLR9, mouse monoclonal anti‐TLR9 antibody (clone 26C593, Catalog No. ab12121, Abcam Inc., Cambridge, MA; 1:1000 dilution), and for TLR7, rabbit polyclonal anti‐TLR7 antibody (Catalog No. IMG‐6070A, Imgenex Corp.; 1:1000 dilution) were used and then incubated with the secondary antibody linked to horseradish peroxidase. The immunoreactive bands were visualised by the enhanced chemiluminescence system (Amersham Biosciences, Pittsburgh, PA). Blots were stripped off and re‐probed with an anti‐GAPDH antibody. Alpha Imager 2200 software version 3.1.2 was used to quantify band density.
Statistical analysis
The data were expressed as mean by considering standard error of mean as error bars. Statistical significance (P < 0·05) was determined with Student's t‐test (two‐tailed) and non‐parametric ANOVA. Statistical analysis of data was performed using GraphPad Prism 5.01 and IBM SPSS Statistics 20.0 software.
Results
Diabetic wounds generally exhibit unresolved chronic inflammation because of the generation of a plethora of endogenous tissue damage products 23. Endosomal TLRs detect these damage‐associated molecular products (DAMPs), and after activation, they induce strong inflammatory response mediated by NF‐κB or TNF 24. Hence, we hypothesised that the persistent hyperglycaemia and tissue damage in T2DM patients may lead to chronic inflammation through activation of endosomal TLRs such as TLR7 and TLR9. We analysed the differential expression of TLR7 and TLR9 both at transcriptional and translational levels in wounds of 84 T2DM patients and 6 control subjects without diabetes using the quantitative RT‐PCR, western blot and IHC. The analysis of quantitative RT‐PCR data indicated that the expression of mRNA of TLR9 and TLR7 was significantly up‐regulated in the lower extremity wounds of DFU patients compared with that of the control wounds (P value = 0·04, mean fold change = 0·98, standard error of mean = 0·11 for TLR9 and P value = 0·01, mean fold change = 1·18, standard error of mean = 0·16 for TLR7) (Figure 1). To evaluate if the endosomal TLR expression was influenced by wound grade, infection status or gender of the subject, the studied endosomal TLR transcripts were compared between different subjects. There was no significant correlation of TLR7 or TLR9 transcripts with the wound grade (P value > 0·05), although the expression of all the endosomal TLR mRNAs was found to decrease drastically in the most severe grade wounds on Wagner's scale or with the infection (Figure 2). The transcript levels of endosomal TLRs were higher in males compared with females (TLR7: males = 0·61 ± 0·22, females = 0·12 ± 0·38; TLR9: males = 0·66 ± 0·16, females = 0·42 ± 0·27) (Figure 3). The infection status of the wound was also a regulatory factor for the expression of endosomal TLRs, and all the endosomal TLRs were found to decrease significantly in the presence of infection (TLR7: P value = 0·02, t = 2·34, R 2 = 0·06; TLR9: P value = 0·01, t = 2·58, R 2 = 0·07) (Figure 4). To determine if the difference in the messages of TLR7 and TLR9 translates to the similar difference in protein, five DFU and five control samples were analysed by IHC using antibodies against TLR7 and TLR9. Immunohistochemical expression analysis among the groups also suggested significant difference of TLR9 and TLR7 between the wound biopsies of DFU cases and controls (Figures 5 and 6). The findings of IHC results were validated by western blot experiments using antibodies against TLR7 and TLR9 (Figure 7). The similar up‐regulation of TLR7 and TLR9 proteins were found in the western blot analysis also (P value ≤ 0·001, t = 4·20 for TLR7 and P value ≤ 0·001, t = 4·27 for TLR9). We also found that TLR9 protein was truncated in the case of DFU patients and was present in three fragments, while the TLR9 protein was intact in the wound samples of non‐diabetic wounds (Figure 8).
Figure 1.
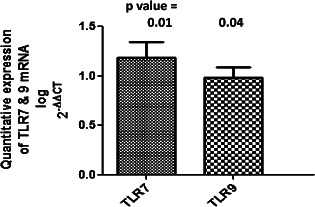
Bar graph of quantitative real‐time polymerase chain reaction (RT‐PCR) analysis showing the higher expression of mRNA of TLR7 and TLR9 in the wounds of 84 diabetic foot ulcer (DFU) patients compared with 6 controls. Fold change in the expression of genes was determined using the ΔΔCT method of relative quantification. Firstly, normalisation of the resulting threshold cycle (CT) values of the target gene was performed with the CT values of the internal control GAPDH in the same samples (ΔCT = CT target − CT GAPDH). It was again normalised with the control (ΔΔCT = ΔCT − ΔCT control). The fold change in the expression was then calculated (2−ΔΔCT). The graph was plotted using log 2−ΔΔCT. The graph clearly show that TLR9 and TLR7 were up‐regulated significantly in the wounds of type 2 diabetes mellitus (T2DM) cases (P value = 0·04, mean log of fold change = 0·98, standard error of mean = 0·11 for TLR9 and P value = 0·01, mean log of fold change = 1·18, standard error of mean = 0·16 for TLR7). [Log (fold change 10) = 1].
Figure 2.
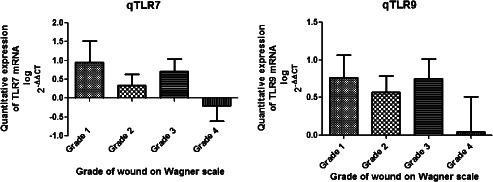
Bar graph showing the comparison of quantitative mRNA expression of endosomal toll‐like receptors (TLRs) with wound grades on Wagner's scale. Non‐parametric one‐way ANOVA was used to check the difference between the mean values of endosomal TLR mRNAs among different wound groups using GraphPad Prism. The graph clearly suggests the reduction of the expression of endosomal TLRs in the higher grade 4 wounds of diabetic cases.
Figure 3.
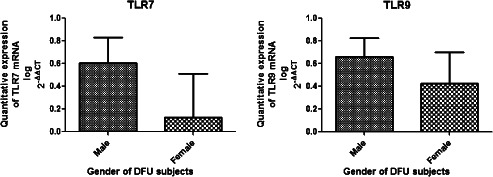
Bar graph showing the comparison of quantitative mRNA expression of endosomal toll‐like receptors (TLRs) with the gender of diabetic foot ulcer (DFU) patients. Unpaired t‐test was used to check the difference between the mean values of endosomal TLR mRNAs in male and female subjects using GraphPad Prism. The analysis showed that the transcript levels of endosomal TLRs were higher in males compared with females (TLR7: males = 0·61 ± 0·22, females = 0·12 ± 0·38; TLR9: males = 0·66 ± 0·16, females = 0·42 ± 0·27).
Figure 4.
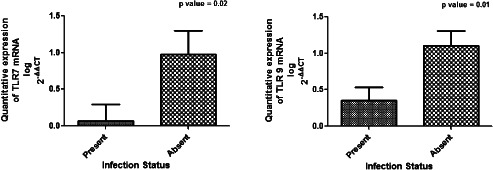
Bar graph showing the comparison of quantitative mRNA expression of endosomal toll‐like receptors (TLRs) with the infection status of the wounds of the diabetic foot ulcer (DFU) subjects. Unpaired t‐test was used to check the difference between the mean values of transcripts of endosomal TLRs. The analysis of the data showed that all the endosomal TLRs decreased significantly in the presence of infection (TLR7: P value = 0·02, t = 2·34, R 2 = 0·06; TLR9: P value = 0·01, t = 2·58, R 2 = 0·07).
Figure 5.
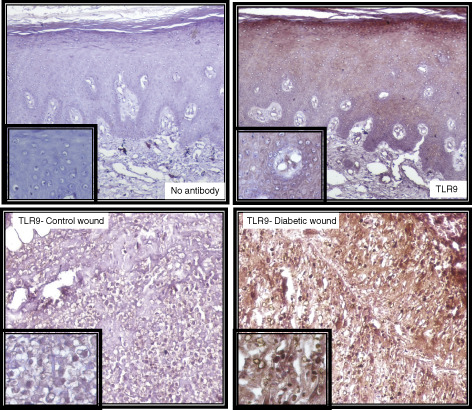
Immunohistochemistry of TLR9 in wound samples (3‐µm‐thick dewaxed paraffin sections) with insets showing detail of staining (40× magnifications). Upper panel shows the negative (left) and positive (right) staining. In the negative control, serum and secondary antibodies were applied but no primary TLR9 antibody was added to the staining solution to check the non‐specific binding of the primary antibody. Lower panel shows the immunohistochemistry for TLR9 in non‐diabetic control wound (left) and diabetic wound (right).
Figure 6.
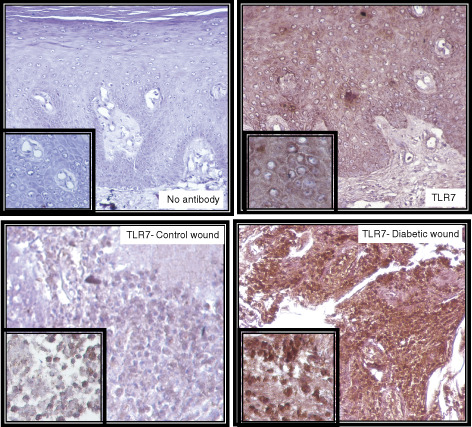
Immunohistochemistry of TLR7 in wound samples was carried out in a similar fashion to that of TLR9. Upper panel shows the negative (left) and positive (right) staining. In the negative control, serum and secondary antibodies were applied but no primary TLR7 antibody was added to the staining solution to check the non‐specific binding of the primary antibody. Lower panel shows the immunohistochemistry for TLR7 in non‐diabetic control wound (left) and diabetic wound (right).
Figure 7.
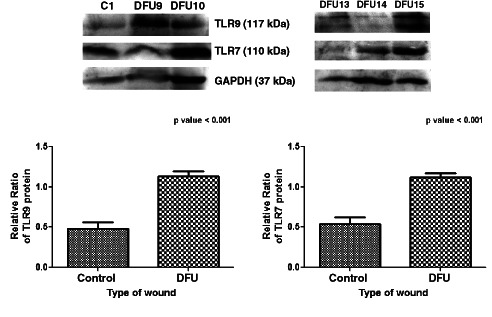
Western blot assay showing that protein expression of TLR9 and TLR7 was elevated in diabetic foot ulcer (DFU) patients. Tissue samples collected during debridement process from control (C) and DFU patients were homogenised, and Western blot analysis was performed for expression of TLR9, TLR7 and GAPDH proteins in 43 DFU subjects and 6 controls. Alpha Imager 2200 software version 3.1.2 was used to quantify band density. The relative percent ratios of proteins (toll‐like receptors, TLRs, versus GAPDH) were calculated. Unpaired t‐test was used to check the difference between the mean values of TLR7 and TLR9 proteins in DFU and control subjects using GraphPad Prism. A two‐tailed P value < 0·05 was considered as statistically significant. Bar graph showing up‐regulation of TLR7 and TLR9 proteins in wounds of type 2 diabetes mellitus (T2DM) patients compared with controls (P value ≤ 0·001, t = 4·20 for TLR7 and P value ≤ 0·001, t = 4·27 for TLR9).
Figure 8.
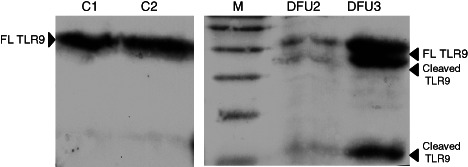
Lysate preparations of wound tissues taken from diabetic foot ulcer (DFU) and control subjects were separated by SDS–PAGE and probed with antibody specific to TLR9. Full‐length (FL TLR9) and the truncated protein (cleaved TLR9) are shown in the figure. It was interesting to note that this truncated version of the protein was common in the case of DFU compared with the control subjects (C).
Discussion
The diabetic wounds are generally chronic wounds characterised by sustained inflammatory phase. Persons with DFU often suffer from neuropathy in which the sensation power of neurons against external stimuli is either diminished or fully lost 25. The wound bed of a diabetic wound is overpopulated by several proinflammatory cells and inflammatory cytokines 26. The reason for this prolonged inflammation may be attributed to hyperglycaemia and endogenous ligands 27, 28. Recently, some members of the innate immunity have been identified to play an important role in the pathogenesis of abnormalities in healing of chronic wounds 29.
Inappropriate endosomal TLR signalling is shown to be involved in some chronic inflammatory diseases such as SLE, T1DM, multiple sclerosis, rheumatoid arthritis and hepatitis 30, 31, 32. The basis for this proinflammatory role is the ability of these endosomal TLRs to activate the plasmacytoid dendritic cells, conventional dendritic cells and B cells, thus producing IFN I and III, which add up to the inflammation 11. Activation of TLR9 and TLR7 on these dendritic cells suppresses the immunosuppressive, anti‐inflammatory Treg cells, thereby promoting inflammation 33. Duramad et al. and Walker et al. also supported the inflammatory nature of TLR9 and suggested that this molecule is involved in causing systemic inflammation in both mice and humans 34, 35. TLR9 when activated also plays an important role in the spontaneous onset of diabetes in non‐obese diabetic (NOD) mice by activating diabetogenic CD8+ T cells and up‐regulating IFN‐α production 36. TLR7 also adds up to the inflammation by induction of IFN I after interaction with their respective ligands polyriboinosinic polyribocytidylic acid [poly (I:C)] and ssRNA 37.
The expression levels of TLR7 and TLR9 were checked in the wounds of patients with T2DM. The up‐regulation of TLR9 and TLR7 both at the mRNA level and the protein level in the wounds of these patients compared with the wounds of non‐diabetic controls clearly suggests the detrimental role of excess TLR9 and TLR7 in the wound microenvironment. The increase in the expression of endosomal TLRs with the increase in the wound grade suggested that the expression levels of these TLRs directly modulate the severity of diabetic wounds. This finding may be supported by the neurotoxic effects of TLR9 and the neurodegenerative effect of TLR7 12, 38. The secretion of nerve growth factor by the neurons nearby into the wound milieu is important for healing of wounds 39, 40. In case of over‐expression of TLR9 and TLR7, there is extensive neurotoxicity, especially directed against neuritis mediated by downstream signalling molecules such as TNF‐α and NO 12. This endosomal TLR–mediated neurotoxicity may be a guiding force of neuropathic ulcers in T2DM cases, and hence, over‐expression of TLR7 and TLR9 in diabetic wounds may be one of the risk factors for the non‐healing nature of the ulcers.
The present study has some limitations as well. First, in our study, we could not perform proteomic analysis of all the 84 samples because of sample limitation (over‐degradation of wound tissue leading to smeared protein). In addition, the number of control samples was less compared with DFU cases, the reason being the unwillingness of controls to provide tissue biopsy because of pain and fear associated with the process.
In conclusion, we found that up‐regulation of TLR9 and TLR7 both at the mRNA and the protein levels in wounds of T2DM patients compared with the non‐diabetic patients may lead to an unresolved inflammatory response and ultimately to non‐healing chronic ulcers. Further research is required to see the effect of antagonists and agonists of these endosomal TLRs in diabetic wound healing models for proper therapeutic interventions.
Acknowledgements
We would like to acknowledge the real‐time PCR facility from the Interdisciplinary School of Life Science, Banaras Hindu University. We thank Nilu Prasad and Shanti Besra, Laboratory Superintendents, Indian Railway Cancer Hospital and Research Centre, N.E.R., Varanasi, for their technical assistance during IHC work. This work was funded by the Department of Science and Technology, New Delhi, India (P‐07/523). The financial assistance by the Department of Biotechnology, Ministry of Science and Technology, New Delhi, India, in the form of Senior Research Fellowship to the first author is gratefully acknowledged. The authors declare no conflicts of interest.
References
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1998;341:738–46. [DOI] [PubMed] [Google Scholar]
- 2. Calvin M. Cutaneous wound repair. Wounds 1998;10:12–32. [Google Scholar]
- 3. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 4. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361:1545–51. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Z, Schluesener HJ. Mammalian toll‐like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci 2006;63:2901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akira S, Takeda K. Toll‐like receptor signaling. Nat Rev Immunol 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 7. Stefan K, Drexler SK. The role of toll‐like receptors in chronic inflammation. Int J Biochem Cell Biol 2010;42:506–18. [DOI] [PubMed] [Google Scholar]
- 8. Alzabin S, Kong P, Medghalchi M, Palfreeman A, Williams R, Sacre S. Investigation of the role of endosomal toll‐like receptors in murine collagen‐induced arthritis reveals a potential role for TLR7 in disease maintenance. Arthritis Res Ther 2012;14:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004;5:190e8. [DOI] [PubMed] [Google Scholar]
- 10. Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of toll‐like receptor 9 is cleaved to generate a functional receptor. Nature 2008;456:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med 2010;207:2931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iliev AI, Stringaris AK, Nau R, Neumann H. Neuronal injury mediated via stimulation of microglial toll‐like receptor‐9 (TLR9). FASEB J 2004;18:412–4. [DOI] [PubMed] [Google Scholar]
- 13. Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll‐like receptor 9 inhibition confers protection from liver ischemia–reperfusion injury. Hepatology 2010;51:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh K, Agrawal NK, Gupta SK, Singh K. A functional SNP‐1562C > T in the matrix metalloproteinases‐9 promoter is associated with type 2 diabetes and diabetic foot ulcers. Int J Low Extrem Wounds 2013;12:199–204. [DOI] [PubMed] [Google Scholar]
- 15. Dasu MR, Ramirez S, Isseroff RR. Toll‐like receptors and diabetes: a therapeutic perspective. Clin Sci 2012;22:203–14. [DOI] [PubMed] [Google Scholar]
- 16. Kanhaiya S, Agrawal NK, Gupta SK, Singh K. Differential expression of toll like receptor 4 in type 2 diabetic patients with impaired wound healing. J Diabetes Metab 2013;4:260. [Google Scholar]
- 17. Singh K, Singh VK, Agrawal NK, Gupta SK, Singh K. Association of toll‐like receptor 4 polymorphisms with diabetic foot ulcers and application of artificial neural network in DFU risk assessment in type 2 diabetes patients. Biomed Res Int 2013;2013:318686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh K, Singh VK, Agrawal NK, Gupta SK, Singh K. Genetic alterations in toll‐like receptor 4 signaling pathway and impairment of wound healing in patients with type 2 diabetes. Int J Low Extrem Wounds 2014;13:162–3. [DOI] [PubMed] [Google Scholar]
- 19. Falck‐Hansen M, Kassiteridi C, Monaco C. Toll‐like receptors in atherosclerosis. Int J Mol Sci 2013;14:14008–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner FW. The dysvascular foot: a system of diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- 21. Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of toll‐like receptors 1–9 in the human term placenta and changes associated with labor at term. Biol Reprod 2009;80:243–8. [DOI] [PubMed] [Google Scholar]
- 22. Souil E, Capon A, Mordon S, Dinh‐Xuan AT, Polla BS, Bachelet M. Treatment with 815‐nm diode laser induces long‐lasting expression of 72‐kDa heat shock protein in normal rat skin. Br J Dermatol 2001;144:260–6. [DOI] [PubMed] [Google Scholar]
- 23. Hirabara SM, Gorjao R, Vinolo MA, Rodrigues AC, Nachbar RT, Curi R. Molecular targets related to inflammation and insulin resistance and potential interventions. J Biomed Biotechnol 2012;2012:379024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010;2010:672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reiber GE. The epidemiology of diabetic foot problems. Diabetes Med 1996;13(S1):S6–11. [PubMed] [Google Scholar]
- 26. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabetes Med 2006;23:594–608. [DOI] [PubMed] [Google Scholar]
- 27. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goova M , Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of RAGE restores effective wound healing in diabetic mice. Am J Pathol 2001;159:513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kluwe J, Mencin A, Schwabe RF. Toll‐like receptors, wound healing, and carcinogenesis. J Mol Med 2009;87:125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKelvey KJ, Highton J, Hessian PA. Cell‐specific expression of TLR9 isoforms in inflammation. J Autoimmun 2011;36:76–86. [DOI] [PubMed] [Google Scholar]
- 31. Xiao X, Zhao P, Rodriguez‐Pinto D, Qi D, Henegariu O, Alexopoulou L, Flavell RA, Wong FS, Wen L. Inflammatory regulation by TLR3 in acute hepatitis. J Immunol 2009;183:3712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marshak‐Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll‐like receptors in the development of chronic inflammatory disease. Annu Rev Immunol 2007;25:419–41. [DOI] [PubMed] [Google Scholar]
- 33. Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 2008;29:637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duramad O, Fearon KL, Chang B, Chan JH, Gregorio J, Coffman RL, Barrat FJ. Inhibitors of TLR‐9 act on multiple cell subsets in mouse and man in vitro and prevent death in vivo from systemic inflammation. J Immunol 2005;174:5193–200. [DOI] [PubMed] [Google Scholar]
- 35. Walker WE, Booth CJ, Goldstein DR. TLR9 and IRF3 cooperate to induce a systemic inflammatory response in mice injected with liposome: DNA. Mol Ther 2010;18:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Lee AS, Shameli A, Geng X, Finegood D, Santamaria P, Dutz JP. TLR9 blockade inhibits activation of diabetogenic CD8+ T cells and delays autoimmune diabetes. J Immunol 2010;184:5645–53. [DOI] [PubMed] [Google Scholar]
- 37. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 38. Liu HY, Hong YF, Huang CM, Chen CY, Huang TN, Hsueh YP. TLR7 negatively regulates dendrite outgrowth through the Myd88–c‐Fos–IL‐6 pathway. J Neurosci 2013;33:11479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pradhan L, Andersen ND, LoGerfo FW, Veves A. Molecular targets for promoting wound healing in diabetes. Recent Pat Endocr Metab Immune Drug Discov 2007;1:1–13. [Google Scholar]
- 40. Dasu MR, Isseroff RR. Toll‐like receptors in wound healing: location, accessibility, and timing. J Invest Dermatol 2012;132:1955–8. [DOI] [PubMed] [Google Scholar]


