Abstract
In stalled, chronic wounds, more aggressive and proactive wound closure efforts are needed. We describe adjunctive use of epidermal grafting in patients with chronic wounds. Wound bed preparation consisted of surgical necrotectomy or sharp debridement, hyperbaric oxygen therapy, negative pressure wound therapy, compression therapy, platelet‐rich plasma therapy and/or heparan sulphate agents. Epidermal grafts were harvested from the patient's thigh and applied to the wound. Wound and donor site healing was monitored. A total of 78 patients (average age = 64·1 ± 15·6 years) were included in the study. Common comorbidities included hypertension (47·4%), venous insufficiency (37·2%) and obesity (28·2%). Average wound duration was 13·2 months (range: 0·3–180 months). The most common wound types were dehiscence (29·5%), radiation ulcer (24·4%) and venous ulcer (17·9%). Total time from epidermal grafting to wound closure was 10·0 ± 7·3 weeks. Of the 78 wounds, 66 (84·6%) reached full wound closure (49 < 3 months, 16 > 3 months, 1 without time data). Of 78 wounds, 10 (12·8%) underwent partial wound healing, while 2 wounds (2/78; 2·6%) remained unhealed. These results suggest that wound surface reduction can be achieved by proactive early application of biological therapies and epidermal skin grafts, which may help decrease time to wound healing.
Keywords: Chronic wounds, Epidermal grafting, Wound care
Introduction
Chronic wounds are a burden to patients and the health care system. In 2009, it was estimated that approximately 1–1·5% of the population in the industrialised world have a wound and that 2–4% of the total health care expenditure in Europe is spent on wound management 1. As the population and costs of health care have increased worldwide, chronic wound prevalence and money spent on wound care has undoubtedly increased as well. In response to the growing need for wound care products, many advanced wound care options have been developed, which include hyperbaric oxygen therapy 2, 3, negative pressure wound therapy (NPWT) 4, 5, 6, platelet‐rich plasma therapy 7 and heparan sulphate agents 8.
However, despite the variety of advanced therapies, the healing of chronic and complex wounds can stall or the wounds become unresponsive to treatment. Here, health care providers should become more proactive in wound surface reduction and consider the application of advanced wound therapies at an early stage. When wounds fail to respond to treatment, it is recommended that the wound be re‐assessed and a new treatment initiated 9, 10, 11. Here, we describe the adjunctive use of epidermal grafting in 78 patients with stalled wounds.
Materials and methods
Patient and wound evaluations
Patients were referred by general hospitals, University medical centres or general practitioners for treatment. During the first consultation, patient medical history and medication was reviewed by a physician and the wound assessed by a tissue viability nurse and a surgeon. After the multidisciplinary meeting reviewing aetiology, duration of wound, comorbidities, wound cultures and imaging results convened, a medical treatment plan was initiated.
Surgical necrotectomy and debridement
Every wound underwent a surgical necrotectomy performed by a vascular surgeon in the wound care treatment centre.
Sharp debridements were performed by the tissue viability nurse or a physician, with the use of local anaesthesia if indicated. All wounds required multiple debridement procedures twice weekly (range: 9–47 procedures).
Wound bed preparation
Each patient received multiple and/or different types of wound bed preparations based on individualised treatment plans. The wound bed preparation procedures are described below.
Hyperbaric oxygen therapy
A multi‐place hyperbaric chamber (IHC Hytech, Raamsdonkveer, The Netherlands) was used, in which 12 patients can be treated simultaneously. Patients were treated once daily at an absolute pressure of 2·5 atmospheres. At this pressure, 100% of oxygen was breathed through a mask during four periods for a total of 85 minutes.
Compression therapy
Compression therapy consisted of single‐layer, double‐layer or compression stockings. Patients could receive one or more compression treatments.
Negative pressure wound therapy
Wounds with exposed bone or tendon were covered with a non‐adherent wound contact layer (ADAPTIC TOUCH™ Non‐Adhering Silicone Dressing, Systagenix, an ACELITY Company, Gargrave, UK). NPWT dressings were cut to fit the wound size. Peri‐wound skin was covered with drape for protection. NPWT [V.A.C. VIA™ Therapy (KCI, an ACELITY Company, San Antonio, TX), NANOVA™ Therapy System (KCI, an ACELITY Company), PICO™ System (Smith & Nephew Inc., Hull, UK)] or another NPWT system was initiated following the manufacturer's instructions.
Platelet‐rich plasma (PRP) therapy
Wounds that did not show a tendency to heal after debridement, compression and/or NPWT (i.e. >10% of wound size reduction in 2 weeks) were selected for PRP therapy. At point of care, 55 ml of whole blood was pre‐donated and prepared for neutrophil‐poor PRP production (Protocol A), using a platelet concentrating system with dedicated sterile disposables and a 544E centrifuge (PurePRP®; EmCyte Corporation, Ft. Myers, FL). From the sequestered blood components, autologous thrombine was produced to activate the PurePRP® to form a wound topical clot to cover the wound area after a sharp debridement to stimulate and microfracture the wound bed. The platelet clot lysed, secreting platelet‐derived growth factors, cytokines and other proteins in the wound bed to induce cell signalling to regenerate tissue healing 12, 13. Initially, two PRP treatments were performed weekly. Thereafter, the procedures were switched to bi‐weekly. After a PurePRP® procedure, the primary dressing was covered with a naturally derived porcine intestinal submucosa matrix (OASIS® wound matrix; Cook Biotech, Inc, West Lafayette, IN) followed a hydrocolloid secondary dressing (DuoDERM® extra thin dressing; ConvaTec, Greensboro, NC).
Heparan sulphate agent
Following sharp debridement, a heparin sulphate agent (Cacipliq®, OTR3®; Paris, France) was added to a sterile gauze dressing and applied to the wounds, remaining in contact with the wound bed for at least 5 minutes. The gauze dressing was removed and the wound dressed with a hydrofibre dressing (AQUACEL®; ConvaTec, Greensboro, NC).
Epidermal harvesting
The donor sites (inner thigh, contra lateral to the wound sites) were prepared for graft harvesting by hair removal and cleansing with 70% ethanol. The epidermal harvesting system (CELLUTOME™ Epidermal Harvesting System; KCI, an ACELITY Company, San Antonio, TX) was attached to the donor site. Epidermal microdomes were created using heat and negative pressure (30–45 minutes, depending on skin thickness). The epidermal micrografts were transferred to the wound using a non‐adherent contact layer (ADAPTIC TOUCH™ dressing). The epidermal grafts were bolstered with NPWT. The donor sites were covered with a hydrocolloid dressing (DuoDERM® extra thin dressing) and left in place for at least 7 days. Wound and donor site healing were monitored at each dressing change.
Results
Patient demographics
A total of 78 patients with an average age of 64·1 ± 15·6 years and average body mass index (BMI) of 28·0 ± 5·2 kg/m2 were included in the study (Table 1). Common patient comorbidities included hypertension (47·4%), venous insufficiency (37·2%), obesity (28·2%) and diabetes (20·5%). Wound duration was 13·2 months (range: 0·3–180 months) with an average wound surface area of 14·9 ± 20·6 mm2 (Table 2). The most common wound types were wound dehiscence (29·5%), radiation ulcer (24·4%) and venous ulcer (17·9%).
Table 1.
Patient demographics
| Characteristic | n = 78 |
|---|---|
| Age (years ± sd) | 64·1 ± 15·6 |
| Gender | |
| Female | 56 (71·8%) |
| Male | 22 (28·2%) |
| BMI (kg/m2 ± sd) | 28·0 ± 5·2 |
| Comorbidities | |
| Hypertension | 37 (47·4%) |
| Venous insufficiency | 29 (37·2%) |
| Obesity (BMI > 30 kg/m2) | 22 (28·2%) |
| Diabetes mellitus | 16 (20·5%) |
| Peripheral arterial disease | 14 (17·9%) |
| Stroke | 10 (12·8%) |
| AMI | 9 (11·5%) |
| COPD | 7 (9·0%) |
| CHD | 4 (5·1%) |
AMI, acute myocardial infarction; BMI, body mass index; CHD, congestive heart disease; COPD, chronic obstructive pulmonary disease; sd, standard deviation.
Table 2.
Wound characteristics
| Wound characteristic | n = 78 |
|---|---|
| Wound duration (months ± sd) | 13·2 ± 25·2 |
| Wound area (mm2 ± sd, n = 76) | 14·9 ± 20·6 |
| Wound type | |
| Dehiscence | 23 (29·5%) |
| Radiation ulcer | 20 (25·7%) |
| Venous ulcer | 14 (17·9%) |
| Diabetic ulcer | 7 (9·0%) |
| Arterial ulcer | 4 (5·1%) |
| Crush injury | 3 (3·8%) |
| Osteomyelitis | 2 (2·6%) |
| Compromised flap | 2 (2·6%) |
| Martorell ulcer | 2 (2·6%) |
| Immunosuppressive medication | 1 (1·3%) |
Sd, standard deviation.
Wound bed preparation
Prior to epidermal grafting, the wounds underwent various means of wound bed preparation, including hyperbaric oxygen therapy (39·7%), NWPT (85·7%), compression therapy (55·7%) and PRP (29·2%) (Table 3).
Table 3.
Wound bed preparation
| Wound bed preparation | N (%) |
|---|---|
| Hyperbaric oxygen therapy (n = 70) | 31 (44·3) |
| Negative pressure wound therapy (n = 70) | 60 (85·7) |
| Compression therapy (n = 70)* | 39 (55·7) |
| Single‐layer compression | 7 (17·9) |
| Double‐layer compression | 35 (89·7) |
| Compression stocking | 11 (28·2) |
| Oedema therapy | 1 (2·6) |
| Platelet‐rich plasma (n = 72) | 21 (29·2) |
| Heparan sulphate agent (n = 77) | 23 (29·9) |
Patients could have one or more compression therapy treatment.
Wound‐healing outcomes
Total duration of wound healing from the first debridement to final wound closure was 26 weeks. Average time after epidermal grafting to wound closure was 10·0 ± 7·3 weeks, with 66 wounds (84·6%) reaching full wound closure (Table 4). Of the healed wounds, 49 healed within 3 months, 16 healed after 3 months, and the remaining healed wound was missing time to heal data. Ten wounds (12·8%) underwent partial wound healing, showing an average wound reduction of 44·5% ± 20·1%. Two wounds showed no wound surface area reduction after 3 months follow up.
Table 4.
Wound closure outcomes
| Wound closure | |
|---|---|
| Closure within 3 months (n = 72) | 49 (68·1%) |
| Closed after 3 months (n = 72) | 16 (22·2%) |
| Total wound healing time (weeks ± sd, n = 72) | 10 ± 7·3 |
| Full wound closure (n = 78) | 66 (84·6%) |
| Partial wound closure (n = 78) | 10 (12·8%) |
| Percent of wound closure (mean ± sd; n = 10) | 44·5% ± 20·1% |
| Non‐healing (n = 78) | 2 (2·6%) |
sd, standard deviation.
Cases
Two cases representative of wound care in this patient population are presented below.
Case 1
A 63‐year‐old male presented with chronically recurring wounds on his right foot with underlying osteomyelitis following Haglundse exostose removal over 20 years earlier (Figure 1A). The current wounds had been present for 33 weeks. Oral antibiotics (amoxicillin/clindamycine acid) were initiated. The patient underwent sharp debridement twice a week for 3 months followed by 6 weeks of compression therapy (Figure 1B). The patient also underwent 39 sessions of HBOT and three PRP applications. The wound was closed 3 weeks following epidermal grafting (Figure 1C). The epidermal harvesting system was attached to the patient's prepared thigh (Figure 2A). The donor site showed signs of healing 1 week post‐epidermal harvesting (Figure 2B) and was fully healed without complications 2 weeks post‐epidermal harvesting (Figure 2C).
Figure 1.

Chronic recurring skin infection on right foot with osteomyelitis. (A) Wound at presentation. (B) Wound after multiple debridements. (C) Wound fully healed 3 weeks post‐grafting.
Figure 2.
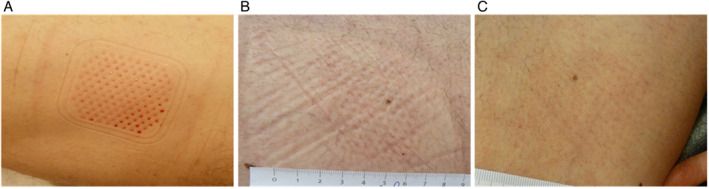
Case 1 donor site post‐graft harvesting. (A) Donor site immediately after graft harvesting. (B) Donor site 1 week post‐graft harvesting. (C) Donor site fully healed 2 weeks post‐graft harvesting.
Case 2
A 51‐year‐old female presented with a 4‐week‐old wound following a breast reconstruction post‐radiation wound (Figure 3A). Oral antibiotics (amoxicillin and clavulanic acid) were initiated. The wound underwent sharp debridement twice per week for 16 weeks followed by treatment with a heparin sulphate agent to stimulate extracellular matrix repair. The patient also underwent 40 sessions of HBOT and NPWT for 2 weeks. The wound showed improvement 2 weeks post‐epidermal grafting (Figure 3B) and was fully healed 6 weeks post‐grafting (Figure 3C). Epidermal grafts were harvested from the patient's prepared thigh (Figure 3D). The donor site showed signs of healing 1 week post‐epidermal harvesting (Figure 3E) and was fully healed without complications 2 weeks post‐epidermal harvesting (Figure 3F).
Figure 3.
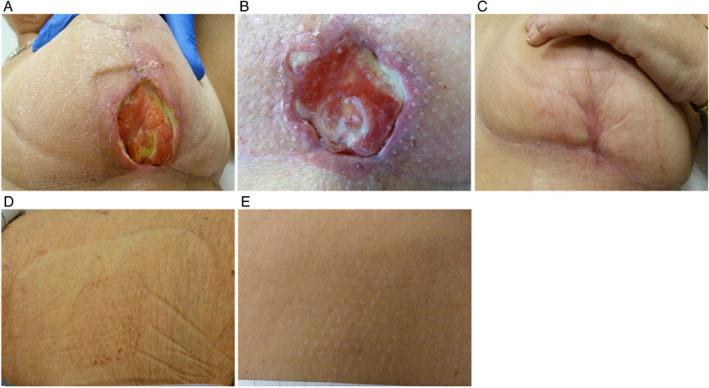
Wound after breast reconstruction post‐radiation therapy. (A) Wound at presentation. (B) Wound 2 weeks post‐grafting. (C) Wound fully healed 6 weeks post‐grafting. (D) Donor site 1 week post‐graft harvesting. (E) Donor site fully healed 2 weeks post‐graft harvesting.
Discussion
During wound healing, epithelialisation in the shortest possible time is critical to avoid potential infections and improve the patient's quality of life. As such, we recommend that health care providers should become more aggressive and proactive in wound bed preparation and consider using therapies aimed at complete wound closure at an early stage. We report that adjunctive use of epidermal grafting, along with adequate wound bed preparation, bolster dressings and NPWT, in 78 patients with chronic wounds resulted in complete closure of 68% of wounds within 3 months.
Early published experiences using epidermal grafts harvested with a commercially available harvesting system to manage wounds have been promising. Richmond et al. first used epidermal grafts in patients with pyoderma gangrenosum, a disorder characterised by recurrent skin ulcers 14. In these patients, wound closure was observed within 3 months in three out of five patients, and wound improvement was seen in the remaining two patients. Gabriel et al. described their initial experiences using epidermal grafts in four patients with various wound types and comorbidities, including diabetes, obesity and cancer. They reported complete wound healing in three out of four wounds and a 50% reduction in the fourth wound 15. Similarly, Serena et al. utilised epidermal grafting in patients with chronic wounds in a resource‐poor country. Six out of seven wounds either improved or completely healed within 4 weeks of grafting 16.
Based on the wound‐healing results in those early reports, larger case studies featuring complex patients and wounds have been recently published. Fearmonti utilised epidermal grafting in 22 patients with 23 lower extremity wounds and who had multiple comorbidities, including diabetes, obesity, peripheral arterial disease and coronary artery disease 17. The average percentage of reepithelialisation was 88·1% in these patients. Eleven wounds (52·4%) showed complete reepithelialisation, while 10 wounds showed partial reepithelialisation ranging from 50% to 99%. The remaining two wounds experienced complete graft loss due to patient non‐compliance 17. In a slightly larger case series, Bhatia used epidermal grafting in 34 patients with multiple comorbidities and chronic wounds. Following epidermal grafting, 82·4% (28/34) showed complete healing, 1 wound showed improved healing, 4 wounds did not heal and 1 wound was lost to follow up 18. In these patients, the mean epithelialisation rate was 7 weeks. Similar results were seen in the more recently published Lincoln and colleague case series. The authors examined the use of epidermal micrografts in 82 patients with 90 complex wounds and comorbidities, such as hypertension, obesity, peripheral vascular disease and diabetes 19. Following application of epidermal micrografts, 75 wounds (83·3%) showed complete reepithelialisation, 4 wounds (4·4%) improved, and 11 (12·2%) failed to heal. The mean time to wound healing was 16·9 weeks 19.
Our use of epidermal grafting in patients with stalled wounds achieved similar results as the published studies above, with a majority of the wounds reaching full closure without complications. Our findings are also supported by two recent international studies using epidermal grafting to manage wounds. Prakash et al. treated 18 patients with epidermal grafts in India, a resource‐poor country 20. Of the 18 wounds, 16 (88·9%) showed complete healing, with a mean time to wound epithelialisation of 3·7 weeks. The UK multicentre study examined epidermal grafting in 35 patients with either acute wounds (n = 10) or chronic wounds (n = 25) 21. Complete wound healing was achieved in 22 (62·9%) patients, two wounds showed improvement, three wounds showed no change, one wound showed improved wound bed activity, and seven wounds became infected, resulting in graft loss. Time to complete wound healing ranged from 6 to 20 weeks, and no differences in wound‐healing times were observed between the acute and chronic wounds.
Minimal pain at the donor sites during harvesting was reported by our patients. This is supported by the findings of the healthy‐human study by Osborne and colleagues. This study measured pain using the Wong‐Baker FACES pain rating scale during epidermal graft harvesting 22, 23. The healthy subjects (n = 15) reported minimal pain during and immediately after graft harvesting and were completely pain free by 7 days post‐harvesting. Minimal pain during harvesting in patients was also reported by Hachach‐Haram et al. 21 and Lincoln et al. 19. A majority of studies using epidermal grafting in wound management did not set out to directly measure patient‐reported pain. While the published literature suggests the patients experience minimal to no pain, planned pain studies are needed. Additionally, the donor sites in our study also healed without complications 1‐2 weeks post‐epidermal harvesting, similar to what has been reported in the published literature 14, 15, 16, 17, 18, 19, 20, 21.
For chronic or complex wounds, aggressive and proactive efforts are needed to reach full wound closure. A multidisciplinary panel from Kirsner and colleagues provided guidelines for the use of epidermal grafting to achieve wound closure 24. The panel recommended that all patients be assessed and the appropriate patient‐specific treatment plan selected 24. Once the patient and wound have been optimised (i.e. controlled blood glucose, wound infection management), preparation of the wound bed should be undertaken followed by wound closure methods 24. In our patients, we utilised epidermal grafting following various wound bed preparation therapies, such as hyperbaric oxygen treatment, NPWT, compression therapy, heparan sulphate agents and/or PRP therapy. Based on the presented data, health care providers should become more proactive in expediting wound surface reduction, initiate biological therapies early for optimal wound bed preparation and consider the application of epidermal grafting at an early stage.
References
- 1. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care 2009;18:154–61. [DOI] [PubMed] [Google Scholar]
- 2. Kranke P, Bennett M, Roeckl‐Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2004;2:CD004123. [DOI] [PubMed] [Google Scholar]
- 3. Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. PM R 2009;1:471–89. [DOI] [PubMed] [Google Scholar]
- 4. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 5. Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118:390–7; discussion 398–400. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium . Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 7. Lacci KM, Dardik A. Platelet‐rich plasma: support for its use in wound healing. Yale J Biol Med 2010;83:1–9. [PMC free article] [PubMed] [Google Scholar]
- 8. Papanas N, Demetzos C, Pippa N, Maltezos E, Tentolouris N. Efficacy of a new heparan sulfate mimetic dressing in the healing of foot and lower extremity ulcerations in type 2 diabetes: a case series. Int J Low Extrem Wounds 2016;15:63–7. [DOI] [PubMed] [Google Scholar]
- 9. Snyder RJ, Kirsner RS, Warriner RA III, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010;56:S1–24. [PubMed] [Google Scholar]
- 10. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 11. Snyder RJ, Fife C, Moore Z. Components and quality measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in wound care. Adv Skin Wound Care 2016;29:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everts PA, Knape JT, Weibrich G, Schonberger JP, Hoffmann J, Overdevest EP, Box HA, van Zundert A. Platelet‐rich plasma and platelet gel: a review. J Extra Corpor Technol 2006;38:174–87. [PMC free article] [PubMed] [Google Scholar]
- 13. Everts PA, Jakimowicz JJ, van Beek M, Schonberger JP, Devilee RJ, Overdevest EP, Knape JT, van Zundert A. Reviewing the structural features of autologous platelet‐leukocyte gel and suggestions for use in surgery. Eur Surg Res 2007;39:199–207. [DOI] [PubMed] [Google Scholar]
- 14. Richmond NA, Lamel SA, Braun LR, Vivas AC, Serena T, Kirsner RS. Epidermal grafting using a novel suction blister‐harvesting system for the treatment of pyoderma gangrenosum. JAMA Dermatol 2014;150:999–1000. [DOI] [PubMed] [Google Scholar]
- 15. Gabriel A, Sobota RV, Champaneria M. Initial experience with a new epidermal harvesting system: overview of epidermal grafting and case series. Surg Technol Int 2014;25:55–61. [PubMed] [Google Scholar]
- 16. Serena T, Francius A, Taylor C, Macdonald J. Use of a novel epidermal harvesting system in resource‐poor countries. Adv Skin Wound Care 2015;28:107–12. [DOI] [PubMed] [Google Scholar]
- 17. Fearmonti RM. Efficacy of epidermal skin grafts over complex, chronic wounds in patients with multiple comorbidities. Wounds 2016;28:226–32. [PubMed] [Google Scholar]
- 18. Bhatia A. Epidermal skin grafting in patients with complex wounds: a case series. J Wound Care 2016;25:150–3. [DOI] [PubMed] [Google Scholar]
- 19. Lincoln K, Hyde J. Evaluation of epidermal skin grafts for the treatment of complex wounds in a wound care center: a 94‐patient case series. Wounds 2016;28:347–53. [PubMed] [Google Scholar]
- 20. Prakash TV, Chaudhary A, Purushothaman S, Arvind KVS. Epidermal grafting for chronic complex wounds in India: a case series. Cureus 2016;8:e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hachach‐Haram N, Bystrzonowski N, Kanapathy M, Smith O, Harding K, Mosahebi A, Richards T. A prospective, multicentre study on the use of epidermal grafts to optimise outpatient wound management. Int Wound J 2017;14:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osborne SN, Schmidt MA, Harper JR. An automated and minimally invasive tool for generating autologous viable epidermal micrografts. Adv Skin Wound Care 2016;29:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osborne SN, Schmidt MA, Derrick K, Harper JR. Epidermal micrografts produced via an automated and minimally invasive tool form at the dermal/epidermal junction and contain proliferative cells that secrete wound healing growth factors. Adv Skin Wound Care 2015;28:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirsner RS, Bernstein B, Bhatia A, Lantis J, Le L, Lincoln K, Liu P, Rodgers L, Shaw M, Young D. Clinical experience and best practices using epidermal skin grafts on wounds. Wounds 2015;27:282–92. [PubMed] [Google Scholar]


