ABSTRACT
Chronic wounds are associated with financial and personal costs. The system level expense associated with chronic wounds has been established, however, the out‐of‐pocket cost incurred by individuals who self‐fund has not been the focus of extensive investigation. Recently, there has been renewed interest in evaluating quality of life, in line with the shift to patient enablement and self‐care in chronic disease management.
The objectives of this research were to describe the out‐of‐pocket wound treatment costs and the quality of life of people who have chronic wounds. A questionnaire incorporating the Cardiff Wound Impact Schedule and purpose‐designed instruments was completed by a non‐probability, convenience sample of 113 people in Australia and Wales. Data was analysed using descriptive statistics.
The sample was on average 63·6 years of age and had wounds that were on an average 109 weeks duration. Participants had spent on average AU$2475 on wound dressing products since the wound started, and AU$121·82 in the most recent 28 days which represented 10% of their disposable income. Health‐related quality of life was sub‐optimal, 6/10 (ave) according to the Cardiff Wound Impact Schedule. Younger participants reported significantly poorer quality of life on all CWIS sub‐scales when compared to older participants.
This study found that chronic wounds present a significant financial cost to individuals who must self‐fund their wound dressings and other wound treatment related expenses. Participants who had access to wound product subsidisation also experienced personal financial costs. People who have chronic wounds experience sub‐optimal quality of life therefore this condition is also costly to the individual's well‐being. The quality of life of younger people has not received adequate attention and requires further consideration given the many years that younger people may have to live with this debilitating and often recurrent condition.
Continued action is required to reduce the financial and personal costs experienced by people who have chronic wounds. It is imperative that healthcare funding is directed to people who have chronic wounds, in particular to alleviate the out‐of‐pocket costs experienced by self‐funders. Continued attention to the quality of life of people who have chronic wounds is required to minimise the negative effects of this condition and enhance well‐being.
Keywords: Chronic wound, Community, Cost, Quality of life
Introduction
Chronic wounds are costly to those affected, particularly in the financial and quality of life domains. A better understanding of the out‐of‐pocket financial costs and the quality‐of‐life cost of chronic wounds will help to direct interventions and care to reduce expense and improve patient well‐being.
Investigation of the financial cost of chronic wounds has tended to focus on the system‐level cost of this condition, and the out‐of‐pocket costs to individuals have not been the focus of extensive research. This evidence is required because wound‐dressing products and other treatment‐related costs are not universally subsidised. A better understanding of the out‐of‐pocket costs of chronic wounds will provide a more comprehensive understanding of the overall economic burden of this condition. This evidence can be used to identify opportunities to reduce the personal financial burden of chronic wounds and to support lobbying for subsidisation of wound products and other treatment expenses.
Chronic wounds affect quality of life; therefore, this condition is costly to the individual's well‐being. There has been renewed interest in evaluating quality of life given the shift to patient enablement and self‐care in chronic disease management. Evidence of the negative effect of chronic wounds on quality of life was established several decades ago; however, more recently published research on this topic has been less plentiful and has arisen from typically small samples. A contemporary understanding of the disease‐specific quality of life of people who have chronic wounds is required.
Literature review
Cost of chronic wounds
Chronic wounds are recognised as a significant and growing economic burden on health care systems 1. In some countries, for example, Australia, the financial cost of wound products is typically carried by the individual as there is no national reimbursement system in this setting 2. In other countries, for example, Wales, wound products are funded by the National Health Service and supplied via pharmacy using a script system 3. Reimbursement is reported to vary greatly by setting and funding streams in different areas of the world 4.
Investigation of the monetary cost of chronic wounds has tended to focus on the system‐level cost and has established that chronic wounds are an economic burden. For example, a cost modelling conducted in Australia suggested that the three most common chronic wounds (pressure injuries, foot wounds associated with diabetes and leg ulcers) present a cost of US$2·85 billion per annum across hospital, community and residential aged care settings. A cost modelling study conducted in Denmark suggested that chronic wounds cost DKK56m (€8 m) in 2009, and this cost will increase to DKK224m (€30 m) in the year 2020 5.
These system costs, however, only present one perspective of the cost of chronic wounds as they do not include out‐of‐pocket costs experienced by people who live in the community. A small pilot study in Australia found an out‐of‐pocket cost of AU$228 (AU$57–751) for 2 months of community‐based leg ulcer treatment 6, with average costs the highest for wound dressings (approx. $150 over 2 months as indicated by graphed data) and lower for fees, transport, medication and other costs. A recent economic evaluation commissioned by Wounds Australia estimated that older individuals who have venous leg ulcers pay AU$27·5 million dollars in self‐funded expenses for consumables (defined as bandages, compression stockings and skin care products) each year 7.
Health‐related quality of life
Foundational research conducted in the United Kingdom in the 1990s established the significant negative effect of leg ulcers on quality of life 8. This study also found that younger people experienced poorer quality of life in all domains when compared to older people. More recent research has used a range of generic quality‐of‐life tools and established poor quality of life among people who have leg ulcers 9, 10, 11, 12, foot wounds associated with diabetes 13, 14, 15, 16, 17, pressure injuries 18, 19 and malignant fungating wounds 20. Research comparing the chronically wounded with controls has determined poorer quality of life for the chronically wounded 21, 22, and one study has shown that an initial improvement in quality of life was not sustained over the longer term 23.
Research investigating disease‐specific quality of life has increased in line with the development and validation of disease‐specific instruments, and of interest to this review is the Cardiff Wound Impact Schedule (CWIS) 24 as this was the tool used in our study.
Several studies using the CWIS have investigated quality of life at one time point or over time. Moderate to good quality of life among older men with gout‐related foot wounds has been reported for the social life score (mean 61·0, SD 24·3), well‐being score (mean 73·3, SD 15·4) and physical symptoms and daily living score (mean 66·7, SD 28·5) 25. A study investigating the quality of life of leg, foot and pressure injury patients receiving negative pressure wound therapy found that people receiving the intervention had improved social life scores during the first 2 weeks when compared to controls; however, there were no other significant differences for other sub‐scales of the CWIS at a range of time points up to 12 weeks 26. Poorer quality of life was determined in a small sample of people who had scleroderma and wounds when compared to those with scleroderma who did not have wounds; the well‐being score and physical symptoms and daily living score in particular 27. A randomised controlled crossover trial with individuals who had venous leg ulcers and who received two‐layer and four‐layer compression bandaging found a significant difference pre‐crossover for the CWIS physical symptoms and daily living score for the two‐layer system 28. Another RCT found a significant difference in CWIS physical symptom and daily living score, well‐being score, overall quality of life and satisfaction with quality of life scores in favour of pilonidal sinus patients who received rhomboid excision and flap when compared to unroofing and marsupialisations 29. No significant differences in any components of the CWIS were found at 14 weeks for a small sample of patients who had positive healing outcomes following treatment with non‐cultured autologous cell grafting 30.
Study objectives
The objectives of this study were to investigate:
The out‐of‐pocket wound dressing and treatment‐related costs incurred by people who have chronic wounds.
The quality of life of people who have chronic wounds.
Methodology
Design
A quantitative, descriptive survey study was conducted with 113 community patients who had a chronic wound.
Population and setting
The study was conducted in four Australian States and also in Cardiff, Wales.
Eligibility
Individuals were eligible to participate if aged 18 years or older, resided in Australia or Wales, were English speaking, currently or previously had a chronic wound, did not have moderate or significant cognitive impairment and were not in the terminal stage of an illness.
All participants in this study were engaged in self‐treatment of their wound. Self‐treatment was defined as the participant conducting wound cleansing, wound inspection, applying wound dressings, removing wound dressings and/or applying and removing compression bandaging.
Sample
A non‐probability convenience sample was sought. A representative sample was not feasible in the study time frame.
Screening and recruitment
General media, professional and consumer networks and social media were used to advertise the study in Australia. Potential participants were screened for eligibility by the researcher. Recruitment commenced in Australia on 12 November 2014 and was completed on 27th September 2015. Participants were recruited directly from participating wound clinics in Wales over a 2‐week period in May 2015. Individuals who met the eligibility criteria and chose to participate were provided with the Plain Language Statement and signed a consent form.
Data collection
A survey was administered via a self‐report questionnaire. The questionnaire was completed on paper or online via the survey administrator SurveyMonkey (SurveyMonkey Inc, Palo Alto, CA).
Measurement tools
The questionnaire included purpose‐designed questions and validated instruments where available. The purpose‐designed questions included demographics, wound status and treatment cost. Regarding the latter, this included participant reports of the out‐of‐pocket (self‐funded) costs of wound‐dressing products (i.e. wound dressings and bandages) and treatment‐related expenses that were directly related to the wound (appointment costs, travel costs, loss of wages, communication costs, additional treatment costs, equipment costs and clothing costs). Cost data were collected in Australian dollar and GBP. 30 June 2015 was selected as the reference date for the currency conversion, and the rate of £1·00 = AU$2·07 was applied. Of note, wound products are typically self‐funded in the community setting in Australia and are subsidised by the government in Wales.
The CWIS is a disease‐specific, self‐report tool 24. The CWIS has five sections that can be individually scored: physical symptoms and daily living (12 questions, single response, 5‐point Likert scale), social life (14 questions single response, 5‐point Likert scale), well‐being (7 questions, single response, 5‐point Likert scale), overall quality of life (1 questions, 10‐point visual analogue scale) and satisfaction with quality of life (1 questions, 10‐point visual analogue scale). The tool asks participants to reflect on the previous 7 days when rating tool items. The first three sub‐groups of the scale are scored by sum and conversion of the sum to a 0–100 scale. A global health‐related quality of life and satisfaction with quality of life score (also 0–100) is also obtained. A high score represents good quality of life, and a low score represents poor quality of life 24.
Expert opinion regarding appropriate wound outcome measures and methods 31, 32, 33 were considered during development of the questionnaire, and the survey tool was piloted (n = 10) and refined before use in the study.
Analysis
Data were analysed in SPSS (IBM SPSS Statistics for Mac, Version 22·0., IBM Corp, Armonk, NY). Descriptive statistics were used to describe the characteristics of the sample. Frequencies have been reported for categorical variables, and the mean, median and standard deviation have been reported for continuous variables.
Because of the larger‐than‐expected number of young people, the larger number of participants who had leg ulcers and the larger‐than‐expected number of participants sourced from specialist wound clinics, analysis was conducted to consider differences between these groups. The analysis included non‐parametric tests (chi‐square) for categorical data and parametric tests (t‐tests) for continuous data. An alpha level of 0·05 was used to classify findings as significant.
The results for the sample overall have been presented as well as the results for three groups: Australian participants who currently had wounds, Cardiff participants who currently had wounds and Australian participants who previously had a wound. This presentation enables a comparison of the findings for these groups and discussion of the similarities and differences associated with these characteristics.
Ethics
The University Human Ethics Committee approved the study, and it was conducted in accordance with the Australian Code for the Responsible Conduct of Research 34 and the National Statement on Ethical Conduct in Research 35.
Results
Screening, inclusion and recruitment
The study recruited 113 individuals. The conversion of eligible individuals to participants of the study was high (n = 113, 77·6%). Further details of screening, inclusions and exclusions are shown in Figure 1.
Figure 1.
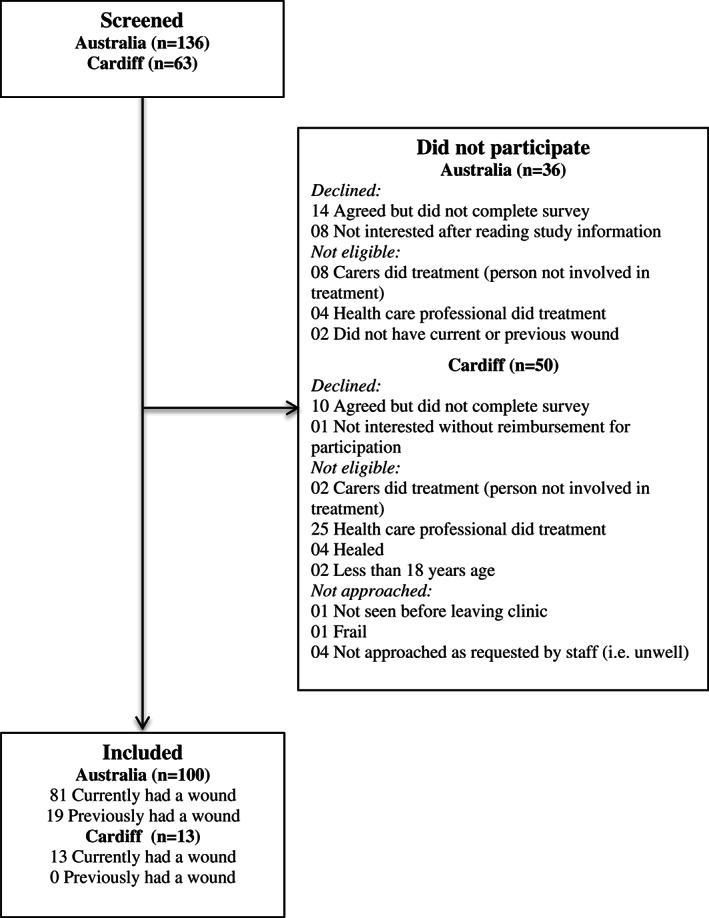
Study flow chart.
Two thirds of participants (n = 74, 65%) completed the survey online. Online completion was higher for the 57 participants who were aged 65 years and younger (online n = 46, 40·7%) when compared to paper completion (n = 11, 9·7%). Online and paper completion was similar for 56 participants aged 66 years and older (online n = 28, 24·8% and paper n = 27, 23·9%). The remaining participant (n = 1, 1%) completed the survey over the phone.
Sample characteristics
The majority of the sample was Australians who had a chronic wound at the time of participation (n = 81, 71·7%), fewer were Australians who previously had a chronic wound (n = 19, 16·8%) and the minority were participants from Cardiff who had a chronic wound at the time of participation (n = 13, 11·5%).
Nearly all participants (n = 110, 97·3%) had received professional care at some stage during their wound episode. Those participants that had not were Australians who had a chronic wound (n = 2) and an Australian who previously had a chronic wound (n = 1). In line with the eligibility criteria, all participants had engaged in self‐treatment on some or all occasions (n = 94, 83%) or previously (n = 19, 17%).
There were no missing data in this study, and the results that follow represent the sample of 113 unless otherwise specified.
Participants were 63·6 years of age on average (min. 23 years, max. 95 years). The Cardiff participants who had a chronic wound were on average 7 years younger than the Australian participants who had a chronic wound; this difference was not statistically significant [t(92) = 1·574, P = 0·119]. Australians who previously had a chronic wound were on average 10 years older than the Australians who currently had a chronic wound, and this difference was also not statistically significant [t(98) = −2·428, P = 0·023]. There was a similar representation of female participants (n = 55, 48·7%) and male participants (n = 58, 51·3%) in the sample. Table 1 displays the demographic and personal characteristics of the sample.
Table 1.
Demographic and personal characteristics (n = 113)
| AU current (n = 81) | Cardiff current (n = 13) | AU previous (n = 19) | Total (n = 113) | ||
|---|---|---|---|---|---|
| Age (years) | Ave | 62·7 | 55·7 | 72·8 | 63·6 |
| Min/max | 23–91 | 28–75 | 30–95 | 23–95 | |
| SD | 14·5 | 16·9 | 16·8 | 15·7 | |
| Gender (%) | |||||
| Male | 50·6 | 61·5 | 47·4 | 51·3 | |
| Female | 49·4 | 38·5 | 52·6 | 48·7 | |
| Marital status (%) | |||||
| Married/defacto | 54·3 | 46·1 | 36·8 | 50·5 | |
| Single, widowed, divorced, sep'd. | 45·7 | 53·9 | 63·2 | 49·5 | |
| Residential location (%) | |||||
| Victoria | 84 | 0 | 52·6 | 69·0 | |
| New South Wales | 3·7 | 0 | 10·5 | 4·4 | |
| Western Australia | 4·9 | 0 | 31·6 | 8·8 | |
| Queensland | 7·4 | 0 | 5·3 | 6·2 | |
| Wales | 0 | 100 | 0 | 11·5 | |
| Country of birth (%) | |||||
| Australia | 90·1 | 0 | 63·2 | 75·2 | |
| United Kingdom | 2·4 | 84·7 | 15·8 | 14·1 | |
| Other | 7·2 | 15·4 | 26·4 | 10·7 | |
| Main language at home (%) | |||||
| English | 97·5 | 100 | 100 | 98·2 | |
| Other | 2·4 | 0 | 0 | 1·8 | |
| Education level | |||||
| Completed secondary school | 48·1 | 30·8 | 52·6 | 46·9 | |
| Income | |||||
| At or below pension rate | 69·0 | 77·0 | 52·7 | 67·1 | |
| Source of income | |||||
| Government pension | 58·0 | 46·2 | 47·4 | 54·9 | |
| Employment status | |||||
| Unemployed/not in workforce | 70·4 | 61·5 | 78·9 | 70·8 | |
The sample reported a total of 166 wounds. Just under one third of participants (n = 34, 30%) reported more than one wound, most commonly multiple lower leg wounds (n = 24, 70·6%). The results that follow refer to the primary wound, reported by the participant as the most significant wound.
The number of primary wounds experienced by the sample overall at each anatomical location is displayed in Figure 2. More than three quarters of participants (n = 89, 78·8%) had a lower leg wound defined as situated at or below the knee. Wound duration was on average 109 weeks, calculated as time to the survey date or healing. Cardiff participants had wounds that were on average three times the duration of the wounds experienced by Australian participants who currently had wounds; this difference was significant [t(18·299) − 4·546, P = 0·000]. Refer Table 2.
Figure 2.
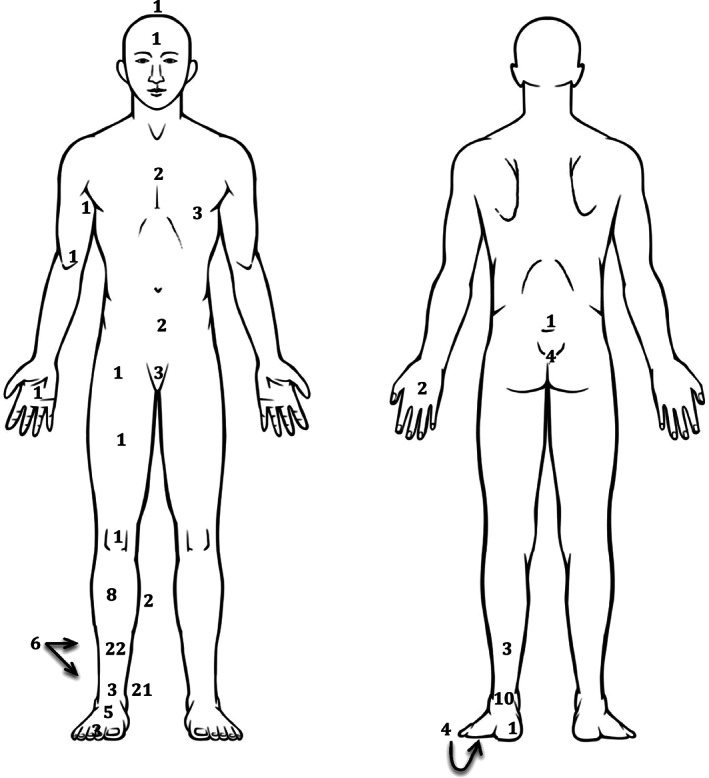
Anatomical location of primary wound (n = 113).
Table 2.
Primary wound duration
| AU current (n = 81) | Cardiff current (n = 13) | AU previous (n = 19) | Total (n = 113) | ||
|---|---|---|---|---|---|
| Wound duration (weeks) | |||||
| Ave | 85·4 | 264·6 | 104·0 | 109·1 | |
| Min/max | 4–676 | 23–892 | 4–572 | 4–892 | |
| SD | 125·1 | 254·3 | 144·9 | 157·2 | |
Quality of life
Global health‐related quality of life and satisfaction with that quality of life according to the Cardiff Wound Impact Scheduled was sub‐optimal, 6 of 10 on average for both measures. The overall quality of life scores for physical symptoms and daily living were reasonable, on average 64/100. The scores were slightly higher on average for social life (72 of 100) and much lower for well‐being (40/100). There was a trend for lower average scores for the Cardiff participants when compared to the Australian participants for all three sub‐group scores. The difference was only significant for the well‐being score as determined by a one‐way between‐groups analysis of variance F(4·908) = 2, P = 0·009 (refer Table 3).
Table 3.
Cardiff Wound Impact Schedule (CWIS) quality of life
| AU current (n = 81) | Cardiff current (n = 13) | AU previous (n = 19) | Total (n = 113) | ||
|---|---|---|---|---|---|
| Phys. Symp. & Daily Liv. (sum) | |||||
| Ave | 84·7 | 69·2 | 98·2 | 85·2 | |
| Min/max | 31–120 | 24–108 | 26–120 | 24–120 | |
| SD | 25·5 | 28·0 | 25·3 | 26·7 | |
| Phys. Symp. & Daily Liv (score) | |||||
| Ave | 63·2 | 47·1 | 77·3 | 63·7 | |
| Min/max | 7–100 | 0–87 | 2–100 | 0–100 | |
| SD | 26·6 | 29·1 | 26·4 | 27·8 | |
| Social life (sum) | |||||
| Ave | 54·3 | 50·5 | 57·9 | 54·5 | |
| Min/max | 23–70 | 20–70 | 28–70 | 20–70 | |
| SD | 13·8 | 16·9 | 12·6 | 14·0 | |
| Social life (score) | |||||
| Ave | 72·1 | 65·2 | 78·4 | 72·3 | |
| Min/max | 16–100 | 10–100 | 25–100 | 10–100 | |
| SD | 24·7 | 30·2 | 22·5 | 25·1 | |
| Well‐being (sum) | |||||
| Ave | 18·4 | 15·9 | 20·0 | 18·4 | |
| Min/max | 7–34 | 8–25 | 10–31 | 7–34 | |
| SD | 6·6 | 1·6 | 5·9 | 6·4 | |
| Well‐being (score) | |||||
| Ave | 41·0 | 31·8 | 46·6 | 40·0 | |
| Min/max | 0–96. | 3–64 | 10–85 | 0–96 | |
| SD | 23·7 | 21·1 | 21·2 | 23·1 | |
| Global HRQoL (score) | |||||
| Ave | 6·20 | 5·23 | 6·05 | 6·06 | |
| Min/max | 0–10 | 0–6 | 5–7 | 0–10 | |
| SD | 2·6 | 1·8 | 0·4 | 2·0 | |
| Satisfaction with HRQoL (score) | |||||
| Ave | 6·1 | 5·5 | 5·8 | 6·0 | |
| Min/max | 0–10 | 1–6 | 4–7 | 0–10 | |
| SD | 2·8 | 1·3 | 0·7 | 2·4 | |
Self‐funded wound product cost
The cost of wound products since the wound started for the sample overall was an average of $2475; however, there was variation between the Australian and Cardiff participants, which was expected given the different funding models that apply in these settings. The cost of products in the most recent 28 days (the last 28 days that the wound was present in the case of Australians who previously had a chronic wound) for the sample overall was an average of $121·82, and as expected, the cost of products in the most recent 28 days was lower for Cardiff participants ($69·70) and higher for the Australians who had a chronic wound at the time of participation (AU$127·02) (Table 4).
Table 4.
Self‐funded: wound product cost
| AU current (n = 81) | Cardiff current (n = 13) | AU previous (n = 19) | Total (n = 113) | |
|---|---|---|---|---|
| Cost since started ($AU) | ||||
| Ave | 2934·93 | 324·88 | 1987·60 | 2475·37 |
| Min/max | 0–65 000 | 0–3272·42 | 0–30 000 | 0–65 000 |
| SD | 9145·24 | 898·21 | 6814·02 | 8247·3031 |
| Cost last 28 days ($AU) | ||||
| Ave | 127·02 | 69·70 | 135·32 | 121·82 |
| Min/max | 0–2000 | 0–307 | 0–1500 | 0–2000 |
| SD | 243·01 | 103·01 | 336·85 | 248·84 |
| % disposable income last 28 days | ||||
| Ave | 12·09 | 5·69 | 6·68 | 10·44 |
| Min/max | 0–100 | 0–50 | 0–60 | 0–100 |
| SD | 17·27 | 14·41 | 13·43 | 16·47 |
The percentage of disposable income spent on wound products in the most recent 28 days (the last 28 days that the wound was present in the case of Australians who previously had a chronic wound) for the sample overall was an average of 10·4%. More specifically, the finding of 12·1% for Australians who had a chronic wound at the time of participation was nearly double the finding for Australians who previously had a chronic wound (6·7%) and more than double the finding for Cardiff participants (5·7%).
Self‐funded treatment‐related costs
Treatment‐related costs are presented in Table 5. Appointment costs (e.g. fees for consultations with doctors, nurses and podiatrists) were an average of AU$309 for the sample overall. The results for Cardiff participants specifically were minimal (AU$1·73). Travel costs (e.g. fuel used to visit the doctor, train fares to visit specialists, parking and accommodation if an over night stay was needed) were AU$679·96 on average for the sample overall, an expense that was much greater for Cardiff participants (AU$1829). Loss of wages (e.g. when taking time off work to visit or be visited by health care professionals and hospitalisation because of the wound) was an average of AU$8946 for the sample overall. The amount for Cardiff participants (AU$31 646) was nearly five times higher than for Australians who had a chronic wound at the time of participation (AU$6620).
Table 5.
Self‐funded: other related costs
| AU Current (n=81) | Cardiff Current (n=13) | AU Previous (n=19) | Total (n=113) | ||
|---|---|---|---|---|---|
| Appointment costs* | |||||
| Ave | 324·46 | 1·73 | 454·84 | 309·12 | |
| Min/max | 0–8000 | 20·45 | 0–4000 | 0–8000 | |
| SD | 1036·78 | 5·65 | 990·34 | 968·99 | |
| Travel costs* | |||||
| Ave | 496·94 | 1829·28 | 664·21 | 679·96 | |
| Min/max | 0–10 000 | 0–10226·31 | 0–8000 | 0–10226·31 | |
| SD | 1453·72 | 3019·90 | 1853·60 | 1796·12 | |
| Loss of wages* | |||||
| Ave | 6620·65 | 31646·51 | 3210·5263 | 8946·93 | |
| Min/max | 0–180 000 | 0–409052·57 | 0–36000 | 0–409052·57 | |
| SD | 28461·91 | 113397·18 | 9789·89 | 45300·32 | |
| Communication costs* | |||||
| Ave | 58·38 | 194·30 | 137·37 | 87·55 | |
| Min/max | 0–1500 | 0–1227·16 | 0–2000 | 0–2000 | |
| SD | 204·55 | 418·98 | 465·15 | 293·63 | |
| Additional treatment costs* | |||||
| Ave | 540·68 | 5·81 | 1078·84 | 569·89 | |
| Min/max | 0–20 000 | 0–40·91 | 0–10 000 | 0–20 000 | |
| SD | 2356·67 | 12·70 | 3144·23 | 2374·49 | |
| Equipment costs | |||||
| Ave | 352·60 | 39·49 | 463·15 | 335·17 | |
| Min/max | 0–6000 | 0–511·32 | 0–8000 | 0–8000 | |
| SD | 942·61 | 141·76 | 1830·66 | 1090·21 | |
| Clothing costs | |||||
| Ave | 215·07 | 586·05 | 321·58 | 275·66 | |
| Min/max | 0–2000 | 0–4908·63 | 0–5000 | 0–5000 | |
| SD | 385·70 | 1351·94 | 1155·69 | 728·65 | |
Sample n = 112 (missing data, AU current).
Communication costs (e.g. telephone calls and postage) were relatively low, AU$87·55 on average for the sample overall. The result for Cardiff participants (AU$194) was considerably higher than Australians who had a chronic wound at the time of participation (AU$58). Additional treatments (e.g. antibiotics and nutritional supplements) were an average of AU$569 for the sample overall. Again, there was variation in the results, with Australians who previously had a chronic wound reporting AU$1078, nearly double that of Australians who had a chronic wound at the time of participation (AU$540). Additional treatment costs for Cardiff participants were low (AU$5).
Equipment costs (e.g. pressure redistributing cushions) were an average of AU$335 for the sample overall. This result was similar for Australians who had a chronic wound at the time of participation (AU$352) and Australians who previously had a chronic wound (AU$463); however, for the Cardiff participants, the average was much lower (AU$39). The cost of clothing or footwear to facilitate healing was, on average, AU$275 for the sample overall. This cost was the highest for Cardiff participants (AU$586).
Sub‐group analysis
Sub‐group analysis was conducted to compare younger and older participants, participants who had leg wounds and other wounds and participants who had and had not presented to a specialist wound clinic. These groups were compared on quality of life, cost of wound dressings, treatment‐related costs and percentage of income spent on dressings. The younger group demonstrated significantly poorer quality of life across all five CWIS scores and significantly higher lost wages (refer Table 6). The leg wound group demonstrated significantly older age and percentage income spent on wound dressings in the most recent 28 days (refer Table 7). The specialist wound clinic group was younger, had longer wound duration and greater costs associated with wound dressings and treatment‐related costs (refer Table 8).
Table 6.
Sub‐group analysis: younger and older age.
| Participant age | Group comparisons | ||
|---|---|---|---|
| ≤65 years (n = 57, 50·4%) | ≥66 years (n = 56, 49·6%) | ||
| Demographic and personal characteristics | |||
| Social support score [M(SD)] | 63·64 (30·30) | 73·66 (22·75) | t(103·874) −1·990 P = 0·049 |
| Self‐efficacy score [M(SD)]* | 32·52 (5·32) | 34·78 (3·87) | t(102·332) −2·558 P = 0·012 |
| Global HRQoL score [M(SD)] | 5·16 (2·47) | 6·98 (1·76) | t(101·24) −4·516 P = 0·000 |
| Satisfaction with HRQoL score [M(SD)] | 5·18 (2·51) | 6·88 (2·098) | t(108·264) −3·910 P = 0·000 |
| QoL, Physical symptoms and daily living score [M(SD)] | 51·08 (27·73) | 76·71 (21·35) | t(105·05) −5·511 P = 0·000 |
| QoL, Social life score [M(SD)] | 62·34 (27·11) | 82·62 (17·97) | t(97·463) −4·693 P = 0·000 |
| QoL, Well‐being score [M(SD)] | 31·95 (20·74) | 50·0 (22·06) | t(110·299) −4·477 P = 0·000 |
| Lost wages $ [M(SD)] † | 17528·25 (63162·11) | 365·62 (1463·08) | t(55·059) 2·033 P = 0·047 |
Missing data (n = 0, n = 2).
Missing data (n = 1, n = 0).
Table 7.
Sub‐group analysis: had a leg ulcer or had other type of wound
| Wound type | Group comparisons | ||
|---|---|---|---|
| Leg wound (n = 89, 78·8%) | Other wound (n = 24, 21·2%) | ||
| Demographic and personal characteristics | |||
| Age years | 65·7 (15·13) | 55·80 (16·07) | t(111) 2·808 P = 0·006 |
| % income last 4 weeks [M(SD)] | 12·35 (17·62) | 3·38 (8·21) | t(82·293) 3·574 P = 0·001 |
Table 8.
Sub‐group analysis: had or had not seen a specialist wound clinic
| Had seen a specialist wound clinic | Group comparisons | ||
|---|---|---|---|
| Yes (n = 69, 62·7%) | No (n = 41, 37·3%) | ||
| Demographic and personal characteristics | |||
| Age (years) | 61·3 (16·3) | 67·4 (14·01) | t(108) −1·984 P = 0·050 |
| Wound duration weeks [M(SD)] | 143·36 (179·84) | 58·31 (94·32) | t(106·668) 3·248 P = 0·002 |
| Cost to date $ [M(SD)] | 3524·26 (10275·40) | 769·31 (2368·82) | t(79·625) 2·134 P = 0·036 |
| Cost previous 28 days $ [M(SD)] | 153·76 (308·67) | 75·54 (81·36) | t(82·903) 1·992 P = 0·050 |
| Travel costs $ [M(SD)]* | 1000·05 (2223·65) | 178·53 (293·50) | t(72·034) 3·024 P = 0·003 |
| Communications cost $ [M(SD)]* | 132·28 (367·13) | 13·20 (25·94) | t(69·168) 2·683 P = 0·009 |
Missing data (n = 0, n = 1).
Discussion
The objectives of this research were to describe the out‐of‐pocket wound dressing and treatment‐related costs as well as quality of life of people who have chronic wounds. The results provide insight about the cost to Australians who had a chronic wound at the time of participation (n = 81, 71·7%), Australians who previously had a chronic wound (n = 19, 16·8%) and Cardiff participants who had a chronic wound at the time of participation (n = 13, 11·5%).
Sample characteristics
In addition to the diversity noted above, the sample was diverse with respect to gender, age and socio‐economic background; this finding is similar to other reports of the characteristics of the chronically wounded 36, 37. The majority of participants had received professional care, which is a characteristic of people who are typically engaged in wound research as research is often conducted in health care settings. Higher‐complexity wounds and the tendency for patients to not present early in their episode may explain the longer wound duration of participants who had seen a specialist wound clinic.
This study recruited a high proportion of young people, and this may be associated with online completion of the survey. The younger people in this study experienced significantly lower quality of life when compared to older people. These findings are similar to earlier research conducted in the UK 8. Given that young people are of pre‐retirement age, loss of wages may be substantial in this group, and this was indicated in our study. It may be that younger people do not normalise their chronic illness and therefore do not cope as well as older people, and this has a theoretical foundation 38. It is essential that health care professionals detect coping and intervene to mitigate these effects among younger people.
The large proportion of leg ulcers experienced by participants of this study (particularly the older participants) were unsurprising given that lower leg ulcers are the most common in the community setting 39, 40. Participants had experienced their wounds for a long period of time, more than 2 years on average. Those who had leg ulcers did not experience significantly longer wound duration when compared to those who had other types of wounds in the sub‐group analysis; this study therefore contrasts with evidence suggesting that leg ulcers are the most enduring chronic wound 36, 37. While this may be the result of a non‐representative sample, this finding nonetheless provides support for the notion that not enough is known about the comparative duration of different types of chronic wounds that are commonly treated in the community. The Cardiff participants had significantly longer wound durations, possibly reflecting higher‐complexity wounds or a limitation associated with the small sample in this setting.
Cost of wound products and other related costs
The personal financial cost of chronic wounds has received limited attention in the literature, likely because subsidisation is relatively common in the settings and countries from which most published literature arises. People who have chronic wounds and live in the community in Australia typically self‐fund wound products, and this was the case among all but one participant of this study. Our finding of a weekly wound dressing cost of AU$31·75 (avg.) for Australians who had a chronic wound at the time of participation was 83% higher than reported in an earlier pilot study conducted in Australia with people who had venous leg ulcers specifically 6. The cost of $17·45 per week for Cardiff participants was unexpected given that wound products are subsidised in this setting. The reasons for this were not explored in the survey but may reflect constraints of the subsidisation scheme, or participant's self‐funded wound products that they preferred or which they could not get via the subsidisation scheme.
Our study suggests that the cost of wound‐dressing products is significant for people who have chronic wounds given the amount that they must spend and their low socio‐economic status. The effect that this cost had on disposable income highlights the potential effect that this cost may have on living affordability. Regarding the cost in the most recent 28 days, this expense consumed 10·4% of the disposable income of the sample overall. Australians who had a chronic wound at the time of participation experienced the highest rate (12%) and a maximum of 100% for one participant, suggesting that his entire income from the most recent 4‐week period was spent on wound treatment expenses. For Cardiff participants, their expense equated to 5·7% of their disposable income, which although less is still significant. The percentage of income spent by the leg wound group was higher than the group that included all other types of wounds, indicating that this type of wound may be particularly costly to self‐funders. If leg ulcers are the most commonly presenting wounds in specialist clinics, this may explain the higher costs in the group that attended this setting.
People who have chronic wounds and live in the community in Australia typically self‐fund other costs associated with receiving wound treatment, and this was found to be the case among the Cardiff participants also. There were, however, some major variations in the findings associated with these costs, including differences between the Australian and Cardiff participants, some of which were unexpected and difficult to explain.
Appointment costs were much higher for participants from Australia, likely reflecting costs associated with general practice visits and domiciliary nursing, the latter attracting a co‐payment of up to AU$70 per week for pensioners in this setting 41. Travel costs were much higher for Cardiff participants, and the longer duration of wounds in this group, and therefore the longer period of time during which travel was required, may explain this finding. The higher lost wages in the Cardiff group may also be associated with wound duration or could be associated with the younger age of this group given that they were younger than the local pension age on average. Additional treatment and equipment costs were much lower for Cardiff participants, possibly reflecting the availability of additional funding for these expenses in this setting. This study suggests that these associated costs may be significant for people who have chronic wounds. The effect of these costs may be overlooked by the patient and health care provider because the cost of wound dressings, which the patient and health care provider are presented with on a regular basis, are at the front of the mind.
Continued effort is required to reduce the cost of treating chronic wounds, particularly in settings where subsidisation is not available. This may be achieved through appropriate wound‐dressing selection, finding the best possible price for wound products and savings associated with reduced healing time. The effect of self‐treatment on the cost of wound treatment should be considered, and future publication arising from this programme of research explores this in detail. Continued lobbying for subsidisation is required in Australia, and our evidence of the out‐of‐pocket cost of chronic wounds, together with earlier cost modelling data 7, will be helpful. Evaluation of the effectiveness, constraints and patient satisfaction with subsidisation of wound products is required so that the reasons for out‐of‐pocket costs in subsidised environments can be determined and the best benefit from this valuable resource achieved.
Generating interest in addressing the economic burden of chronic wounds in Australia has been difficult, and this is a challenge that has been identified in other parts of the world 4. One approach that may be helpful is to contrast the cost of chronic wounds with other more well‐known and funded health conditions 2, and our study provides an opportunity to do this. For example, the average self‐funded treatment cost for people with chronic obstructive pulmonary disease is AU$487 per quarter 42. Calculation of the average weekly spend of Australians who had a wound at the time of participation (specifically wound product costs and all related costs except loss of wages over the 109‐week average wound duration) results in an average quarterly out‐of‐pocket spend of AU$664·95 in this study, a finding that is 30% higher than the reported out‐of‐pocket expense associated with COPD.
These data provide preliminary evidence that chronic wounds may be more costly on a quarterly basis than one of the major contributors to chronic disease in Australia. The cost of chronic disease and effect on quality of life are determinants of health care funding allocation; therefore, further research is required to build on the findings of this study to ensure appropriate allocation of health care resources.
Cost of illness is a complex area of enquiry that requires sophisticated economic analysis; therefore, retrospectively investigating wound treatment cost, and using a non‐validated tool, has some limitations. While some participants had kept detailed records of their costs, others were required to estimate. There was great variability in the data, occurring across a number of findings. The key differences between participants, particularly recency of treatment costs and differences in wound product subsidisation between Australia and Wales, are factors that must be considered when interpreting these findings. It is for this reason that this discussion has focused on the findings associated with Australia participants who had wounds at the time of participation, for whom the sample was the largest and the costs most current.
Our findings are, however, consistent with the exploratory intent of this research and nonetheless give some indication of the size and scope of out‐of‐pocket wound treatment costs, and other related costs that are commonly overlooked among this health care consumer group. Importantly, this study has tested a methodology for ascertaining the out‐of‐pocket cost of wound dressing and treatment‐related costs in the community setting, which can be refined for use in prospective research in the future.
Quality of life
Our study found sub‐optimal health‐related quality of life, the CWIS global rating and satisfaction with this rating, 6 of 10 on average. This finding is not dissimilar to baseline scores reported in other research 28, 43. Our sample reported higher scores for all sub‐scales of the CWIS than people who have gout‐related foot wounds 25 and slightly higher physical symptom and daily living scores than people with lower leg ulcers 27. Our sample reported similar baseline CWIS sub‐scales and overall scores for participants of a compression therapy RCT 28, a cell‐grafting evaluation 30 and a pilonidal sinus disease study 29. A lower well‐being score for Cardiff participants was the only significant difference found. This may be associated with the low sample size or could be associated with other differences that were determined in this study, such as longer wound duration.
Health‐related quality of life is an important outcome measure as the symptoms of chronic conditions can have a prolonged effect on well‐being 44. A growing interest in patient‐reported outcomes in wound management has resulted in greater efforts to measure and understand quality of life among the chronically wounded in the UK 45. Standardised, chronic wound‐specific patient outcome measures in the Australian setting, however, are lacking, and more attention should be paid to measuring this construct.
The relationship between financial cost and quality of life
This study has presented evidence of the financial and quality‐of‐life costs to people who have chronic wounds. A further consideration is the relationship between financial cost and quality of life given that research conducted with people who have other chronic conditions, such as COPD 46 and Alzheimer's disease 47, has shown that poorer quality of life can be associated with higher medical health care costs. It is suggested that increased use of services, associated with better quality of life and help seeking behaviours, is accountable for these findings. Research is required to investigate the potential relationship between these two constructs in the chronically wounded. This is particularly the case for self‐treaters given their sub‐optimal level of quality of life, considerable out‐of‐pocket expense and engagement in self‐treatment.
The findings for Australian and Cardiff participants
This study provided an opportunity to contrast Australian and Cardiff participants who reside in settings that have different health care systems and funding schemes. Cardiff participants had higher costs associated with treatment‐related expenses and this may be explained by service factors, including the accessibility of clinics to regional participants. Cardiff participants had lower out‐of‐pocket costs for wound dressings, and this is likely explained by the availability of subsidisation in this setting. The lower appointment costs, additional treatment costs and equipment costs reported by Cardiff participants may reflect the availability of subsidisation for these expenses in Cardiff, a situation that is generally not the case in Australia.
Limitations
This study involved self‐reporting; therefore, the results may be influenced by response bias. Recall bias in particular, with respect to wound dressing and treatment cost data, is a consideration. A representative sample was not sought; therefore, any generalisations to other groups of chronically wounded people must be made with caution. Furthermore, there are a number of important groups that are not represented in this study, including individuals from culturally and linguistically diverse backgrounds and people with cognitive impairment. The small number of participants from Cardiff, and the associated recruitment period of 2 weeks, may account for some of the differences between the Australian and Cardiff participants. Further research with a larger sample is required to better understand these differences.
Conclusion
Chronic wounds present a significant out‐of‐pocket cost to individuals who must self‐fund their wound treatments and treatment‐related expenses. Individuals who receive wound treatment that is subsidised also experience out‐of‐pocket costs. While subsidisation may be the best solution to the personal financial cost of chronic wounds, this resource may not resolve the cost or the associated effect on disposable income. Continued action is required to ensure that health care funding for chronic wound management is directed in the most effective manner.
People who have chronic wounds experience sub‐optimal quality of life; therefore, this condition is costly to the individual's well‐being. This situation continues to be reported despite advances in the wound management field. The quality of life of younger people has not received adequate attention and requires further consideration given the many years that younger people may have to live with this debilitating and often recurrent condition. Continued attention to the quality of life of all people who have chronic wounds is required to minimise the negative effects of this condition and enhance well‐being.
References
- 1. Sen C, Gordillo G, Sashwati R, Kirsner R, Lambert L, Hunt T, Gottrup F, Gurtner G, Longaker M. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapp S, Santamaria N. Chronic wounds should be an Australian National Health Priority Area. Aust Health Rev 2015;39:600–2. [DOI] [PubMed] [Google Scholar]
- 3. Morgan T. Are your wound management choices costing you money? J Commun Nurs 2015;29:17–20. [Google Scholar]
- 4. World Union of Wound Healing Societies . Reimbursement of dressings: a WUWHS statement. Int Wound J 2006;3:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Denmark over the next decade. J Wound Care 2010;19:173–8. [DOI] [PubMed] [Google Scholar]
- 6. Smith E, McGuiness W. Managing venous leg ulcers in the community: personal financial cost to sufferers. Wound Pract Res 2010;18:134–9. [Google Scholar]
- 7. KPMG . An Economic Evaluation of Compression Therapy for Venous Leg Ulcers. Melbourne: KPMG, 2013. [Google Scholar]
- 8. Franks P, Moffatt C. Who suffers most from leg ulceration? J Wound Care 1998;7:383–5. [DOI] [PubMed] [Google Scholar]
- 9. Faria E, Blanes L, Hochman B, Mesquita Filho M, Ferreira L. Health‐related quality of life, self‐esteem, and functional status of patients with leg ulcers. Wounds 2011;23:4–10. [Google Scholar]
- 10. Farias Dias T, Medeiros Melo M, de Oliveira TS, Fernandes Costa I, Chaves Maia E, de Vasconcelos Torres G. Quality of life assessment of patients with and without venous ulcer. Rev Latino‐Am Enfermagem 2014;22:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franks P, McCullagh L, Moffatt C. Assessing quality of life in patients with chronic leg ulceration using the Medical Outcomes Short Form‐36 Questionnaire. Ostomy Wound Manage 2003;49:26–32. [PubMed] [Google Scholar]
- 12. Jull A, Walker N, Hackett M, Jones M, Rodgers A, Birchall N, Norton R, Macmahon S. Leg ulceration and perceived health: a population based case–control study. Age Ageing 2004;33:236–41. [DOI] [PubMed] [Google Scholar]
- 13. Siersma V, Thorsen H, Holstein P, Kars M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Jirkovska A, Mauricio D, Tennvall GR, Reike H, Spraul M, Uccioli L, Urbancic V, van Acker K, van Baal J, Schaper N. Importance of factors determining the low health‐related quality of life in people presenting with a diabetic foot ulcer: the Eurodialestudy. Diabet Med 2013;30:1382–7. [DOI] [PubMed] [Google Scholar]
- 14. Alzahrani H, Sehlo M. The impact of religious connectedness on health‐related quality of life in patients with diabetic foot ulcers. J Religion Health 2013;52(iii):840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García‐Morales E, Lázaro‐Martínez J, Martínez‐Hernández D, Aragón‐Sánchez J, Beneit‐Montesinos J, González‐Jurado M. Impact of diabetic foot related complications on the health related quality of life of patients. a regional study in Spain. Int J Lower Extremity Wounds 2011;10:6–11. [DOI] [PubMed] [Google Scholar]
- 16. Hoban C, Sareen J, Henriksen C, Kuzyk L, Embil J, Trepman E. Mental health issues associated with foot complications of diabetes mellitus. Foot Ankle Surg 2015;21:49–55. [DOI] [PubMed] [Google Scholar]
- 17. Siersma V, Thorsen H, Holstein P, Kars M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Jirkovská A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, van Acker K, van Baal J, Schaper NC. Health‐related quality of life predicts major amputation and death, but not healing, in people with diabetes presenting with foot ulcers: the eurodiale study. Diabetes Care 2014;37:694–700. [DOI] [PubMed] [Google Scholar]
- 18. Franks P, Winterberg H, Moffatt C. Health‐related quality of life and pressure ulceration assessment in patients treated in the community. Wound Repair Regen 2002;10:133–40. [DOI] [PubMed] [Google Scholar]
- 19. Sebba Tosta de Souza D. Health‐related quality of life in elderly patients with pressure ulcers in different care settings. J Wound Ostomy Continence Nurs 2015;42:352–9. [DOI] [PubMed] [Google Scholar]
- 20. Lo S, Hayter M, Hu W, Tai C, Hsu M, Li Y. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012;68:1312–21. [DOI] [PubMed] [Google Scholar]
- 21. Ramshorst G, Eker H, Voet J, Jeekel J, Lange J. Long‐term outcome study in patients with abdominal wound dehiscence: a comparative study on quality of life, body image, and incisional hernia. J Gastrointest Surg 2013;17:1477–84. [DOI] [PubMed] [Google Scholar]
- 22. Ribu L, Birkeland K, Hanestad B, Moum T, Rustoen T. A longitudinal study of patients with diabetes and foot ulcers and their health‐related quality of life: wound healing and quality‐of‐life changes. J Diabetes Complications 2008;22:400–7. [DOI] [PubMed] [Google Scholar]
- 23. Franks P, Moffatt C, Doherty D, Smithdale R, Martin R. Longer‐term changes in quality of life in chronic leg ulceration. Wound Repair Regen 2006;14:536–41. [DOI] [PubMed] [Google Scholar]
- 24. Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rome K, Erikson K, Otene C, Sahid H, Sangster K, Gow P. Clinical characteristics of foot ulceration in people with chronic gout. Int Wound J 2016;13:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ousey K, Milne J, Cook L, Stephenson J, Gillibrand W. A pilot study exploring quality of life experienced by patients undergoing negative pressure wound therapy as part of their wound care treatment compared to patients receiving standard wound care. Int Wound J 2012;11:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shanmugam V, Price P, Attinger C, Steen V. Lower extremity ulcers in systemic sclerosis: features and response to therapy. Int J Rheumatol 2010;2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moffat C, Edwards L, Collier M, Treadwell T, Miller M, Shafer L, et al. A randomised controlled 8‐week crossover clinical evaluation of the 3 M Coban 2 layer compression system versus Profore to evaluate the product performance in patients with venous leg ulcers. Int Wound J 2008;5:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karakayali F, Karagulle E, Karabulut Z, Oksuz E, Moray G, Haberal M. Unroofing and marsupialization vs. rhomboid excision and Limberg flap in pilonidal disease: a prospective, randomized, clinical trial. Dis Colon Rectum 2009;52:496–502. [DOI] [PubMed] [Google Scholar]
- 30. Seghers A, Goh B, Tan S, Tang B. Simplified noncultured autologous cell grafting for the treatment of chronic nonhealing ulcers: the six‐well plate technique. Clin Exper Dermatol 2014;39:620–3. [DOI] [PubMed] [Google Scholar]
- 31. Firth J, Nelson E, Hale C, Hill J, Helliwell P. A review of design and reporting issues in self‐reported prevalence studies of leg ulceration. J Clin Epidemiol 2010;63:907–13. [DOI] [PubMed] [Google Scholar]
- 32. Gottrup F, Apelqvist J, Price P. Outcomes in controlled and comparative studies on non‐healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care 2010;19:239–68. [DOI] [PubMed] [Google Scholar]
- 33. Harding K, Posnett J, Vowden K. A new methodology for costing wound care. Int Wound J 2013;10:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Health & Medical Research Council . Australian Code for the Responsible Conduct of Research. Canberra: Commonwealth of Australia, 2007. [Google Scholar]
- 35. National Health & Medical Research Council . National Statement on Ethical Conduct in Human Research. Canberra: Commonwealth of Australia, 2007. [Google Scholar]
- 36. Baker S, Stacey M, Hoskin S, Thompson B. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864–7. [DOI] [PubMed] [Google Scholar]
- 37. McDermott‐Scales L, Cowin S, Gethin G. Prevalence of wounds in a community care setting in Ireland. J Wound Care 2009;18:405–17. [DOI] [PubMed] [Google Scholar]
- 38. Strack S, Feifel H. Age differences, coping, and the adult life span. In: Zeidner M, Endler N, editors. Handbook of Coping: Theory, Research, and Applications. USA: John Wiley & Sons, Inc, 1996. [Google Scholar]
- 39. Graves N, Zheng H. The prevalence and incidence of chronic wounds: a literature review. Wound Pract Res 2014;22:4–19. [Google Scholar]
- 40. Körber A, Klode J, Al‐Benna S, Wax C, Schadendorf D, Steinstraesser L, Dissemond, J. Etiology of chronic leg ulcers in 31·619 patients in Germany analyzed by an expert survey. J German Soc Dermatol 2011;9:116–21. [DOI] [PubMed] [Google Scholar]
- 41. Australian Government . MyAgedCare. Help at home: costs explained Canberra, Australia 2016. URL http://www.myagedcare.gov.au/financial‐and‐legal/help‐home‐costs‐explained [accessed on 11 June 2016].
- 42. Essue B, Kelly P, Roberts M, Leeder S, Jan S. We can't afford my chronic illness! The out‐of pocket burden associated with managing chronic obstructive pulmonary disease in western Sydney, Australia. J Health Serv Res Policy 2011;16:226–31. [DOI] [PubMed] [Google Scholar]
- 43. Price P, Harding K. The impact of foot complications on health‐related quality of life in patients with diabetes. J Cutan Med Surg 2000;4:45–50. [DOI] [PubMed] [Google Scholar]
- 44.Australian Commission on Safety and Quality in Health Care. Standard 2: Partnering with Consumers. Safety and Quality Improvement Guide 2012. URL http://www.safetyandquality.gov.au/ [accessed on 4 March 2016].
- 45. Ousey K, Cook L. Understanding patient reported outcome measures (PROMs). Br J Commun Nurs 2011;16:80–2. [DOI] [PubMed] [Google Scholar]
- 46. Wu M, Zhao Q, Chen Y, Fu C, Xu B. Quality of life and its association with direct medical costs for COPD in urban China. Health Quality Life Outcomes 2015;13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller E, Rosenheck R, Schneider L. Assessing the relationship between health utilities, quality of life, and health care costs in Alzheimer's disease: the CATIE‐AD study. Curr Alzheimer Res 2010;7:348–57. [DOI] [PubMed] [Google Scholar]


