Abstract
Pathological scars, such as keloids and hypertrophic scars, readily cause physical and psychological problems. Combination 5‐fluorouracil (5‐FU) with triamcinolone acetonide (TAC) is presumed to enhance the treatment of pathological scars, although supportive evidence is lacking. We aimed to compare the efficacy and safety of TAC alone and in combination with 5‐FU for the treatment of hypertrophic scars and keloids. Five databases (PubMed, Medline, Cochrane databases, Embase and CNKI) were searched with the limitations of human subjects and English‐language text. Mean differences (MDs), odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. The Cochrane Collaboration's Risk of Bias Tool was used to assess the risk of bias. The control group received intralesional TAC alone, and the experimental group received TAC combined with 5‐FU injection. A pooled analysis of the effectiveness based on patient self‐assessment after treatment showed that the experimental group achieved better results than the control group (OR = 2·92, 95% CI = 1·63–5·22, P = 0·0003). Similarly, a pooled analysis of the effectiveness based on observer assessment following treatment produced the same conclusion (OR = 4·03, 95% CI = 1·40–11·61, P = 0·010). A meta‐analysis of scar height after treatment showed that the experimental group performed better than the control group (MD = −0·14, 95% CI = −0·23–0·05, P = 0·002). The erythema score of the experimental group after treatment was superior (MD = −0·20, 95% CI = −0·34–0·06, P = 0·004). The heterogeneity test showed no heterogeneity among the studies (P > 0·1, I 2 = 0%). TAC combined with 5‐FU is more suitable for the treatment and prevention of hypertrophic scars and keloids, with greater improvement in scar height and patient satisfaction as well as fewer side effects.
Keywords: 5‐Fluorouracil, Hypertrophic scars, Keloids, Meta‐analysis, Triamcinolone acetonide
Introduction
Pathological scars are quite common among people with hereditary factors and those who sustain large‐wound surgical procedures, burns and injuries occurring in unsanitary environments. The excessive proliferation of connective tissue, defined as benign skin tumours, following skin injury leads to the extension of the pathological scars beyond the lesion area 1, 2, 3. The reasons for the development of keloids and hypertrophic scars are complex but are primarily related to dermal collagen fibre disorders, excessive fibroblast proliferation and excessive collagen deposition, which differ from the normal wound‐healing process 4. During normal wound healing, fibroblasts produce new extracellular matrix, including types I and III collagen, fibronectin and proteoglycans in an orderly fashion. When the formation of the tissue structure and the scar are complete, the wound is repaired 5, 6. Pathological scarring often causes itching, pain, erythema and persistent growth of a mass beyond the original site of injury. All these symptoms ultimately lead to deformities of function and appearance, psychological stress and patient dissatisfaction 7, 8.
Many methods are clinically available for the treatment of these two types of pathological scars, such as intralesional corticosteroid injections, cryotherapy, radiotherapy, pressure therapy and laser therapy. However, the results and recurrence rates are not satisfactory 9. Currently, comprehensive surgical treatment is advocated; however, surgery is invasive and is associated with a high recurrence rate 10. Corticosteroids, particularly the intralesional injection of triamcinolone acetonide (TAC), is the most prevalent and effective treatment method, given its low recurrence rate and its non‐invasive nature, and has been used since 1961 11. Although infrequent, adverse glucocorticoid reactions following the intralesional injection of large doses of TAC alone occur, such as hypopigmentation, tissue atrophy and terminal arterial dilatation 12, 13. Some data indicated that the clinical efficacy of glucocorticoids may be improved and that fewer adverse reactions occurred in combination with 5‐fluorouracil (5‐FU) 12. However, the use of 5‐FU may be questioned because systemic 5‐FU may cause anaemia, leukopenia and thrombocytopenia 14. Recently, randomised controlled trials (RCTs) provided some evidence regarding the superiority of a combination of 5‐FU and TAC compared with TAC alone. Yet, almost without exception, the number of cases in these studies was small, and no solid evidence emerged to support a conclusive final analysis. To help clarify whether a relative benefit of TAC combined with 5‐FU in the treatment of both hypertrophic scars and keloids existed, we performed a systematic review and meta‐analysis of all published RCTs that compared TAC alone versus TAC in combination with 5‐FU for the treatment of hypertrophic scars and keloids.
Materials and methods
Data sources and search strategy
A computerised search was conducted using the PubMed, Medline, Cochrane, Embase and CNKI databases through September 2015 with combinations of the following six keywords: triamcinolone acetonide or TAC, 5‐fluorouracil or 5‐FU, hypertrophic scars and keloids. The search was limited to human subjects and English‐language studies. The titles and abstracts of potentially relevant studies that were identified by the computerised search were reviewed. Furthermore, the reference lists of the retrieved studies were also assessed to identify additional relevant articles.
Inclusion and exclusion criteria
The inclusion criteria were as follows 1: study types including RCTs, cluster‐randomised trials, controlled clinical trials (CCTs) and prospective controlled trials (PCTs) regardless of allocation concealment and blinding 2; participant types including patients of both genders with healed full‐thickness wounds, newly healed wounds and established scarring with no history of treatment 3; intervention types including TAC alone and in combination with 5‐FU for the treatment of hypertrophic scars and keloids; and 4 outcome measures including changes in scar size in terms of length, width and height, the erythema score, itch reduction, pliability score, pruritus score, adverse events and patient self‐assessment and observer assessments.
The exclusion criteria included the following 1: non‐PCT trials, non‐CCT trials, non‐RCT trials and retrospective studies 2; data description or sampled information that was insufficiently clear 3; women who were pregnant, lactating or planning pregnancy in the near future 4; surgical excision before treatment or other drugs during the treatment; and 5 non‐English‐language articles. After the hospital's ethics committee approved the consent forms and the study protocol, all of the studies were conducted.
Assessment of quality and data extraction
Two reviewers (WL and BYF) independently screened, extracted and assessed the research data to ensure consistency. First, the titles and abstracts were read by the reviewers to directly screen for the relevant articles. The full texts of the relevant articles were downloaded, and the full texts of the articles that could not be downloaded were obtained directly from the authors. For studies that were difficult to judge, the reviewers downloaded and read the full texts for screening. The inclusion and exclusion criteria were strictly observed during the assessment of the potentially eligible studies and data extraction.
Risk of bias
The risk of bias was assessed using the Cochrane Collaboration tool, which includes random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other criteria. Two reviewers independently assessed the included studies.
Statistical analysis
The statistical analyses were conducted using RevMan analysis software (version 5.3), provided by the Cochrane collaboration network. Heterogeneity among the studies was assessed by the chi‐square test and I 2 statistic. Fixed‐effect or random‐effect models were selected according to clinical homogeneity, the P value and the I 2 value. If P > 0·1 or I 2 < 50%, the study was analysed using a fixed‐effect model. If P ≤ 0·1 or I 2 ≥ 50%, we used the random‐effect model. The effectiveness outcome data from all studies were dichotomous variables, and the odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated as effect indexes. The scar heights and erythema scores of all studies were taken as continuous variables, and the mean differences (MDs) and 95% CI were calculated as effect indexes. Publication bias was analysed by constructing a funnel plot to display the specific data distributions, and the presence of publication bias was subsequently judged. We also performed sensitivity analysis to assess the stability of the results and to investigate the influence of each study by omitting a single study.
Results
Data extraction
A total of 113 articles were collected, and 62 papers that were not relevant to the subject were excluded based on readings of the titles and abstracts. Preliminarily, 51 articles, including 46 Chinese articles and five English articles, were included. Based on further full‐text evaluation, four of the English articles 12, 15, 16, 17 were considered to be in strict accordance with the inclusion and exclusion criteria (Figure 1).
Figure 1.
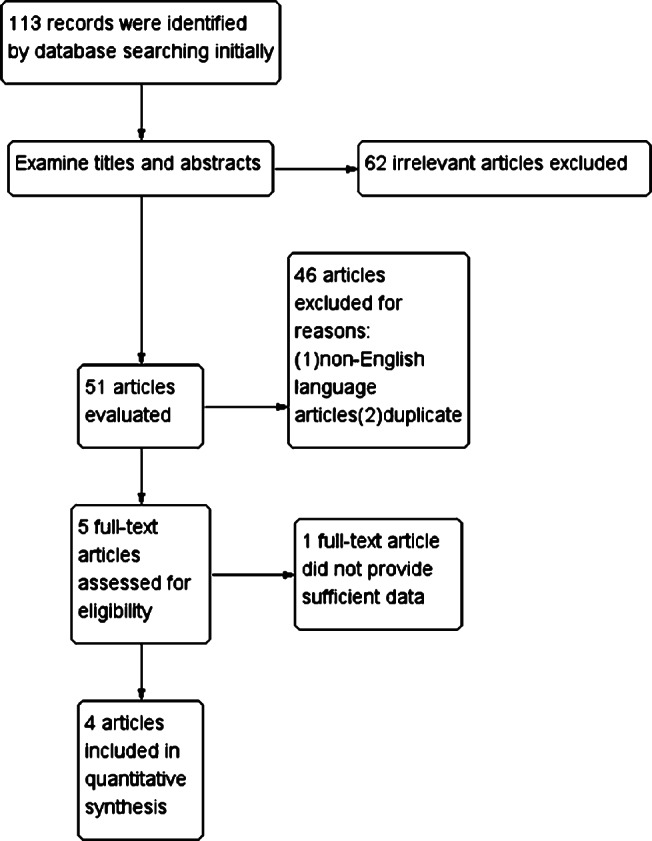
Review flow diagram.
Risk of bias
The risks of bias of the included studies 12, 15, 16, 17 were estimated using the Cochrane Collaboration tool. Khan et al. 17 used a random number table to generate sequences and group divisions. Darougheh's study 12 cited double blinding and parallel grouping. A computer‐generated table of random numbers was used for allocation. Asilian's study 16 cited single blinding and parallel grouping. In the studies of Darougheh and Asilian 12, 16, the dermatologists were blinded to assess the results of the patient groups. However, the random method and the allocation concealment were not described in the studies of Woraphong and Asilian 15, 16, which may give rise to high risks of selection bias and may affect the study results. More detailed information is presented in Figures 2 and 3.
Figure 2.
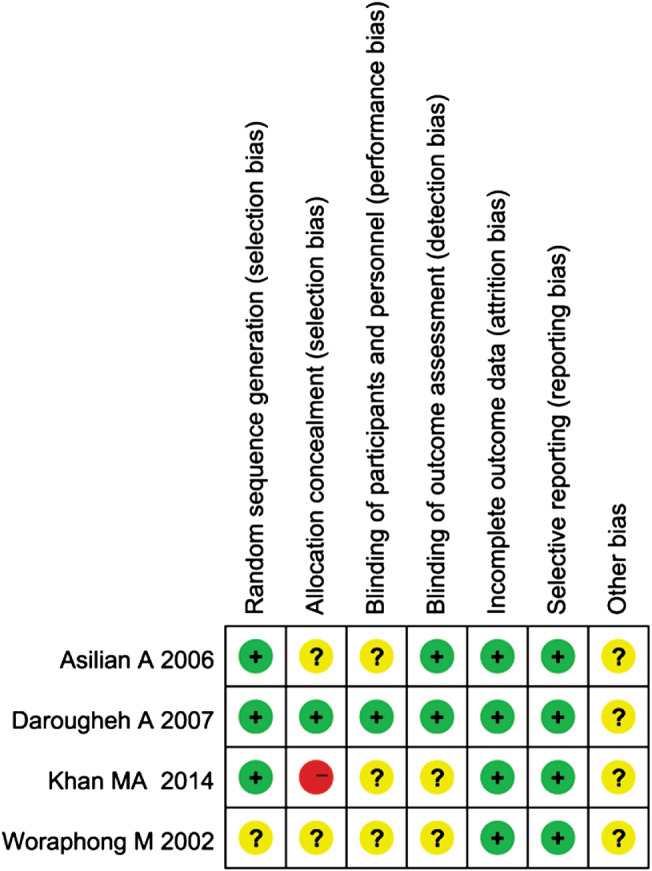
Risk of bias summary: this risk of bias tool incorporates the assessment of randomisation (sequence generation and allocation concealment), blinding (participants and outcome assessors), incomplete outcome data, selective outcome reporting and other risk of bias. The items were judged as ‘low risk’, ‘unclear risk’ or ‘high risk’. Green means ‘low risk’, red means ‘high risk’ and yellow means ‘unclear risk’.
Figure 3.
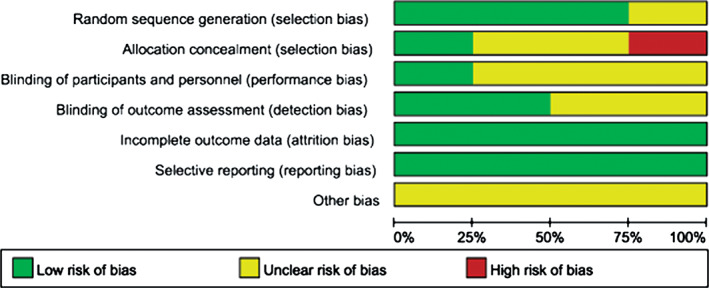
Risk of bias graph: each risk of bias assessment was presented as the percentage across all the included studies, which indicates the proportion of different levels of risk of bias for each item.
Characteristics
The characteristics of the included studies are presented in Table 1. Four RCT studies 12, 15, 16, 17 were included, and the sizes and locations of the scars were not identical. The control group received intralesional TAC alone, and the experimental group received TAC combined with 5‐FU injection therapy. Hypertrophic scars or keloids of the included patients could not be clearly distinguished and defined. Intralesional TAC 10 mg (0·25 ml of 40 mg/ml TAC diluted with 0·75 ml injectable normal saline) was administered once weekly for a total of eight sessions, and an intralesional injection of TAC 4 mg (0·1 ml of 40 mg/ml TAC) mixed with 5‐FU 45 mg (0·9 ml of 50 mg/ml 5‐FU) was administered once weekly for a total of eight sessions in the studies by Khan, Darougheh and Asilian 12, 16, 17. The maximum injection doses were also the same in these three studies 12, 16, 17. However, in Woraphong's study 15, intralesional TAC 20 mg was administered four times a week for a total of six sessions, and the intralesional injection of TAC 1 mg mixed with 5‐FU 45 mg was administered twice weekly for a total of ten sessions. The follow‐up duration of the trials ranged from 12 to 32 weeks. The scars were assessed on a 5‐point observer scar assessment scale in which 0, no improvement (no reduction in scar height); 1, poor (0–25% reduction in height); 2, fair (25–50% reduction in height); 3, good (50–75% reduction in height); 4, excellent (75–100% reduction in height). Effectiveness as an outcome index was defined as a greater than 50% reduction in the initial scar height 15. Data pooling was conducted according to effectiveness, scar height and erythema score, which were extracted from each trial. Additional outcome indexes included the pruritus score and pliability score, which could not be compared.
Table 1.
Characteristics of studies included
| Year | Sample size (E/C) | Female (%) | Mean age (years) | Disease duration (month) | Intervention | Definition keloid | Measurement method | Conc. (mg/ml) | Max dose/ injection (mg) | Injections (n) | Injection interval (weeks) | Follow‐up (weeks) | Study design | Relevant outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | C | |||||||||||||||
| Khan et al. 17 | 2014 | 75/75 | 56·60 | 29·45 ± 9·27 | 6 | TAC + 5‐FU | TAC | NR | R |
E:4/45 C:10 |
E:8/90 C:20 |
E:8 C:8 |
E:1 C:1 |
26 | RCT | Scar height; good to excellent |
| Darougheh et al. 12 | 2007 | 20/20 | 62·50 | 5–70 | 12·5 ± 7·4 | TAC + 5‐FU | TAC | NR | R |
E:4/45 C:10 |
E:8/90 C:20 |
E:8 C:8 |
E:1 C:1 |
12 | RCT | Scar height; erythema score; pruritus score; patient self‐assessment and observer assessment |
| Asilian et al. 16 | 2006 | 23/23 | 60 | 24·7 ± 11·0 | 12·2 ± 7·3 | TAC + 5‐FU | TAC | NR | R |
E:4/45 C:10 |
E:8/90 C:20 |
E:8 C:8 |
E:1 C:1 |
12 | RCT | Scar height; erythema score; pliability score; pruritus score; patient self‐assessment and observer assessment |
| Woraphong et al. 15 | 2002 | 10/10 | 60 | 25–74 | 6·0–11·5 | TAC + 5‐FU | TAC | NR | R |
E:1/45 C:20 |
NR |
E:10 C:6 |
E:2 C:4 |
32 | RCT | Scar height; erythema score; pliability score; patient self‐assessment |
5‐FU, 5‐fluorouracil; C, control group; Conc, concentrations; E, experimental group; NR, not reported; R, reported; RCT, randomised controlled trials; TAC, triamcinolone acetonide.
Patient self‐assessment and observer assessment
In the pooled analysis results regarding effectiveness after the treatment, four randomised controlled studies 12, 15, 16, 17 provided effectiveness data in terms of patient self‐assessment after treatment, which included 128 experimental group cases and 128 control group cases. A heterogeneity test showed no heterogeneity among the studies (P = 0·39, I 2 = 0%); thus, the fixed‐effect model was used to combine the statistical data of the two groups. A pooled analysis showed that the difference between the experimental and control groups was significant (OR = 2·92, 95% CI = 1·63–5·22, P = 0·0003). The total event percentage (96/128) of TAC + 5‐FU was more than that (70/128) of TAC; hence, the experimental group exhibited superior results compared with the control group (Figure 4).
Figure 4.
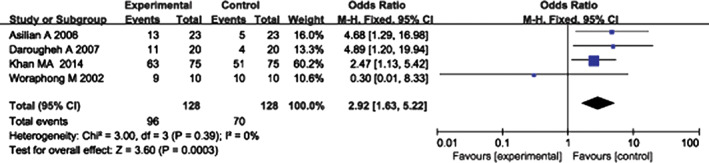
Forest plot of comparison: clinical effectiveness in terms of patient self‐assessment of experimental group versus control group. Experimental group, triamcinolone acetonide combined with 5‐FU; control group, triamcinolone acetonide alone.
Two randomised controlled studies 12, 16 provided effectiveness data in terms of observer self‐assessments following treatment. These studies included 43 experimental group cases and 43 control group cases. A heterogeneity test showed no heterogeneity between the studies (P = 0·91, I 2 = 0%), and thus, a fixed‐effect model was used to combine the statistical data of the two groups. The pooled analysis showed that the difference between the experimental and control groups was significant (OR = 4·03, 95% CI = 1·40–11·61, P = 0·010). The total event percentage (17/43) of TAC + 5‐FU was greater than that (6/43) of TAC, and similarly, the experimental group performed better than the control group (Figure 5).
Figure 5.
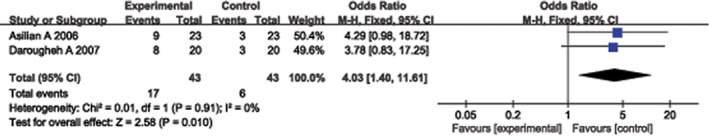
Forest plot of comparison: clinical effectiveness in terms of observer assessment of experimental group versus control group.
Scar height
Three randomised controlled studies 12, 16, 17 provided useful data regarding significant decreases in scar height. These studies included 118 experimental group cases and 118 control group cases. A heterogeneity test showed no heterogeneity among the studies (P = 1·00, I 2 = 0%); thus, a fixed‐effect model was used to combine the statistical data from the three groups. The pooled analysis showed that the difference between the experimental and control groups was significant (MD = −0·14, 95% CI = −0·23–0·05, P = 0·002). The experimental group exhibited results that were superior to the control group (Figure 6).
Figure 6.
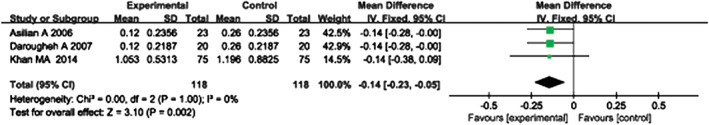
Forest plot of comparison: scar height of experimental group versus control group.
Erythema score
Two randomised controlled studies 12, 16 provided useful data regarding significant decreases in erythema scores. These studies included 43 experimental group cases and 43 control group cases. A heterogeneity test showed no heterogeneity between the studies (P = 1·00, I 2 = 0%); thus, a fixed‐effect model was used to combine the statistical data of the two groups. The pooled analysis showed that the difference between the experimental and control groups was significant (MD = −0·20, 95% CI = −0·34–0·06, P = 0·004). The experimental group results were superior to the control group (Figure 7).
Figure 7.
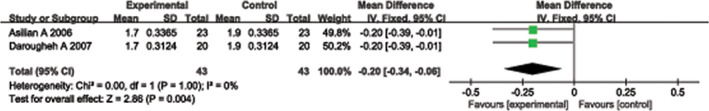
Forest plot of comparison: erythema scores of experimental group versus control group.
Sensitivity analysis and publication bias
Because the injection concentration and the interval in Woraphong's low‐quality study 15 were different from the other three studies, we omitted it to test the stability of the results. By omitting it, we found that the pooled outcome of heterogeneity (P = 0·58, I 2 = 0%) did not change, indicating that our results were statistically reliable. The publication biases of the four included articles were evaluated. The points indicating the effectiveness of the treatments in the funnel plot were not symmetrically distributed, as shown in Figure 8, which indicated that a certain degree of publication bias existed.
Figure 8.
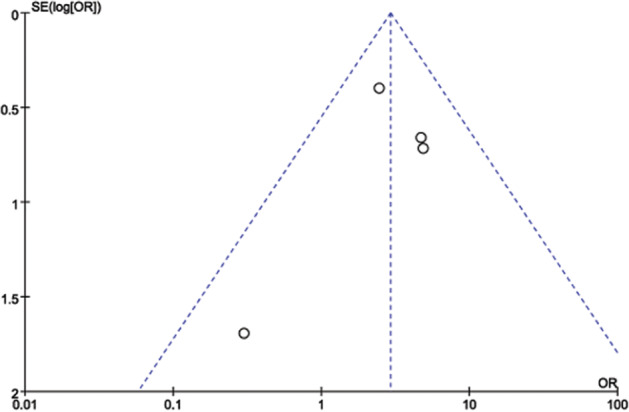
Funnel plot to test for publication bias. Each point represents a separate study for the indicated association. Log OR represents the natural logarithm of the OR. The vertical line represents the mean effects size. OR, odds ratio; SE, standard error.
Discussion
Pathological scarring, including keloids and hypertrophic scars, is the inevitable outcome of an abnormal wound‐healing process and is caused by the overreaction of fibrillar connective tissue because of wound stimulation. The treatment methods are various but not ideal 18. Recurrence rates typically persist at 50–70% 19. Glucocorticoid suspension injection remains a first‐line treatment; however, this treatment has substantial side effects when glucocorticoid is applied alone, which limits its clinical utility. Because 5‐FU inhibits cell proliferation, the combination of 5‐FU with TAC produces good results, and this combination has been used for clinical treatment 20. A meta‐analysis based on four RCTs 12, 15, 16, 17 was performed. The degrees of scarring in 256 patients were evaluated, and the results showed that the combined application of TAC and 5‐FU for the treatment of scars significantly improved the recovery rate or the effectiveness of scar recovery, which indicates that the combined application of TAC and 5‐FU for the treatment of scars is more effective than the application of TAC alone.
The combination of 5‐FU and TAC may inhibit scar hypertrophy via numerous mechanisms. TAC may inhibit scar fibroblast proliferation, enhance collagen degradation, reduce scar tissue atrophy and reduce cell migration 21, 22. According to the pharmacological properties of its anti‐tumour activity, combining this drug with 5‐FU may competitively inhibit the synthesis of thymidylate synthase, which inhibits the synthesis of thymidine and thus DNA. Moreover, 5‐FU is also converted to 5‐fluorouridine, which is a pseudo‐metabolite that penetrates RNA, interferes with the function of RNA, inhibits rRNA and mRNA transcription in the nucleus, create false base pairings during the RNA transcription process and ultimately prevents cell division and inhibits the growth of fibroblasts 23, 24, 25, 26. Wendling et al. showed that 5‐FU blocks the expression of the α2 type I collagen gene (COL1A2), which is induced by TGF‐β in human fibroblasts through c‐Jun NH2‐terminal Kinase/Activator Protein‐1 activation; this result proves that 5‐FU indeed has an inhibitory effect on type I collagen fibres and inhibits the growth of fibroblasts 27, 28. In addition, Bulstrode et al. showed that 5‐FU induces the fibroblastic proliferation of Dupuytren's contracture patients and may selectively inhibit collagen synthesis 29. Angiogenesis is a key factor in the growth and recurrence of keloids, and Vacca et al. indicated that 5‐FU exhibits anti‐angiogenic effects at non‐toxic doses. The inhibitory mechanism of 5‐FU on blood vessels is different, which accounts for the special ability of 5‐FU to inhibit scar formation 30.
The mechanisms of action of TAC and 5‐FU are different and act synergistically in the curing of hypertrophic scars and keloids. Combined treatment with TAC and 5‐FU resulted in G2 cell cycle arrest, the down‐regulation of VEGF and suppressive effects on Col‐1 synthesis and MMP‐2 synthesis in keloid fibroblasts. Moreover, 5‐FU predominantly inhibits cell proliferation and promotes apoptosis 31, 32.
The injection concentration and interval of TAC + 5‐FU or TAC were also important factors during their comparison, although the injection concentration and interval were equivalent in the studies of Khan, Darougheh and Asilian 12, 16, 17. Omitting Woraphong's study 15 by sensitivity analysis, the results of the pooled analysis did not change, which indicated that different injection concentrations and intervals did not affect the results of the effectiveness comparison.
Adverse events were also reviewed in this study. In Asilian's and Darougheh's studies 12, 16, 37% of patients in the TAC group reported some degree of skin atrophy and telangiectasia. Adverse sequelae, including hypopigmentation (20%, two out of ten), telangiectasia (20%, two out of ten), and skin atrophy (10%, one out of ten), were observed in 50% (five out of ten) of the subgroups that received TAC injection alone in Woraphong's study 15. No side effects were observed among the TAC + 5‐FU groups in the four included studies 12, 15, 16, 17. At the conclusion of the studies, 5‐FU indeed could decrease the glucocorticoid reactions of TAC injection alone. Conversely, small concentrations of TAC reduced side effects such as erythema and ulceration when pure 5‐FU was used 33. Superior safety was shown in the TAC + 5‐FU group.
In summary, four RCTs comparing the efficacies of 5‐FU and TAC were identified. The study results showed that 5‐FU suppresses keloids and hypertrophic scars via its effects on anti‐angiogenesis, collagen synthesis, cell proliferation inhibition and fibroblast apoptosis. Sensitivity analysis confirmed the stability of the results. Furthermore, fewer side effects were observed following the application of TAC and 5‐FU.
However, based on a comprehensive analysis, the following limitations were found pertaining to these studies. First, the four articles included in this study primarily adopted random, controlled research and design methods; however, for the random method, blinding and allocation concealment are not described in detail, which may result in high risks of selection biases. Second, based on the results of funnel plots, it is difficult to rule out the effects of publication bias. Third, the four included studies unfortunately did not distinguish keloids and hypertrophic scars before treatment. Moreover, because the injection therapies were given together, we were unable to further assess these studies by subgroup analysis. Fourth, it is difficult to recruit adequate numbers of patients in RCTs concerning injection treatment comparisons. Sometimes, patients prefer to select a more rapid treatment, such as surgical excision. Therefore, the number of included studies and the sample sizes were small. Fifth, we estimated the standard deviations of the scar height and erythema score for two studies using the formula suggested in the Cochrane Handbook for Systematic Reviews of Interventions because a response was unavailable from certain authors. Finally, the follow‐up times and standardised assessment scales for the pathological scars were not unified, which may have hindered the synthesis of the results and analysis.
In conclusion, TAC combined with 5‐FU offers better safety and is appropriate for the treatment and prevention hypertrophic scars and keloids. Additional randomised, controlled, multicentre, large‐sample, high‐quality trials are needed for a more objective analysis of the treatment efficacy and to assess adverse reactions associated with the use of 5‐FU for keloids and hypertrophic scars.
Acknowledgements
This work was supported by the State Program of the National Natural Science Foundation of China (81371957), the State Key Program of the National Natural Science Foundation of China (81330042), the Special Program for Sino‐Russian Joint Research sponsored by the Ministry of Science and Technology, China (2014DFR31210) and the Key Program sponsored by the Tianjin Science and Technology Committee, China (13RCGFSY19000, 14ZCZDSY00044).
References
- 1. Rekha A. Keloids – a frustrating hurdle in wound healing. Int Wound J 2004;1:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 3. Chike‐Obi CJ, Cole PD, Brissett AE. Keloids: pathogenesis, clinical features, and management. Semin Plast Surg 2009;23:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr 2006;18:396–402. [DOI] [PubMed] [Google Scholar]
- 5. Epstein FH, Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 6. Bayat A, Arscott G, Ollier WER, McGrouther DA, Ferguson MWJ. Keloid disease: clinical relevance of single versus multiple site scars. Br J Plast Surg 2005;58:28–37. [DOI] [PubMed] [Google Scholar]
- 7. Baisch A, Riedel F. Hyperplastic scars and keloids. Part I: basics and prevention. HNO 2006;54:893–904. [DOI] [PubMed] [Google Scholar]
- 8. Oliver B, Schmid‐Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 2005;297:433–8. [DOI] [PubMed] [Google Scholar]
- 9. Mutalik S. Treatment of keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol 2005;71:3–8. [DOI] [PubMed] [Google Scholar]
- 10. Zielinski T. Surgical excision and cryosurgery in the treatment of recurrent auricular keloids. Adv Dermatol Allergol 2012;29:152–5. [Google Scholar]
- 11. Muneuchi G, Suzuki S, Onodera M, Ito O, Hata Y, Igawa HH. Long‐term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloid scars in Asian patients. Scand J Plast Reconstr Surg Hand Surg 2006;40:111–6. [DOI] [PubMed] [Google Scholar]
- 12. Darougheh A, Asilian A, Shariati F. Intralesional triamcinolone alone or in combination with 5‐fluorouracil for the treatment of keloid and hypertrophic scars. Clin Exp Dermatol 2009;34:219–23. [DOI] [PubMed] [Google Scholar]
- 13. Hochman B, Locali RF, Matsuoka PK, Ferreira LM. Intralesional triamcinolone acetonide for keloid treatment: a systematic review. Aesthetic Plast Surg 2008;32:705–9. [DOI] [PubMed] [Google Scholar]
- 14. Haurani MJ, Foreman K, Yang JJ, Siddiqui A. 5‐Fluorouracil treatment of problematic scars. Plast Reconstr Surg 2009;123:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woraphong M, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5‐fluorouracil, and 585‐nm flashlamp‐pumped pulsed‐dye laser treatments. Arch Dermatol 2002;138:1149–55. [DOI] [PubMed] [Google Scholar]
- 16. Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5‐fluorouracil, and pulsed‐dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg 2006;32:907–15. [DOI] [PubMed] [Google Scholar]
- 17. Khan MA, Bashir MM, Khan FA. Intralesional triamcinolone alone and in combination with 5‐fluorouracil for the treatment of keloid and hypertrophic scars. J Pak Med Assoc 2014;64:1003–7. [PubMed] [Google Scholar]
- 18. Jones CD, Guiot L, Samy M, Gorman M, Tehrani H. The use of chemotherapeutics for the treatment of keloid scars. Dermatol Reports 2015;7:5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewartiv C, Kim J. Application of mitomycin‐C for head and neck keloids. Otolaryngol Head Neck Surg 2006;135:946–50. [DOI] [PubMed] [Google Scholar]
- 20. Carroll LA, Hanasono MM, Mikulec AA, Kita M, Koch RJ. Triamcinolone stimulates bFGF production and inhibits TGF‐beta1 production by human dermal fibroblasts. Dermatol Surg 2002;28:704–9. [DOI] [PubMed] [Google Scholar]
- 21. Wang XQ, Liu YK, Wang ZY, Jun W, Jiang Y‐Z, Chun Q, Lu SL. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds 2008;7:151–9. [DOI] [PubMed] [Google Scholar]
- 22. Mccoy BJ, Diegelmann RF, Cohen IK. In vitro inhibition of cell growth, collagen synthesis, and prolyl hydroxylase activity by triamcinolone acetonide. Proc Soc Exp Biol Med 1980;163:216–22. [DOI] [PubMed] [Google Scholar]
- 23. Dockery GL. Hypertrophic and keloid scars. J Am Podiatr Med Assoc 1995;85:57–60. [DOI] [PubMed] [Google Scholar]
- 24. Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. Apoptosis induced by 5‐fluorouracil, cisplatin and paclitaxel are associated with p53 gene status in gastric cancer cell lines. Int J Oncol 2005;26:1563–7. [PubMed] [Google Scholar]
- 25. Ocker M, Alajati A, Ganslmayer M, Zopf S, Lüders M, Neureiter D, Hahn EG, Schuppan D, Herold C. The histone‐deacetylase inhibitor SAHA potentiates proapoptotic effects of 5‐fluorouracil and irinotecan in hepatoma cells. J Cancer Res Clin Oncol 2005;131:385–94. [DOI] [PubMed] [Google Scholar]
- 26. Fitzpatrick RE. Treatment of inflamed hypertrophic scars using intralesional 5‐FU. Dermatol Surg 1999;25:224–32. [DOI] [PubMed] [Google Scholar]
- 27. Wendling J, Marchand A, Mauviel A, Verrecchia F. 5‐fluorouracil blocks transforming growth factor‐beta‐induced alpha 2 type I collagen gene (COL1A2) expression in human fibroblasts via c‐Jun NH2‐terminal kinase/activator protein‐1 activation. Mol Pharmacol 2003;64:707–13. [DOI] [PubMed] [Google Scholar]
- 28. Leask A, Abraham DJ. TGF – signaling and the fibrotic response. FASEB J 2004;18:816–27. [DOI] [PubMed] [Google Scholar]
- 29. Bulstrode NW, Mudera V, Mcgrouther DA, Grobbelaar AO, Cambrey AD. 5‐Fluorouracil selectively inhibits collagen synthesis. Plast Reconstr Surg 2005;116:209–21. [DOI] [PubMed] [Google Scholar]
- 30. Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, Pellegrino A, Dammacco F. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood 1999;94:4143–55. [PubMed] [Google Scholar]
- 31. Lin H, Cai YJ, Lung I, Leung BC, Burd A. A study of the combination of triamcinolone and 5‐fluorouracil in modulating keloid fibroblasts in vitro. J Plast Reconstr Aesthet Surg 2013;66:e251–9. [DOI] [PubMed] [Google Scholar]
- 32. Gupta S, Kalra A. Efficacy and safety of intralesional 5‐fluorouracil in the treatment of keloids. Dermatology 2002;204:130–2. [DOI] [PubMed] [Google Scholar]
- 33. Gupta S, Sharma VK. Standard guidelines of care: keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol 2011;77:94–100. [DOI] [PubMed] [Google Scholar]


