Abstract
Vacuum‐assisted closure (VAC) therapy is a new emerging non‐invasive system in wound care, which speeds up wound healing by causing vacuum, improving tissue perfusion and suctioning the exudates, and facilitating the removal of bacteria from the wound. The application of sub‐atmospheric pressure on the lesions seems to alter the cytoskeleton of the cells on the wound bed, triggering a cascade of intracellular signals that increase the rate of cell division and subsequent formation of granulation tissue. The aim of this study is to analyse the results of VAC therapy used as an adjuvant therapy for the treatment of foot wounds in patients affected by critical limb ischaemia (CLI) (Rutherford 6 class) after distal surgical revascularisation, to promote and accelerate the healing of ulcers. Twenty‐nine patients (20 males, 9 females; mean age 68·4) affected by CLI of Rutherford 6 class, after surgical revascularisation of the lower limb, underwent VAC therapy in order to speed up wound healing. Complete wound healing was achieved in 19 patients (65·51%), in an average period of 45·4 ± 25·6 days. VAC therapy is a valid aid, after surgical revascularisation, to achieve rapid healing of foot lesions in patients with CLI.
Keywords: Critical lower limb ischaemia, Vacuum‐assisted closure therapy, Wound healing
Introduction
Critical lower limb ischaemia (CLI) is a widespread disease, and foot ulcers in patients with Rutherford 6 lesions represent a serious problem, with high levels of morbidity, long hospital stay and high costs. Current methods of treatment are represented by endovascular and surgical revascularisation 1, 2 in association with specific antibiotic therapy for infected lesions, repeated local debridement, advanced moist wound dressing 3, bioengineered tissue or skin substitutes 4, growth factors 5, 6 and spinal cord stimulation 7, 8, 9. Vacuum‐assisted closure (VAC) therapy represents a new emerging non‐invasive system for wounds healing, based on localised delivery of continuous negative sub‐atmospheric pressure through a pump which is connected to the resilient, foam‐surface dressing that collects the wound exudates.
Our experience in treatment of foot wounds with VAC therapy in patients affected by CLI with Rutherford 6 lesions, after distal surgical revascularisation, is reported.
Materials and methods
From January 2012 to July 2013, the VAC system was used in 29 patients (20 males, 9 females; mean age 68·4) affected by peripheral arterial disease of Rutherford 6 class, after surgical revascularisation of the lower limb, as an adjuvant therapy to accelerate wound healing. Institutional Review Board (IRB) approval was obtained.
The enrolled patients presented the following risk factors and comorbidities: diabetes mellitus (22, 75·9%), hypertension (19, 65·5%), dyslipidemia (16, 55·2%), heart failure (17, 58·6%), chronic obstructive pulmonary disease (11, 37·9%), smoke (14, 48·3%) and end‐stage renal disease on haemodialysis (8, 27·6%). One patient underwent an axillo‐bifemoral bypass in polytetrafluoroethylene (PTFE) 8 mm; three patients underwent a popliteal–posterior tibial artery bypass in great saphenous vein (GSV); the remaining 25 patients were submitted to a femoro‐distal bypass in GSV (eight femoro‐peroneal artery bypass, eight femoro‐anterior tibial artery bypass, two femoro‐pedideal artery and seven femoro‐posterior tibial artery).
After surgical revascularisation of the lower limb, 12 patients underwent an open transmetatarsal amputation (Figure 1), 9 patients underwent minor amputations, 8 patients were submitted to surgical debridement of the calcaneal lesion.
Figure 1.
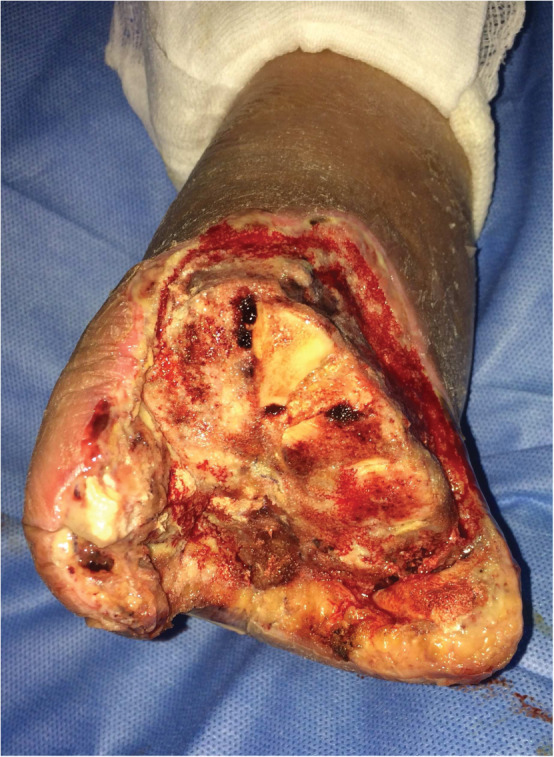
open transmetatarsal amputation.
Selection criteria for the application of VAC therapy were represented by calcaneal, dorsal or plantar foot ulcers with an area of >3 cm2; adequate distal blood flow assessed by ultrasonographic examination after revascularisation; absence of granulation tissue 7 days after surgery.
VAC therapy was applied following the first debridement and washing of wounds in patients with calcaneal lesions (8 cases), after minor amputations (9 cases) or open transmetatarsal amputation (12 cases) (Figure 2). A sub‐atmospheric localised pressure was applied on the wound, in a controlled manner: the system unit is programmed to deliver controlled negative pressure ranging from 50 to 200 mmHg. The sub‐atmospheric pressure generally applied is 100–125 mmHg. Suction effect, generated by a portable, adjustable pump, was applied on the wound cleaned by a sponge made of polyurethane or polyvinyl alcohol. These sponges were closed with an adhesive drape to obtain a sealed environment.
Figure 2.
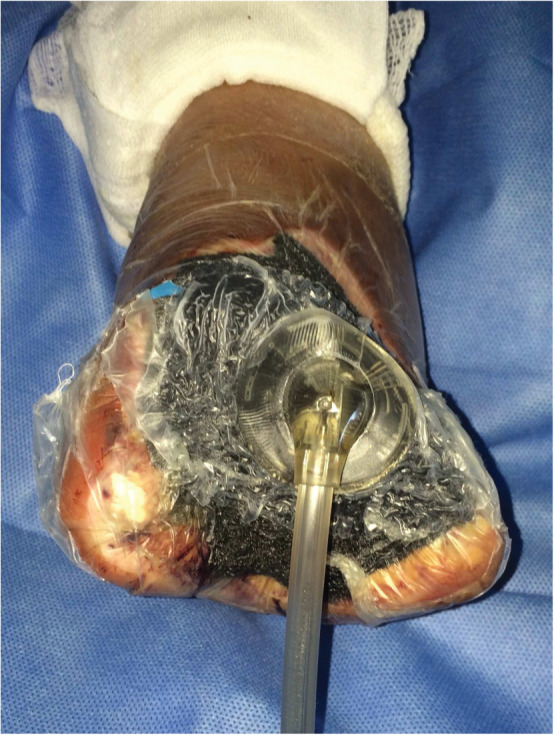
Application of VAC therapy on open transmetatarsal amputation.
Between the drape and the device, an electrical pump is connected to a canister, which collects the wound exudate, using a flexible pipe. The polyurethane sponge has pore sizes ranging from 400 to 600 µm. The polyvinyl alcohol sponge has pore sizes ranging from 200 to 300 µm 10, 11.
A specific antibiotic therapy in accordance with the antibiogram results was started in all patients. In accordance with the severity of the wound, patients were exposed to a continuous negative pressure ranging from −75 to −125 mmHg. Patients with severely infected wounds with discharge and necrosis were submitted to wound culture and subsequently to specific antibiotic therapy in accordance with the antibiogram results. All data for the enrolled patients are summarised in Table 1.
Table 1.
Summary of 29 patients enrolled in this study
| Age | Sex | Main disease | Size and localisation of the wound (mm) | Surgical treatment | Isolated germ | Antibiotic therapy | Time of hospital stay | Results |
|---|---|---|---|---|---|---|---|---|
| 46 | M | DM, Hypertension, smoke, dyslipidemia, heart failure | Left heel 70 × 70 | Femoro‐peroneal artery bp in GSV; surgical debridement of heel lesion | Enterococcus faecalis; Escherichia coli | Meropenem | 45 |
Hyperbaric oxygen therapy; healed |
| 63 | M | DM, dyslipidemia, heart failure | Right heel 30 × 35 | Femoro‐anterior tibial artery bp in GSV; surgical debridement of heel lesion | No bacteria | Cefazolin | 15 | Lost during follow‐up |
| 68 | M | Hypertension, heart failure, smoke, dyslipidemia | Necrosis of the last three toes of the right foot | Femoro‐anterior tibial artery bp in GSV; amputation of the last three toes of the right foot | Staphylococcus epidermidis | Imipenem; trimethoprim sulfamethoxazole | 24 |
Bypass occlusion and restoration Transmetatarsal amputation; healed |
| 81 | M | Hypertension, DM, haemodialysis, dyslipidemia, heart failure | Necrosis of the last two toes of the right foot | Femoro‐pedideal artery bp in GSV; amputation of the last three toes of the right foot | Pseudomonas aeruginosa; Staphylococcus aureus | Vancomycin; imipenem | 16 | Healed |
| 76 | F | Hypertension, DM, dyslipidemia, heart failure | Left heel 40 × 30 | Femoro‐posterior tibial artery bp in GSV; surgical debridement of the left calcaneal lesion | E. faecalis; Morganella morganii | Piperacillin tazobactam; ciprofloxacin | 21 | Healed |
| 72 | M | Hypertension, DM, haemodialysis, heart failure, dyslipidemia | Gangrene of all toes of left foot | Femoro‐anterior tibial artery bp in GSV; open transmetatarsal amputation | S. aureus | Cefazolin | 47 | Healed |
| 57 | F | Hypertension, heart failure, smoke | Gangrene of the first and second toes of the right foot | Popliteal–posterior tibial artery bp in GSV; right first and second toes amputation | No bacteria | Cefazolin | 14 |
Transmetatrsal amputation; healed |
| 88 | M | DM, hypertension, COPD, smoke, heart failure, dyslipidemia | Gangrene of all toes of the left foot | Femoro‐peroneal artery bp in GSV; open transmetatarsal amputation of the left foot | S. epidermidis | Imipenem; trimethoprim sulfamethoxazole | 23 | Healed |
| 72 | M | DM, hypertension, heart failure, smoke | Gangrene of the left first toe | Popliteal–posterior tibial artery bp in GSV; left first toe amputation | P. aeruginosa | Meropenem | 19 |
Transmetatarsal amputation; healed |
| 78 | M | DM, hypertension, COPD | Gangrene of all toes of the right foot | Femoro‐peroneal artery bp in GSV; open transmetatarsal amputation | P. aeruginosa; E. faecalis | Amikacin; ampicillin–sulbactam | 24 | Healed |
| 68 | M | DM, smoke, heart failure | Gangrene of the first and second toes of the left foot | Femoro‐peroneal artery bp in GSV; amputation of the first and second left toes | No bacteria | Cefazolin | 14 | Lost during follow‐up |
| 66 | M | DM, hypertension, smoke | Gangrene of all toes of the left foot | Femoro‐posterior tibial artery bp in GSV; open transmetatarsal amputation | S. epidermidis | Imipenem; trimethoprim sulfamethoxazole | 24 | Healed |
| 58 | M | DM, haemodialysis, hypertension | Calcaneal lesion 50 × 40 | Femoro‐peroneal artery bp in GSV; surgical debridement of the calcaneal lesion | No bacteria | Cefazolin | 21 | Healed |
| 66 | M | Hypertension, heart failure, smoke, dyslipidemia, haemodialysis | Calcaneal right lesion 60 × 70 | Femoro‐posterior tibial artery bp; open transmetatarsal amputation | P. aeruginosa | Meropenem | 10 |
Bypass occlusion; above‐the‐knee amputation |
| 69 | M | Haemodialysis, DM, heart failure | Gangrene of the last three toes of the left foot | Femoro‐pedideal artery bp in GSV; amputation of the last three toes | S. aureus; E. faecalis | Rifampicin; doxiciclin | 20 | Healed |
| 68 | F | Hypertension, COPD, smoke, haemodialysis | Gangrene of all toes of the right foot | Femoro‐peroneal artery bp in GSV; transmetatarsal amputation | Acinetobacter baumanii | Colistimethate sodium; trimethoprim sulfamethoxazole | 11 | Healed |
| 70 | F | DM, COPD, smoke, haemodialysis | Gangrene of all toes of the left foot | Femoro‐posterior tibial artery bp in GSV; open transmetatarsal amputation | P. aeruginosa; E. faecalis | Ampicillin–sulbactam; amikacin | 13 | Healed |
| 87 | F | Heart failure, dyslipidemia, hypertension | Gangrene of the last three toes of the right foot | Femoro‐posterior tibial artery bp in GSV; amputation of the last three toes | Enterococcus casseliflavus | Ampicillin–sulbactam | 20 | Dead |
| 53 | M | Smoke, hypertension, COPD | Calcaneal lesion of the left foot 40 × 35 | Femoro‐anterior tibial artery bp in GSV; surgical debridement of the calcaneal lesion | S. aureus | Cefazolin | 27 | Healed |
| 69 | M | DM, hypertension, dyslipidemia | Gangrene of first and second toes of the right foot | Femoro‐peroneal artery bp in GSV; minor amputation of the right foot | S. aureus; E. faecalis | Rifampicin; doxiciclin | 15 | Healed |
| 72 | M | Hypertension, smoke, COPD | Gangrene of the first toe of the left foot | Femoro‐anterior tibial artery bp in GSV; amputation of the first toe | No bacteria | Cefazolin | 13 | Healed |
| 76 | F | Dyslipidemia, smoke, heart failure, COPD | Calcaneal lesion of the right foot 35 × 45 | Femoro‐anterior tibial artery bp in GSV; surgical debridement of the calcaneal lesion | P. aeruginosa | Meropenem | 20 | Healed |
| 70 | M | Dyslipidemia, DM, COPD, hypertension | Calcaneal lesion of the left foot 40 × 50 | Femoro‐peroneal artery bp in GSV; surgical debridement of the calcaneal lesion | No bacteria | Cefazolin | 21 | Healed |
| 58 | F | Dyslipidemia, smoke, COPD, DM | Calcaneal lesion of the right foot 40 × 35 | Popliteal–posterior tibial artery bp in GSV; surgical debridement of the calcaneal lesion | No bacteria | Cefazolin | 22 | Healed |
| 74 | F | Heart failure, DM, COPD, haemodialysis | Gangrene of all toes of the right foot | Femoro‐posterior tibial artery bp in GSV; open transmetatarsal amputation | A. baumanii | Colistimethate sodium; trimethoprim sulfamethoxazole | 13 | Healed |
| 46 | F | Dyslipidemia, heart failure, tromboangiitis obliterans | Gangrene of all toes of the left foot | Femoro‐anterior tibial artery bp in GSV; open transmetatarsal amputation | P. aeruginosa | Vancomycin | 14 |
Bypass occlusion and restoration; healed |
| 56 | M | Dyslipidemia, DM, heart failure | Gangrene of all toes of the left foot | Femoro‐anterior tibial artery bp in GSV; open transmetatarsal amputation | No bacteria | Cefazolin | 46 | Healed |
| 64 | M | DM, dyslipidemia, hypertension, COPD | Gangrene of all toes of the left foot | Femoro‐posterior tibial artery bp in GSV; open transmetatarsal amputation | E. coli; E. faecalis | Meropenem | 25 | Healed |
| 93 | M | Heart failure, DM, COPD | Gangrene of all toes of the right foot | Axillo‐bifemoral bp in PTFE; open transmetatarsal amputation | Proteus mirabilis; Citrobacter freundii | Cefazolin; piperacillin tazobactam; amikacin | 31 | Dead |
DM, diabetes mellitus; bp, bypass; GSV, great saphenous vein; COPD, chronic obstructive pulmonary disease; PFTE, polytetrafluoroethylene.
Results
The VAC dressing was changed every 3 days in the operation room. Patients had an average of 14 (range: 4–21) treatment sessions. The duration of VAC use ranged from 7 to 51 days, and generally the treatment was continued until sufficient granulation tissue formation, with complete disappearance of signs of local infection, with an average length of stay of 31·5 ± 19·5 days (Figure 3).
Figure 3.
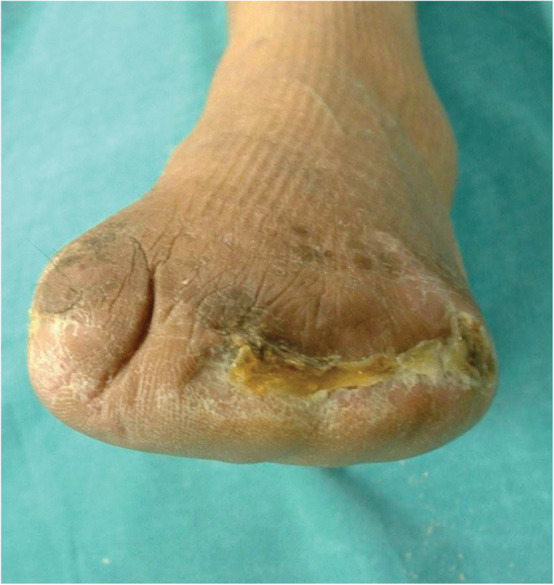
Results at the end of the treatment.
During a mean follow‐up of 17 months, bypass occlusion occurred in three patients. The first patient presenting an open transmetatarsal amputation underwent femoro‐anterior tibial bypass restoration and subsequently wound healing. The second one with transmetatarsal amputation underwent femoro‐posterior tibial bypass restoration failure, followed by above‐the‐knee amputation. The third patient with amputation of three toes needed a transmetatarsal amputation after femoro‐anterior tibial bypass restoration and after obtaining wound healing. Among the remaining 26 patients, 2 patients already submitted to minor amputations with slow healing, underwent transmetatarsal amputation followed by wound healing during follow‐up. One patient received hyperbaric oxygen therapy in addition to VAC therapy. Two patients were lost during follow‐up; one patient died during hospital stay for concomitant comorbidities and another patient died during follow‐up. Complete wound healing was achieved in 19 patients (65·51%) in an average period of 45·4 ± 25·6 days.
Discussion
VAC therapy was initially developed to treat decubitus ulcers and wounds with vascular dysfunction; successively the indications for its use have gradually increased 12. Recently, it has not only been used for chronic pressure ulcers but also prior to graft or flap treatments in cases of acute wounds, diabetic ulcers, burns and osteomyelites 13. In addition, VAC therapy seems to be very effective in accelerating foot wound healing in patients with arterial ulcers, after surgical revascularisation of the lower limbs, with restoration of adequate distal blood flow.
VAC therapy exerts mechanical forces on the wound bed and has positive effects on both the contraction of the wound and the proliferation of granulation tissue. Moreover, it stimulates local blood circulation and it significantly reduces bacterial counts in tissues 14. It also contributes to the healing process by reducing excess interstitial fluid and by keeping the wound moist in a sealed environment.
Various reports on the application of VAC therapy for the treatment of diabetic foot syndrome are available in the literature.
Armstrong et al. 15 carried out a study in 31 patients with diabetic foot ulcers and reported a 90·3% limb salvage rate without amputation, with an average length of stay of 32·9 days. Only 3·2% of patients were amputated below the knee, and the remaining 6·5% underwent transmetatarsal amputation. Nather et al. 16 reported a 100% limb salvage in 11 patients with diabetic foot ulcers treated with VAC therapy, presenting Wagner grade 2 or 3 wounds with an average length of stay of 23·3 days. Ulusal et al. 17 reported an amputation rate of 37% , with an average hospitalisation of 32 days. The reason for this higher amputation rates in comparison to those in the literature is that 80% of the subjects had Wagner Grade 3 and 4 wounds. Beno et al. 18 reported efficient results in the management of venous ulcers and infected wounds, and they concluded that an appropriate revascularisation is necessary prior to VAC therapy in patients with diabetic foot syndrome and peripheral arterial occlusive disease, while further studies are necessary to prove the efficiency of VAC systems in treatment of infected graft material after revascularisation.
Contrasting results on reduction of bacterial counts in tissues are reported in the literature.
Morykwas et al. 14 carried out experiments on animals and demonstrated that VAC therapy decreased bacterial counts in tissues. On the other hand, Weed et al. 19 determined that VAC therapy did not have a consistent effect on bacterial clearance, based on serial bacterial cultures collected in their clinical study.
However, because VAC therapy is a closed therapy system, it facilitates the safe removal of infected drainage, protecting health care personnel and other patients from nosocomial infections 12.
About the use of VAC therapy in the treatment of foot wounds after surgical revascularisation of the lower limb, Nishimura et al. 20 reported a case of severe ischaemic foot in a patient submitted to left axillopopliteal bypass and third, fourth and fifth digital amputation for gangrene, complicated with Staphylococcus aureus infection, in which VAC therapy markedly improved wound healing. Also, Clare et al. 21 reported their experience with VAC device in 17 patients with non‐healing diabetic and dysvascular wounds, 6 of whom previously submitted to lower limbs revascularisation.
In our Institution, which is a primary referral centre for the treatment of peripheral arterial disease, VAC system was applied as an adjuvant therapy in 29 patients with Rutherford 6 lesions (area >3 cm2) after surgical revascularisation of the lower limb followed by transmetatarsal amputation (12 cases), local minor amputations (9 cases) and/or surgical wounds debridement of extensive calcaneal lesions (8 cases), in association with specific antibiotic therapy, to speed up foot wound healing.
Only one patient needed hyperbaric oxygen therapy in addition to VAC therapy; one patient underwent above‐the‐knee amputation after bypass failure; three patients already submitted to minor amputations needed a transmetatarsal amputation of the foot. Two patients were lost to follow‐up and another two patients died. We recorded an average hospital stay of 31·5 ± 19·5 days, and complete wound healing was achieved in 19 patients in an average period of 45·4 ± 25·6 days.
In conclusion, our results suggested that VAC therapy, together with periodical surgical wound debridement and specific antibiotic therapy, could be helpful to promote and accelerate wound healing of foot lesions after restoration of an adequate distal blood flow through surgical revascularisation.
References
- 1. Serra R, Grande R, Scarcello E, Buffone G, de Franciscis S. Angiosome‐targeted revascularisation in diabetic foot ulcers. Int Wound J 2013. In press. DOI: 10.1111/iwj.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Caridi G, Massara M, Villari S, Martelli E, Spinelli F, Grande R, Butrico L, de Franciscis S, Serra R. Extreme distal bypass to improve wound healing in Buerger's disease. Int Wound J 2014. In press. DOI: 10.1111/iwj.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wound Ostomy and Continence Nurses Society . Guideline for management of wounds in patients with lower‐extremity arterial disease. Glenview: Wound Ostomy and Continence Nurses Society, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Veves A, Falanga V, Armstrong DA, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:2001–295. [DOI] [PubMed] [Google Scholar]
- 5. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J 2013;10:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mannari RJ, Payne WG, Ochs DE, Walusimbi M, Blue M, Robson MC. Successful treatment of recalcitrant, diabetic heel ulcers with topical becaplermin (rhPDGF‐BB) gel. Wounds 2002;14:116–21. [Google Scholar]
- 7. De Caridi G, Massara M, David A, Giardina M, La Spada M, Stilo F, Spinelli F, Grande R, Butrico L, de Franciscis S, Serra R. Spinal cord stimulation to achieve wound healing in a primary lower limb critical ischemia referral centre. Int Wound J 2014. In press. DOI: 10.1111/iwj.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil 2001;82:721–5. [DOI] [PubMed] [Google Scholar]
- 9. De Caridi G, Massara M, Benedetto F, Tripodi P, Spinelli F, David A, Grande R, Butrico L, Serra R, de Franciscis S. Adjuvant spinal cord stimulation improves wound healing of peripheral tissue loss due to steal syndrome of the hand: clinical challenge treating a difficult case. Int Wound J 2014. In press. DOI: 10.1111/iwj.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kloth LC. 5 questions‐and answers‐about negative pressure wound therapy. Adv Skin Wound Care 2002;15:226–9. [DOI] [PubMed] [Google Scholar]
- 11. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 12. Wongworawat MD, Schnall SB, Holtom PD, Moon C, Schiller F. Negative pressure dressing as an alternative technique for the treatment of infected wounds. Clin Orthop Relat Res 2003;414:45–8. [DOI] [PubMed] [Google Scholar]
- 13. Hunter S, Langemo D, Hanson D, Anderson J, Thomson P. The use of negative pressure wound therapy. Adv Skin Wound Care 2007;20:90–5. [Google Scholar]
- 14. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong DG, Lavery LA, Abu‐Rumman P, Espensen EH, Vazquez JR, Nixon BP, Boulton AJ. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage 2002;48:64–8. [PubMed] [Google Scholar]
- 16. Nather A, Chionh SB, Han AY, Chan PP, Nambiar A. Effectiveness of vacuum‐assisted closure (VAC) therapy in the healing of chronic diabetic foot ulcers. Ann Acad Med Singapore 2010;39:353–8. [PubMed] [Google Scholar]
- 17. Ulusal AE, Sahin MS, Ulusal B, Cakmak G, Tuncay C. Negative pressure wound therapy in patients with diabetic foot. Acta Orthop Traumatol Turc 2011;45:254–60. DOI: 10.3944/AOTT.2011.2283. [DOI] [PubMed] [Google Scholar]
- 18. Beno M, Martin J, Sager P. Vacuum assisted closure in vascular surgery. Bratisl Lek Listy 2011;112:249–52. [PubMed] [Google Scholar]
- 19. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004;52:276–80. [DOI] [PubMed] [Google Scholar]
- 20. Nishimura K, Kanaoka Y, Ishiguro S, Harada S, Shiraya S, Fujiwara Y, Nakamura Y, Kamihira S, Nishimura M. Vacuum‐assisted closure for bilateral severe ischemic foot after revascularization: a case report. Int Angiol 2009;28:340–3. [PubMed] [Google Scholar]
- 21. Clare MP, Fitzgibbons TC, McMullen ST, Stice RC, Hayes DF, Henkel L. Experience with the vacuum assisted closure negative pressure technique in the treatment of non‐healing diabetic and dysvascular wounds. Foot Ankle Int 2002 Oct;23:896–901. [DOI] [PubMed] [Google Scholar]


