Abstract
Diabetes mellitus is a common metabolic disorder. Among various complications, diabetic neuropathy and peripheral vascular disorders are closely associated with diabetic foot ulcers (DFUs). Lower extremity ulcers and amputations are ongoing problems among individuals with diabetes. There are several classification systems for DFUs; however, no prognostic system has to date been accepted as the gold standard or the optimum prediction tool for amputations. A retrospective study was designed. Demographic data and baseline laboratory data were gathered and scored or evaluated using five representative DFU classification systems. These included (i) the diabetic ulcer severity score (DUSS); (ii) University of Texas (UT) diabetic wound classification; (iii) Meggitt–Wagner classification; (iv) depth of the ulcer, extent of bacterial colonisation, phase of ulcer and association aetiology (DEPA) scoring system; and (v) site, ischaemia, neuropathy, bacterial infection and depth (SINBAD) score. Finally, a statistical analysis was performed. A total of 137 patients were included in this study. During the follow‐up, DFU had healed in 51·1% of subjects and 48·9% of the individuals underwent lower extremity amputations (LEAs). In a univariable logistic regression analysis, history of previous DFU, hypertension, neuropathy, haemoglobin, C‐reactive protein (CRP) and ankle‐brachial index (ABI) showed a statistically significant difference between the healed group and the LEA group. Moreover, the stages, grades or overall prognostic ability of all five classifications were highly associated with the overall occurrence of LEA. On multivariable logistic regression analysis of the risk of LEA, all classifications showed a significant positive trend with an increased number of amputations. All the five classification systems exhibited high sensitivity, specificity, classification accuracy, positive predictive, negative predictive and area under the curve (AUC) values. They showed substantial accuracy and their main variables were associated with LEA occurrence. The Wagner and UT systems, although they are relatively simple to assess, were better predictors of LEA.
Keywords: Amputation, Classification, Diabetic foot ulcer
Introduction
Diabetes mellitus is one of the most common metabolic disorders, with a worldwide prevalence of 6·4% and involving an increasing number of patients globally 1. Patients with diabetes are at high risk of complications, the most important of which are diabetic neuropathy and peripheral vascular disorders, which lead to diabetic foot ulcers (DFUs) 2. DFUs are one of the most serious, expensive and alarming complications and compromise the well‐being and survival of diabetic patients 3.
The decisive factors in the aetiology of DFUs are diabetic neuropathy, macroangiopathy and the combination of neuropathy and macroangiopathy 4. Diabetic foot can present as neuropathic alone, mixed neuropathic and ischaemic, or ischaemic with infection 5. Lower extremity ulcers and amputations are an increasing problem among individuals with diabetes. In the USA, diabetic patients account for approximately 3% of the total population. About 1–4% of diabetic patients develop DFUs annually, and 15–25% of them develop DFUs during the course of their disease. More than 50% of diabetic patients undergo lower extremity amputation (LEA) and 85% of those who have undergone lower limb amputations develop intractable DFUs 6, 7.
DFU classification systems are essential tool for assessing and selecting treatment and for improving communication among health care professionals. They also facilitate standardisation of prognostic estimation and identify patients who require specialised care 8. Thus, a single or simplified classification system of DFUs, which includes the most accurate predictive factors for LEA, would facilitate decision‐making. Nevertheless, no prognostic system has to date been accepted as the gold standard 9. Therefore, to predict LEA occurrence and to determine the factors associated with and predictive of the risk of amputation in DFU patients 10, 11, 12, 13, 14, we aimed to compare the accuracy of the following five classification systems: (i) diabetic ulcer severity score (DUSS); (ii) University of Texas (UT) diabetic wound classification, (iii) Meggitt–Wagner classification, (iv) depth of the ulcer, extent of bacterial colonisation, phase of ulcer and association aetiology (DEPA) scoring system and (v) site, ischaemia, neuropathy, bacterial infection and depth (SINBAD) score.
Patients and methods
Type of study and selection of participants
A retrospective study was conducted via chart and photographic review, consecutively including all subjects with diabetes and active foot ulcer who visited the hospital from January 2010 to December 2014. Patients were defined as subjects who were admitted to the hospital for DFU. A foot ulcer was defined as a full‐thickness skin break at least to Wagner stage 1, occurring distal to the malleolus 15. A total of 158 patients were invited to participate in the study.
Data collection
The authors collected demographic data of patients – including age, sex, history of DFU, duration of hypertension, duration of diabetes, the presence of diabetic complications (retinopathy, nephropathy, neuropathy) and body mass index (BMI) – using medical records. Hypertension was diagnosed when the recorded value of systolic blood pressure was ≥140 mm Hg and that of diastolic blood pressure was ≥90 mm Hg 16. Retinopathy was diagnosed when non‐proliferative or proliferative retinopathy was observed upon fundus examination by an ophthalmologist. Nephropathy was defined as serum creatinine >1·5 mg/dl or a need for dialysis. Peripheral neuropathy was diagnosed when the patients had neuropathic symptoms and signs or objectively abnormal results on the Semmes–Weinstein 5·07/10 g monofilament test, without any other significant disease 17. BMI is a measure of body fat based on height and weight and is defined as the body mass divided by the square of the height, and it is universally expressed in kg/m2.
Baseline laboratory data – including glycosylated haemoglobin A (HbA1c), haemoglobin, white blood cell (WBC) count, total protein, serum creatinine, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), which mainly reflect the level of blood glucose, infection, nutrition and metabolism – were collected. The ankle‐brachial index (ABI) was also determined to confirm the presence of peripheral artery disease (PAD). ABI was calculated using the formula: ABI = (maximum systolic pressure in the dorsalis pedis artery or tibialis posterior)/(maximum systolic pressure in the brachial artery). PAD was defined as an ABI < 0·9 18.
Complete healing was defined as ulcer closure with no further need for any dressing 11. Amputation was defined as the complete loss in the transverse anatomical plane of any part of the lower limb. Minor amputations were defined as removal of a part of the foot distal to the transverse tarsal joint, with preservation of the talus and calcaneus. Major amputations were defined as transtibial amputation 19. The foot area was divided into four regions. Toe was defined as the region distal to the metatarsophalangeal joint. The area from the midshaft of the metatarsals distally was referred to as the forefoot. The midfoot was defined as the region between the proximal tarsal row and the midshaft of the metatarsals. The hindfoot was defined as the region from the malleolus to calcaneal area and the ankle was confined to the malleolar area.
As mentioned earlier, five classification systems available for LEA prediction in subjects with diabetes and active foot ulcer were applied. These systems were: (i) the DUSS, (ii) UT diabetic wound classification, (iii) Meggitt–Wagner classification, (iv) DEPA scoring system and (v) SINBAD score 10, 11, 12, 13, 14. Characterisation variables of all subjects were retrieved from photographic findings and confirmed with the patients' medical records. A total of 158 ulcers were graded using the aforementioned classification systems. DUSS was created using four clinically defined parameters – palpable pedal pulses, probing to bone, ulcer location and presence of multiple ulcerations. Palpable pedal pulses were categorised by the absence (scored as 1) or presence (scored as 0) of pedal pulses, while probing to bone was defined as yes (scored as 1) or no (scored as 0). The site of ulceration was defined as toe (scored as 0) or foot (scored as 1) ulcer. Patients with multiple ulcerations were graded as 1 compared with those with single ulcers (scored as 0) 10. The UT system assesses ulcer depth, the presence of wound infection and the presence of clinical signs of lower extremity ischaemia. This system uses a matrix of grade on the horizontal axis and stage on the vertical axis. The grades of the UT system are as follows: grade 0 (pre‐ or post‐ulcerative site that has healed), grade 1 (superficial wound not involving tendon, capsule or bone), grade 2 (wound penetrating to tendon or capsule) and grade 3 (wound penetrating bone or joint). Within each wound grade, there are four stages: clean wounds (stage A), non‐ischaemic infected wounds (stage B), ischaemic non‐infected wounds (stage C) and ischaemic infected wounds (stage D) 12. The Wagner system assesses ulcer depth and the presence of osteomyelitis or gangrene using the following grades: grade 0 (pre‐ or post‐ulcerative lesion), grade 1 (partial/full‐thickness ulcer), grade 2 (probing to tendon or capsule), grade 3 (deep with osteitis), grade 4 (partial foot gangrene) and grade 5 (whole foot gangrene) 13. The DEPA scoring system includes the depth of the ulcer (D), the extent of bacterial colonisation (E), the phase of ulcer healing (P) and the associated underlying aetiology (A). The depth of the ulcer was given a score of 1 for superficial ulcers, 2 for ulcers extending down to the subcutaneous tissues and tendons and 3 for ulcers reaching the bone. The extent of bacterial colonisation was given 1 point for contamination, 2 points for active infection and 3 points for sepsis or necrotising infection. The phase of healing was graded as 1 for the granulating phase, 2 for the inflammatory phase and 3 for the non‐healing phase. Finally, the associated underlying cause was given 1 point for neuropathic ulcers, 2 points for ulcers in association with structural deformity and 3 points for ulcers in association with chronic lower limb ischaemia. Overall, a patient with a score of 6 or less was deemed to have a low‐grade ulcer, a patient with a score of 7–9 a moderate‐grade ulcer and a patient with a score of 10 or more a high‐grade ulcer 14. In the SINBAD system, the six elements were graded as follows: (i) ulcer site (forefoot: 0; midfoot/hindfoot: 1), (ii) ischaemia (blood flow relatively intact at least one pulse palpable: 0; evidence of ischaemia: 1), (iii) neuropathy (defined as being absent: 0; present: 1, on the basis of routine examination), (iv) bacterial infection (graded as absent: 0; present: 1), (v) area (ulcer < 1 cm2: 0; ulcer ≥ 1 cm2: 1) and (vi) depth (confined to skin and subcutaneous tissue: 0; reaching muscle, tendon or deeper: 1) 11.
Statistical analysis
Among the clinical and laboratory parameters, continuous variables were expressed as means with standard deviation (SD) or medians with interquartile range (IQR). Differences in these variables between groups were assessed using Student's t‐test or the Wilcoxon rank‐sum test as appropriate after testing the normality assumption using the Shapiro–Wilk test and the equal variance assumption using Levene's test. Categorical variables were expressed as frequencies with percentage (%) and compared with the chi‐squared test or Fisher's exact test, as appropriate. The Cochran–Armitage trend test was used to test the significance of the classification of amputation versus healed, according to whether the true status was presented in multiple‐ordered categories.
Univariable logistic regression analysis was performed to examine the association between the candidate predictors and the classification with amputation. For each of the candidate predictors, the odds ratio (OR) for the likelihood of amputation was calculated. Variables significant in univariable logistic regression were subjected to multivariable logistic regression for each classification. The variables considered clinically meaningful were also included in the multivariable logistic regression. The final model was determined by backward selection for initial multivariable logistic regression model.
After the final model was determined for each classification, the predicted probability for each subject was used as input to generate the receiver‐operating characteristic (ROC) curve. The area under the ROC curve (AUC) and the 95% confidence interval (CI) of this area were computed to evaluate the diagnostic ability. When the lower limit of the CI for AUC was >0·5, the diagnostic test was considered to have discriminatory potential. The AUC could distinguish between less predictive (0·5–0·7), moderately predictive (0·7–0·9) and highly predictive (0·9–1). The optimum cut‐off values of the probability for each subject to undergo amputation were determined by Youden's index. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the best cut‐off value were calculated. The AUCs of classifications were compared using the non‐parametric method suggested by DeLong et al. 20. A significance level of 0·05 was selected. All statistical analyses were performed using SPSS 14.0 KO for Windows and R 3.1.3, which is freely available online (http://cran.r‐project.org/).
Results
Initially, a total of 158 patients were included in this study, of which 21 patients who were discharged or lost to follow‐up were excluded, resulting in a final total of 137. Of them, 79 (57·7%) were males and 58 (42·3%) were females. The age of patients ranged from 27 to 89 years, with the mean age being 61·1 years. With respect to the complications of diabetes, 51·8% of patients had retinopathy, 55·5% of patients had nephropathy and 65·7% of patients had neuropathy. The mean BMI of the patients was 23·7 kg/m2. During the follow‐up, DFU had healed in 51·1% (n = 70, the healed group) of subjects and 48·9% (n = 67, the amputation group) of the individuals underwent a LEA. There was no statistically significant difference in the distribution of comorbidities and aforementioned complications among these groups, with the exception of age (P = 0·047), history of previous DFU (P = 0·011), hypertension (P = 0·013), duration of hypertension (P = 0·02) and neuropathy (P = 0·048) (Table 1). In the amputation group, the ratios of previous DFU (88·06% versus 68·57%), hypertension (86·57% versus 67·14%) and nephropathy (74·63% versus 57·14%) were higher and the duration of hypertension was longer (10·2 versus 7·4 years) than those in the healed group. The mean age of the patients who underwent amputation was also higher (63·4 versus 59·1 years).
Table 1.
Patients demographics per group
| Variables | Healed (n = 70) | Amputation (n = 67) | Total (n = 137) | Comparison (P‐value)* |
|---|---|---|---|---|
| Age (years), mean (SD) | 59·06 (14·42) | 63·37 (10·51) | 61·17 (12·8) | 0·047 |
| Sex, n (%) | ||||
| Male | 39 (55·71) | 40 (59·7) | 79 (57·66) | 0·765 |
| Female | 31 (44·29) | 27 (40·3) | 58 (42·34) | |
| Ulcer history, n (%) | 48 (68·57) | 59 (88·06) | 107 (78·1) | 0·011 |
| Hypertension, n (%) | 47 (67·14) | 58 (86·57) | 105 (76·64) | 0·013 |
| Duration of diabetes (years), mean (SD) | 15·94 (8·96) | 19·67(10·08) | 17·77 (9·67) | 0·072 |
| Duration of hypertension (years), mean (SD) | 7·36 (8·25) | 10·22 (8·44) | 8·76 (8·44) | 0·02 |
| Retinopathy, n (%) | 33 (47·14) | 38 (56·72) | 71 (51·82) | 0·342 |
| Nephropathy, n (%) | 36 (51·43) | 40 (59·7) | 76 (55·47) | 0·423 |
| Neuropathy, n (%) | 40(57·14) | 50 (74·63) | 90 (65·69) | 0·048 |
| BMI, mean (SD) | 24·41 (5·16) | 23·05 (3·06) | 23·74 (4·3) | 0·172 |
BMI, body mass index.
Statistically significant values are provided in bold.
In terms of baseline clinical and laboratory characteristics, haemoglobin (P = 0·021), CRP (P = 0·012) and ABI (P < 0·001) showed statistically significant differences between the groups. Distribution of location of DFU (P < 0·001) also showed a statistically significant difference. In this variable, the toe area was more strongly associated with amputation (80·6%) than other locations (Table 2). Univariable logistic regression analysis was performed to examine the association between the candidate predictors. History of previous DFU, hypertension, neuropathy, haemoglobin, CRP and ABI showed statistically significant differences (Table 3). These significant variables were selected for multivariable logistic regression for each DFU classification.
Table 2.
Baseline clinical and laboratory characteristics per group
| Variables | Healed (n = 70) | Amputation (n = 67) | Total (n = 137) | Comparison (P‐value) |
|---|---|---|---|---|
| HbA1c, median (IQR) | 7·5 (7–8·3) | 8 (7·1–9) | 7·7 (7–8·75) | 0·351 |
| Haemoglobin, mean (SD) | 10·48 (1·57) | 9·77 (1·98) | 10·14 (1·81) | 0·021 |
| WBC, median (IQR) | 7575 (5545–10040) | 7990 (6330–9975) | 7900 (6070–10040) | 0·481 |
| Total protein, mean (SD) | 6·49 (0·86) | 6·48 (0·91) | 6·49 (0·88) | 0·966 |
| Serum creatinine, median (IQR) | 1·7 (1–5·53) | 2·9 (1·25–7·65) | 1·9 (1·2–6·9) | 0·088 |
| ESR, median (IQR) | 59·5 (33·25–79·75) | 69 (41·5–87·5) | 65 (36–85) | 0·199 |
| CRP, median (IQR) | 0·97 (0·21–2·57) | 1·84 (0·48–6·57) | 1·27 (0·37–5·3) | 0·012 |
| ABI, n (%) | ||||
| ≥0·9 (normal) | 48 (68·57) | 15 (22·39) | 63 (45·99) | <0·001 |
| <0·9 (PAD) | 12 (17·14) | 34 (50·75) | 46 (33·58) | |
| Missing | 10 (14·29) | 18 (26·87) | 28 (20·44) | |
| Location, n (%) | ||||
| Toe | 23 (32·86) | 54 (80·6) | 77 (56·2) | <0·001 |
| Forefoot | 18 (25·71) | 6 (8·96) | 24 (17·52) | |
| Midfoot | 8 (11·43) | 7 (10·45) | 15 (10·95) | |
| Hindfoot | 8 (11·43) | 0 (0) | 8 (5·84) | |
| Ankle | 13 (18·57) | 0 (0) | 13 (9·49) |
ABI, ankle‐brachial index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HbA1c, glycosylated haemoglobin A; IQR, interquartile range; PAD, peripheral artery disease; WBC, white blood cell.
Table 3.
Logistic regression analysis for amputation
| Variable | Univariable | ||
|---|---|---|---|
| OR | 95% CI of OR | P‐value | |
| Age (years) | 1·03 | 1–1·06 | 0·051 |
| Sex (ref : female); male | 1·18 | 0·6–2·33 | 0·637 |
| Ulcer history | 3·38 | 1·43–8·72 | 0·008 |
| Hypertension | 3·15 | 1·37–7·8 | 0·009 |
| Duration of diabetes (years) | 1·04 | 1·01–1·08 | 0·026 |
| Duration of hypertension (years) | 1·04 | 1–1·09 | 0·049 |
| Retinopathy | 1·47 | 0·75–2·9 | 0·263 |
| Nephropathy | 1·4 | 0·71–2·77 | 0·331 |
| Neuropathy | 2·21 | 1·08–4·63 | 0·033 |
| BMI | 0·92 | 0·84–1 | 0·075 |
| HbA1c* | 1·07 | 0·18–6·45 | 0·938 |
| Haemoglobin | 0·79 | 0·64–0·96 | 0·024 |
| WBC* | 1·23 | 0·58–2·64 | 0·592 |
| Serum creatinine* | 1·35 | 0·94–1·96 | 0·106 |
| Total protein | 0·99 | 0·67–1·46 | 0·966 |
| ESR* | 1·24 | 0·77–2·07 | 0·384 |
| CRP* | 1·33 | 1·08–1·66 | 0·009 |
| Peripheral artery disease [ref : ABI ≥ 0·9 (Normal)] | 9·07 | 3·88–22·6 | 0·001 |
ABI, ankle‐brachial index; BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; OR, odds ratio; WBC, white blood cell.
Natural logarithmic transformations were performed before analysis.
The stages, grades and overall prognostic ability of all five classifications were highly associated with overall LEA occurrence (all P < 0·001; Table 4). For the DUSS and Wagner systems, the authors corrected two score groups that showed low frequency in the logistic regression analysis. A score of 4 on DUSS, which showed no healed patients and 11 amputation patients, was combined with a score of 3 on DUSS (3–4 score group). Also, a score of 0 on Wagner, which showed one healed patient and no amputation patients, was combined with a score of 1 (0–1 score group). Because the UT system uses a matrix of grade on the horizontal axis and stage on the vertical axis, ranking the groups according to severity is problematic. Hence, the criteria of the UT system were divided into two groups: (i) infection and ischaemia and (ii) depth. Both groups showed a significant positive trend with increasing number of amputations (P < 0·001). The DEPA and SINBAD systems were more complex than the other three systems and showed multiple score groups, because each parameter was rated on a point scale according to severity and complexity. Hence, these systems were divided into three groups (DEPA, low grade: 3–6, moderate grade: 7–9 and high grade: 10–12; SINBAD, low grade: 0–2, moderate grade: 3–4 and high grade: 5–6).
Table 4.
Classification score per group
| Variables | Healed (n = 70) | Amputation (n = 67) | Total (n = 137) | Trend (P‐value)* | |
|---|---|---|---|---|---|
| DUSS | 0 | 10 (14·29) | 4 (5·97) | 14 (10·22) | <0·001 |
| 1 | 38 (54·29) | 7 (10·45) | 45 (32·85) | ||
| 2 | 21 (30) | 33 (49·25) | 54 (39·42) | ||
| 3–4 | 1 (1·43) | 23 (34·33) | 24 (17·52) | ||
| UT | A | 22 (31·43) | 2 (2·99) | 24 (17·52) | <0·001 |
| B | 23 (32·86) | 9 (13·43) | 32 (23·36) | ||
| C | 13 (18·57) | 10 (14·93) | 23 (16·79) | ||
| D | 12 (17·14) | 46 (68·66) | 58 (42·34) | ||
| 1 | 29 (41·43) | 2 (2·99) | 31 (22·63) | <0·001 | |
| 2 | 38 (54·29) | 16 (23·88) | 54 (39·42) | ||
| 3 | 3 (4·29) | 49 (73·13) | 52 (37·96) | ||
| A1 | 13 (18·57) | 1 (1·49) | 14 (10·22) | <0·001 | |
| A2 | 9 (12·86) | 1 (1·49) | 10 (7·3) | ||
| B1 | 9 (12·86) | 0 (0) | 9 (6·57) | ||
| B2 | 13 (18·57) | 5 (7·46) | 18 (13·14) | ||
| B3 | 1 (1·43) | 4 (5·97) | 5 (3·65) | ||
| C1 | 6 (8·57) | 0 (0) | 6 (4·38) | ||
| C2 | 5 (7·14) | 2 (2·99) | 7 (5·11) | ||
| C3 | 2 (2·86) | 8 (11·94) | 10 (7·3) | ||
| D1 | 1 (1·43) | 1 (1·49) | 2 (1·46) | ||
| D2 | 11 (15·71) | 8 (11·94) | 19 (13·87) | ||
| D3 | 0 (0) | 37 (55·22) | 37 (27·01) | ||
| Wagner | 0–1 | 30 (42·86) | 2 (2·99) | 32 (23·36) | <0·001 |
| 2 | 36 (51·43) | 15 (22·39) | 51 (37·23) | ||
| 3 | 3 (4·29) | 21 (31·34) | 24 (17·52) | ||
| 4 | 1 (1·43) | 29 (43·28) | 30 (21·9) | ||
| DEPA | 3–6 | 33 (47·14) | 4 (5·97) | 37 (27·01) | <0·001 |
| 7–9 | 35 (50) | 20 (29·85) | 55 (40·15) | ||
| 10–12 | 2 (2·86) | 43 (64·18) | 45 (32·85) | ||
| SINBAD | 0–2 | 19 (27·14) | 2 (2·99) | 21 (15·33) | <0·001 |
| 3–4 | 45 (64·29) | 23 (34·33) | 68 (49·64) | ||
| 5–6 | 6 (8·57) | 42 (62·69) | 48 (35·04) | ||
DEPA, depth of the ulcer, extent of bacterial colonisation, phase of ulcer and association aetiology; DUSS, diabetic ulcer severity score; SINBAD, site, ischaemia, neuropathy, bacterial infection, and depth; UT, University of Texas.
P‐values were derived from Cochran–Armitage trend test.
On multivariable logistic regression analysis adjusted for age, sex, ulcer history, hypertension, neuropathy and log‐transformed CRP for evaluation of the risk of LEA, all classifications showed a significant positive trend with increasing number of amputations (Table 5). For example, using the DEPA scoring system, patients were fourfold more likely to undergo an LEA if the ulcer grade was moderate when compared with low grade (P = 0·036; OR = 4·05; 95% CI: 1·09–15·02). Also, patients with high‐grade ulcers were 160‐fold more likely to undergo an LEA compared with those with low‐grade ulcers (P < 0·001; OR = 160·05; 95% CI: 22·66–1130·49).
Table 5.
Logistic regression analysis for amputation
| Variable | Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI of OR | P‐value | OR | 95% CI of OR | P‐value | ||
| DUSS (ref: 0) | 1 | 0·46 | 0·11–2·04 | 0·282 | 0·31 | 0·06–1·57 | 0·156 |
| 2 | 3·93 | 1·15–15·85 | 0·036 | 2·77 | 0·56–13·69 | 0·212 | |
| 3–4 | 57·5 | 8·03–1222·67 | <0·001 | 68·24 | 5·1–912·23 | 0·001 | |
| UT (ref: A) | B | 4·3 | 0·97–30·36 | 0·081 | 4·24 | 0·71–25·24 | 0·113 |
| C | 8·46 | 1·87–60·94 | 0·012 | 7·9 | 1·32–47·26 | 0·024 | |
| D | 42·17 | 10·53–287·7 | <0·001 | 41·69 | 7·55–230·16 | <0·001 | |
| UT (ref: 1) | 2 | 6·11 | 1·57–40·58 | 0·022 | 3·96 | 0·75–20·94 | 0·106 |
| 3 | 236·83 | 46·43–2066·4 | <0·001 | 372·6 | 43·19–3214·77 | <0·001 | |
| Wagner (ref : 0–1) | 2 | 6·25 | 1·59–41·68 | 0·021 | 4·53 | 0·87–23·6 | 0·073 |
| 3 | 105 | 19·85–933·58 | <0·001 | 92·39 | 10·61–804·71 | <0·001 | |
| 4 | 435 | 55·6–10420·06 | <0·001 | 622·56 | 44·1–8789·65 | <0·001 | |
| DEPA (ref : 3–6) | 7–9 | 4·71 | 1·58–17·51 | 0·01 | 4·05 | 1·09–15·02 | 0·036 |
| 10–12 | 177·37 | 37·57–1409·35 | <0·001 | 160·05 | 22·66–1130·49 | <0·001 | |
| SINBAD (ref : 0–2) | 3–4 | 4·86 | 1·26–32·14 | 0·044 | 6·02 | 1·04–34·93 | 0·045 |
| 5–6 | 66·5 | 14·81–493·95 | <0·001 | 78·5 | 11·08–556·36 | <0·001 | |
CI, confidence interval; DEPA, depth of the ulcer, extent of bacterial colonisation, phase of ulcer and association aetiology; DUSS, diabetic ulcer severity score; OR, odds ratio; SINBAD, site, ischaemia, neuropathy, bacterial infection, and depth; UT, University of Texas.
The multivariable model was adjusted for age, gender, neuropathy, hypertension, ulcer history and log‐transformed C‐reactive protein.
To evaluate the accuracy of the classification systems, their sensitivity, specificity, classification accuracy, PPV, NPV and AUC were calculated (Table 6, Figure 1). The DUSS classification showed a higher sensitivity (0·84) than the other classification systems and the results of the UT system on the aspect of depth showed the highest specificity (0·96). AUC values were higher than 0·80 for all classification systems. The Wagner classification showed the highest accuracy (0·85) and AUC (0·8921), while the DUSS classification showed the lowest accuracy (0·76) and AUC (0·8012). On comparison of pairs of AUCs, the UT system on depth (P = 0·009), Wagner (P = 0·006) and DEPA (P = 0·006) systems showed statistically significant differences compared with the DUSS system. The DEPA (P = 0·003) and Wagner (P = 0·016) systems also showed statistically significant differences compared with the results of the UT system on the aspect of infection.
Table 6.
Diagnostic performance of five categorised score
| Variables | Threshold* | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC | 95% CI of AUC | Comparison (P‐value †) |
|---|---|---|---|---|---|---|---|---|---|
| DUSS | 1·5 | 56/67 (0·84) | 48/70 (0·69) | 104/137 (0·76) | 56/78 (0·72) | 48/59 (0·81) | 0·8012 | 0·7305–0·8719 | DUSS versus UT on wound depth, P = 0·009 |
| UT on infection | 3·5 | 46/67 (0·69) | 58/70 (0·83) | 104/137 (0·76) | 46/58 (0·79) | 58/79 (0·73) | 0·8065 | 0·7367–0·8763 | DUSS versus Wagner, P = 0·006 |
| UT on wound depth | 2·5 | 49/67 (0·73) | 67/70 (0·96) | 116/137 (0·85) | 49/52 (0·94) | 67/85 (0·79) | 0·8856 | 0·8343–0·9369 | DUSS versus DEPA, P = 0·006 |
| Wagner | 3·5 | 50/67 (0·75) | 66/70 (0·94) | 116/137 (0·85) | 50/54 (0·93) | 66/83 (0·8) | 0·8921 | 0·842–0·9422 | UT on infection versus DEPA, P = 0·003 |
| DEPA | 8·5 | 53/67 (0·79) | 59/70 (0·84) | 112/137 (0·82) | 53/64 (0·83) | 59/73 (0·81) | 0·8908 | 0·8375–0·9442 | UT on infection versus Wagner, P = 0·016 |
| SINBAD | 4·5 | 42/67 (0·63) | 64/70 (0·91) | 106/137 (0·77) | 42/48 (0·88) | 64/89 (0·72) | 0·8483 | 0·7857–0·9108 |
AUC, area under the curve; CI, confidence interval; DEPA, depth of the ulcer, extent of bacterial colonisation, phase of ulcer and association aetiology; DUSS, diabetic ulcer severity score; NPV, negative predictive value; PPV, positive predictive value; SINBAD, site, ischaemia, neuropathy, bacterial infection, and depth; UT, University of Texas.
The thresholds were computed by Youden's index.
The P‐values for the comparison of the pairs of AUCs were calculated by Delong's method.
Figure 1.
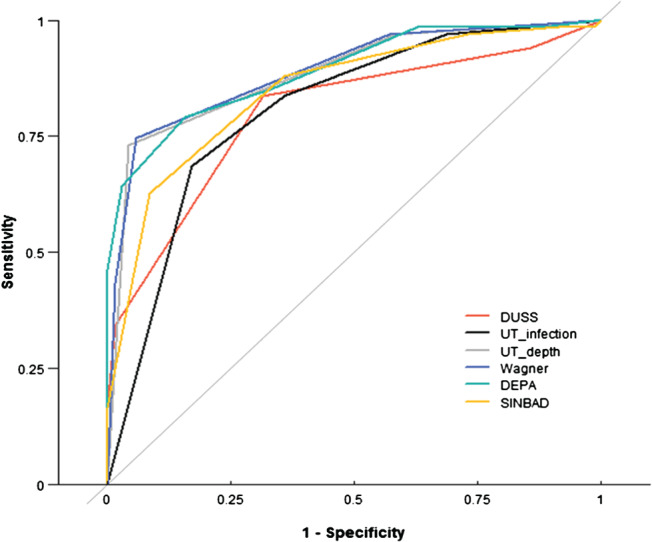
ROC curves of the five classification systems. In all classification systems, AUC values were higher than 0·80; the Wagner system had the highest value (0·8921).
Discussion
DFU is a major indication for lower extremity non‐traumatic amputation worldwide. The Global Lower Extremity Amputation Study Group estimated that 25% to 90% of all amputations were associated with diabetes 21. In this study, the overall amputation rate among diabetic foot patients was 48·9% (n = 67; major amputation: 3·6%, n = 5; minor amputation: 45·3%, n = 62). This indicated a high rate of LEA, as was reported by Won et al., who showed that 47% of DFU patients had undergone LEA (major amputation: 5%; minor amputation: 42%), although this study involved patients who underwent critical limb ischaemia 22. This is likely because almost all patients in this study presented to the department of plastic surgery when infection or ischaemic change had already occurred, and so the overall amputation rate was relatively high. Also, the locations of DFU were concentrated in the toe area (56·2%), which showed the highest amputation rate (70%).
Risk factors for LEA in terms of diabetic complications have been investigated intensively and can be used to define the risk assessment models. Risk factors for LEA among patients with diabetes include age, sex (male) and stroke, comorbidities such as ischaemic heart disease and hypertension, chronic complications such as PAD, nephropathy, duration of diabetes, sensory neuropathy and HbA1c 23, 24. However, inconsistent results have been reported and the risk factors have not yet been clarified. In this study, a history of previous DFU (P = 0·008), hypertension (P = 0·009), duration of diabetes (P = 0·026), duration of hypertension (P = 0·049), sensory neuropathy (P = 0·033), haemoglobin (P = 0·024), CRP (P = 0·009) and PAD (P = 0·001) showed statistically significant differences between the healed and the amputation groups in univariable logistic regression analyses.
A history of previous DFU increased the risk of LEA in univariable logistic regression analysis (P = 0·008; OR = 3·38). Wu and Armstrong reported that patients with a history of ulceration possess all the risk factors necessary for ulceration 25. Helm et al. reported that between 20% and 58% of patients develop another ulcer within a year of wound healing 26. In this study, 88% of patients who had undergone amputation had a history of previous DFU, which is comparable to a previous study that reports 85% of diabetic patients who had undergone lower limb amputations developed intractable DFU 6.
Hypertension and longer duration of hypertension increased the risk of LEA in univariable logistic regression analyses (P = 0·009, OR = 3·15; P = 0·049, OR = 1·04). This result is similar to that reported by Magalhaes et al., that is, amputees had a higher systolic blood pressure (BP) than non‐amputees (P < 0·05) and demonstrated increased arterial stiffness in subjects with LEA. These results supported a stronger correlation between arterial stiffness and systolic BP 27. A chronic increase in blood pressure may accelerate central arterial stiffening, thereby contributing to rapid structural and functional alterations in the walls of these arteries 28. Arterial stiffness is also associated with occurrence of PAD, which involves gradual reduction in blood flow to one or more limbs secondary to atherosclerosis. Although there are inconsistencies among studies regarding the risk factors, PAD is a major risk factor for LEA, in agreement with our results (P = 0·001, OR = 9·07). PAD is commonly seen in individuals with type 2 diabetes, in whom it occurs almost threefold more frequently compared with age‐ and sex‐matched non‐diabetic individuals 29.
An increased duration of diabetes appears to provide important information on LEA risk among DFU patients. The authors estimated that as the duration of diabetes increased, the occurrence of risk factors for LEA – such as infection, neuropathy, peripheral vascular disease and repeated DFU – may increase. In this study, the difference in duration (healed: 15·94 years versus amputation: 19·67 years) was significantly associated with a higher risk of amputation in a univariable logistic regression analysis (P = 0·026, OR = 1·04), as was reported by Resnick et al. 30
Sensory neuropathy, as measured by the 5.07 Semmes–Weinstein monofilament test, was significantly associated with a higher risk of amputation in a univariable logistic regression analysis (P = 0·033, OR = 2·21). In this study, the prevalence of sensory neuropathy was 65·6%, which is comparable with a previous report (69%) 24. Other studies also showed a significant association of sensory neuropathy with amputation 23, 24. Feng et al. reported that diabetic patients with sensory neuropathy have a 1·5–15‐fold higher risk of major amputation 31. Diabetic neuropathy is a consequence of chronically elevated blood sugar levels, which cause vascular and metabolic abnormalities. Elevated intraneural concentrations of sorbitol, a glucose byproduct, are thought to be one of the principle mechanisms of nerve damage. As a result, neuropathic changes, such as foot deformity, decreased protective sensation and skin fissures, caused by diminished sweating lead to formation of DFUs 32.
A lower haemoglobin level increased the risk of LEA in a univariable logistic regression analysis (P = 0·024, OR = 0·79). In terms of physiology, a higher blood haemoglobin level indicates greater delivery of oxygen molecules to local tissue, enhancing anabolism and catabolism occurred. Blood haemoglobin is also a good indicator of nutritional status. These two factors may explain why a lower blood haemoglobin level increased the risk of LEA in DFU 33.
The serum CRP level increases in response to inflammation, infection, trauma, tissue necrosis, malignancies and autoimmune disorders; hence, it is not specific. However, it can be used as a guide for managing diabetic foot disease and monitoring of disease progression as a rapid decrease in serum CRP level indicates a good response to treatment 34. Baseline levels of acute‐phase reactants are associated with increased amputation risk. Univariable analysis showed that baseline CRP levels were independent predictors of overall and major amputations, with the exception of baseline ESR. Indeed, it has been reported that CRP level decreased more rapidly than ESR after appropriate treatment, reflecting the effectiveness of therapy with a higher sensitivity than ESR 35. Similarly, Volaco et al. reported that elevated CRP levels were strongly predictive of major amputation in long‐standing diabetic patients with ischaemic foot lesions 36. Lipsky et al. found that elevated baseline levels of acute‐phase reactants (WBC, CRP and ESR) were associated with clinical treatment failure in diabetic foot infections treated with broad‐spectrum antibiotics 34. Moreover, patients with PAD had increased levels of inflammatory markers. Elevated levels of CRP have been associated with poor long‐term prognosis in patients with PAD 37.
Five classification systems showed a significant positive trend with increasing number of LEA in a multivariable logistic regression analysis (Table 5). This indicates that as the score or grade of classification was high at baseline for DFUs, the probability of predicting the risk of LEA was also increased. To compare the accuracy of these five classification systems, the authors evaluated the sensitivity, specificity, accuracy, PPV, NPV and AUC (Figure 1). Among the aforementioned values, the authors supposed that the available DFU classification systems have a high NPV, PPV and accuracy for the overall LEA prediction. Because the PPV (or NPV) was defined as the probability that the disease is (or not) present when the test is positive (or negative), high results can be interpreted to indicate the accuracy of such a statistic. The definitions of these values can be used to predict LEA occurrence, which facilitated comparison of the accuracy of classification systems for predicting LEA occurrence using baseline data. In this study, Wagner and UT on depth classification systems showed the highest PPVs (0·94 and 0·93), NPVs (0·79 and 0·80) and accuracies (both 0·85). ROC curves and AUC were used to compare the prediction accuracy. The AUC values of all systems were >0·80, and the Wagner system showed a higher AUC (0·8921) than DUSS (0·8012) and UT (0·8065) on infection. On comparison of pairs of AUCs, the UT on depth, Wagner and DEPA systems showed a statistically significant difference when compared with the DUSS system, and the DEPA and Wagner systems also showed a difference compared with UT on infection. Therefore, the Wagner system was most predictive of LEA. Also, Wagner 3.5 had the highest Youden's index and so may be considered as one of the referral criteria for high‐risk patients with DFUs. A study by Wang et al. published in 2010 by Chinese Journal of Diabetes also reported a poor prognosis of ulcer in patients with Wagner grade 3. Because UT on depth showed a similar predictive value to the Wagner system, the UT system, which combines grade and stage, is also more descriptive and shows a stronger association with increased risk of amputation.
This study had several limitations. First, the patient data were derived retrospectively from medical and laboratory records and photographic analysis, and hence, some information might have been lost. Second, as unified therapy was not administered for all ulcers, difference in medical care levels among physicians might have influenced the outcome, leading to bias. Third, there is a possibility of excess OR in the multivariable analysis because of assessment of a small number of patients with DFU and their uneven distribution. Despite these limitations, this study reported factors predictive of the risk of amputation in DFU patients and compared the accuracy of five classification systems for predicting LEA occurrence.
The authors conclude that all of the available systems have substantial accuracy and their main variables are associated with LEA occurrence. Above all, the Wagner and UT systems, which are simple and easy to use, are better predictors of LEA. Also, a history of previous DFU, hypertension, duration of diabetes, sensory neuropathy, haemoglobin, CRP and PAD were significant predictive variables for LEA.
Acknowledgement
This work was supported by the Soonchunhyang University Research Fund.
References
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 2. O'Loughlin A, McIntosh C, Dinneen SF, O'Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds 2010;9:90–102. [DOI] [PubMed] [Google Scholar]
- 3. Jeffcoate WJ. Stratification of foot risk predicts the incidence of new foot disease, but do we yet know that the adoption of routine screening reduces it? Diabetologia 2011;54:991–3. [DOI] [PubMed] [Google Scholar]
- 4. Stiegler H. Diabetic foot syndrome. Herz 2004;29:104–15. [DOI] [PubMed] [Google Scholar]
- 5. Lauterbach S, Kostev K, Kohlmann T. Prevalence of diabetic foot syndrome and its risk factors in the UK. J Wound Care 2010;19:333–7. [DOI] [PubMed] [Google Scholar]
- 6. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 7. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 8. Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24:84–8. [DOI] [PubMed] [Google Scholar]
- 9. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV. American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45:S1–66. [DOI] [PubMed] [Google Scholar]
- 10. Beckert S, Witte M, Wicke C, Konigsrainer A, Coerper S. A new wound‐based severity score for diabetic foot ulcers: a prospective analysis of 1,000 patients. Diabetes Care 2006;29:988–92. [DOI] [PubMed] [Google Scholar]
- 11. Ince P, Abbas ZG, Lutale JK, Basit A, Ali SM, Chohan F, Morbach S, Mollenberg J, Game FL, Jeffcoate WJ. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care 2008;31:964–7. [DOI] [PubMed] [Google Scholar]
- 12. Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg 1996;35:528–31. [DOI] [PubMed] [Google Scholar]
- 13. Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- 14. Younes NA, Albsoul AM. The DEPA scoring system and its correlation with the healing rate of diabetic foot ulcers. J Foot Ankle Surg 2004;43:209–13. [DOI] [PubMed] [Google Scholar]
- 15. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ, North‐West Diabetes Foot Care S. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabet Med 2002;19:377–84. [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure , National Heart, Lung, and Blood Institute , National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Y, Ran X, Jia L, Yang C, Wang P, Ma J, Chen B, Yu Y, Feng B, Chen L, Yin H, Cheng Z, Yan Z, Yang Y, Liu F, Xu Z. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in China. Int J Low Extrem Wounds 2015;14:19–27. [DOI] [PubMed] [Google Scholar]
- 18. Shahbazian H, Yazdanpanah L, Latifi SM. Risk assessment of patients with diabetes for foot ulcers according to risk classification consensus of International Working Group on Diabetic Foot (IWGDF). Pak J Med Sci 2013;29:730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int 2013;34:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 21. Global Lower Extremity Amputation Study Group . Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. The Global Lower Extremity Amputation Study Group. Br J Surg 2000;87:328–37. [DOI] [PubMed] [Google Scholar]
- 22. Won KB, Chang HJ, Hong SJ, Ko YG, Hong MK, Jang Y, Choi D. Prognostic usefulness of metabolic syndrome compared with diabetes in Korean patients with critical lower limb ischemia treated with percutaneous transluminal angioplasty. Yonsei Med J 2014;55:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hennis AJ, Fraser HS, Jonnalagadda R, Fuller J, Chaturvedi N. Explanations for the high risk of diabetes‐related amputation in a Caribbean population of black African descent and potential for prevention. Diabetes Care 2004;27:2636–41. [DOI] [PubMed] [Google Scholar]
- 24. Nather A, Bee CS, Huak CY, Chew JL, Lin CB, Neo S, Sim EY. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications 2008;22:77–82. [DOI] [PubMed] [Google Scholar]
- 25. Wu S, Armstrong DG. Risk assessment of the diabetic foot and wound. Int Wound J 2005;2:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helm PA, Walker SC, Pullium GF. Recurrence of neuropathic ulceration following healing in a total contact cast. Arch Phys Med Rehabil 1991;72:967–70. [PubMed] [Google Scholar]
- 27. Magalhaes P, Capingana DP, Silva AB, Capunge IR, Goncalves MA. Arterial stiffness in lower limb amputees. Clin Med Insights Circ Respir Pulm Med 2011;5:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005;45:652–8. [DOI] [PubMed] [Google Scholar]
- 29. Pradeepa R, Chella S, Surendar J, Indulekha K, Anjana RM, Mohan V. Prevalence of peripheral vascular disease and its association with carotid intima‐media thickness and arterial stiffness in type 2 diabetes: the Chennai urban rural epidemiology study (CURES 111). Diab Vasc Dis Res 2014;11:190–200. [DOI] [PubMed] [Google Scholar]
- 30. Resnick HE, Carter EA, Sosenko JM, Henly SJ, Fabsitz RR, Ness FK, Welty TK, Lee ET, Howard BV, Strong HS. Incidence of lower‐extremity amputation in American Indians: the strong heart study. Diabetes Care 2004;27:1885–91. [DOI] [PubMed] [Google Scholar]
- 31. Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination is a significant predictor of the risk of foot ulceration and amputation in patients with diabetes mellitus. J Vasc Surg 2011;53:220–6.e1‐5. [DOI] [PubMed] [Google Scholar]
- 32. Clemens MW, Colen LB, Attinger CE. Foot reconstruction. In: Neligan PC, editor. Plastic surgery, 3rd edn. London: Elservier, 2013:189–219. [Google Scholar]
- 33. Golinko MS, Margolis DJ, Tal A, Hoffstad O, Boulton AJ, Brem H. Preliminary development of a diabetic foot ulcer database from a wound electronic medical record: a tool to decrease limb amputations. Wound Repair Regen 2009;17:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lipsky BA, Sheehan P, Armstrong DG, Tice AD, Polis AB, Abramson MA. Clinical predictors of treatment failure for diabetic foot infections: data from a prospective trial. Int Wound J 2007;4:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roine I, Faingezicht I, Arguedas A, Herrera JF, Rodriguez F. Serial serum C‐reactive protein to monitor recovery from acute hematogenous osteomyelitis in children. Pediatr Infect Dis J 1995;14:40–4. [DOI] [PubMed] [Google Scholar]
- 36. Volaco A, Chantelau E, Richter B, Luther B. Outcome of critical foot ischaemia in longstanding diabetic patients: a retrospective cohort study in a specialised tertiary care centre. Vasa 2004;33:36–41. [DOI] [PubMed] [Google Scholar]
- 37. Violi F, Criqui M, Longoni A, Castiglioni C. Relation between risk factors and cardiovascular complications in patients with peripheral vascular disease. Results from the A.D.E.P. study. Atherosclerosis 1996;120:25–35. [DOI] [PubMed] [Google Scholar]


