Abstract
Amnion and chorion allografts have shown great promise in healing diabetic foot ulcers (DFUs). Results from an interim analysis of 40 patients have demonstrated the accelerated healing ability of a novel aseptically processed, dehydrated human amnion and chorion allograft (dHACA). The goal of this study was to report on the full trial results of 80 patients where dHACA was compared with standard of care (SOC) in achieving wound closure in non‐healing DFUs. After a 2‐week screening period, during which patients with DFUs were unsuccessfully treated with SOC, patients were randomised to either SOC alone or SOC with dHACA applied weekly for up to 12 weeks. At 12 weeks, 85% (34/40) of the dHACA‐treated DFUs healed, compared with 33% (13/40) treated with SOC alone. Mean time to heal within 12 weeks was significantly faster for the dHACA‐ treated group compared with SOC, 37 days vs 67 days in the SOC group (P = .000006). Mean number of grafts used per healed wound during the same time period was 4.0, and mean cost of the tissue to heal a DFU was $1771. The authors concluded that aseptically processed dHACA heals DFUs significantly faster than SOC at 12 weeks.
Keywords: amnion/chorion graft, complete wound healing, diabetic foot ulcer, randomised controlled trial, time to heal
1. INTRODUCTION
Diabetic foot ulcerations are a common complication of individuals with long‐standing diabetes. Because of the nature of wound‐healing impairment induced by diabetes, such wounds can become chronic and challenging to heal.1 In addition, the presence of such a wound increases the risk of complications, including the development of osteomyelitis, severe infection with concurrent hospitalisation, lower extremity amputation, and mortality.2, 3, 4, 5 Moreover, as Armstrong and his colleagues have reported, even when a diabetic foot ulcer (DFU) is healed, the odds of having a recurrence concomitantly increase with an estimated probability of 40% within 1 year and 65% within 5 years.6 In this respect, episodes of foot ulceration are akin to the appearance of cancer, successful treatment, and remission.6
Foot ulcerations are also costly in economic terms. Recent estimates of the Medicare population suggest that the treatment of DFUs can range from $6.2 to $18.7 billion annually in the United States, with nationwide estimates that include all diabetes‐related wounds likely to be far higher.7 Consequently, it is important to heal chronic DFUs as expeditiously as possible to prevent complications and maintain quality of life for patients.
Dehydrated human amnion and chorion allograft (dHACA, AmnioBand Membrane, Musculoskeletal Transplant Foundation [MTF], Edison, New Jersey) is an aseptically processed amnion and chorion tissue form approved for use as a Human Cellular and Tissue‐Based Product (HCT/P) under FDA 21 CFR 1271 and Section 361 of the Public Health Service (PHS) Act. The amnion and chorion membranes of the human placenta have been used for decades in the clinical setting—their observed efficacy owing to their natural role as a barrier with self‐restorative properties.8, 9 As the native habitat for a wide variety of cells retaining stem‐like abilities (Figure 1), the extracellular matrix is inherently rich in growth factors, cytokines, and unique proteins like beta defensins and lactoferrin.9, 10 The MTF method for aseptically procuring and processing dHACA is designed to retain as much of the native state of these complex tissues through simple treatment steps and by avoiding irradiation sterilisation, while the preservation technique renders it shelf stable at ambient temperature for up to 3 years.
Figure 1.
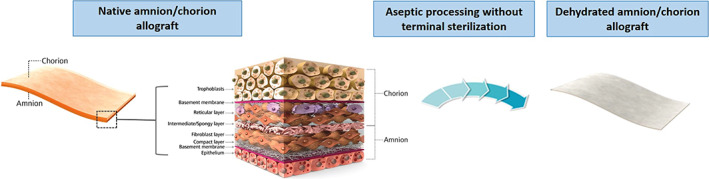
Photograph of amnion and chorion structure schematic and dehydrated human amnion and chorion allograft (dHACA)
Interim results of a randomised controlled trial (RCT) that tested random allocation of 40 patients with DFUs to dHACA plus standard of care (SOC) or SOC alone over a study period of 12 weeks demonstrated that dHACA can substantially improve the time to healing of these chronic ulcers.11 In the current study, we report the results of the full trial in which 80 patients were randomised with a primary objective of evaluating the percentage of wounds healed at 6 weeks and the number of applications, wastage, and cost to closure for healed wounds in the dHACA group.
2. METHODS
The trial was a parallel, 2‐group RCT in which eligible patients with 1 or more neuropathic DFUs randomised 1:1 to receive either dHACA plus SOC or SOC alone. The study protocol and patient consent form were reviewed and approved by the Western Institutional Review Board on January 29, 2015 (20150073). Compliance with applicable regulatory requirements in accordance with the provisions of the Declaration of Helsinki was maintained at all times. In addition, the study adhered to Good Clinical Practice and was pre‐registered at ClinicalTrials.gov (NCT02399826). Written consent was obtained from all participants prior to any study‐related procedure. The trial was conducted between March 23, 2015 and January 21, 2018 at 5 outpatient wound care centres in the United States.
2.1. Patient screening, eligibility, and SOC
On the first day of screening, potentially eligible patients received a full physical examination with documentation of their medical history as well as wound evaluation. Blood was also drawn for serum creatinine and glycosylated haemoglobin (HbA1c) analysis. If multiple eligible DFUs were present in a single patient, the largest DFU was selected as the index wound. Wound evaluation began with infection assessment based on the guidelines of Woo and Sibbald.12 After cleaning of the index wound, surgical debridement was accomplished with a 15 blade or curette to remove all necrotic tissue. Screening for osteomyelitis was carried out using a sterile, ophthalmological probe to determine if the index wound could be probed to bone; confirmation of osteomyelitis required a foot X‐ray and bone biopsy. The index wound was photographed digitally from a distance of 30 cm with a graded centimetre ruler in the viewing field and a legible label adjacent to the ulcer. Surface area of the index wound was calculated using acetate sheet tracing. The lower extremity harbouring the index wound was also subjected to vascular assessment using transcutaneous oximetry measurement (TCOM), ankle brachial index (ABI), or Doppler arterial waveform tests. All DFUs received a collagen alginate primary dressing applied daily. Finally, off‐loading of the index wound was performed using a removable diabetic offloading cam‐walker (Royce Medical, Inc., Camarillo, California; or similar generic device)—the removable walker could be converted instantly to a total contact cast if patients were non‐compliant. A true total contact cast was only required if the patient could not be fitted with a removable diabetic walker.
During the 2‐week screening or “run‐in” period, further debridement was performed as necessary, and the index wound was assessed weekly and its area calculated. If the area had not reduced by more than 20%, and all inclusion and exclusion criteria (Table 1) continued to be met 2 weeks after screening, the patient was then randomised.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Male or female aged 18 years or older | Wound probing to bone (UT grade IIIA‐D). |
| Type 1 or type 2 diabetes mellitus (american diabetes association (ADA) diagnostic criteria) | Index wound greater than 25 cm2 |
| Signed informed consent | HbA1c greater than 12% within previous 90 d |
| Patient's wound diabetic in origin and larger than 1 cm2 | Serum creatinine level 3.0 mg/dL or greater |
| Wound present for a minimum of 4‐week duration, with documented failure of prior treatment to heal the wound | Patients with a known history of poor compliance with medical treatments |
| Wound has no signs of infection | Patients previously randomised into this study or presently participating in another clinical trial |
| Wound present anatomically on the foot as defined by beginning below the malleoli of the ankle | Patients currently receiving radiation therapy or chemotherapy |
| Additional wounds may be present but not within 3 cm of the study wound | Patients with known or suspected local skin malignancy to the index wound |
| Serum creatinine less then 3.0 mg/dL | Patients with uncontrolled autoimmune connective tissues diseases |
| HbA1c less than 12% at randomisation | Non‐revascularisable surgical sites |
| Patient has adequate circulation to the affected extremity as demonstrated by 1 of the following within the past 60 d: Dorsum transcutaneous oxygen test (TCOM) ≥30 mm Hg or ABI with results of ≥0.7 and ≤1.2 in conjunction with Doppler arterial waveforms, which are triphasic or biphasic at the ankle of affected leg | Active infection at index wound site |
| Patient is of legal consenting age | Any pathology that would limit the blood supply and compromise healing |
| Patient is willing to provide informed consent and is willing to participate in all procedures and follow‐up evaluations necessary to complete the study | Patients who have received a biomedical or topical growth factor for their wound within the previous 30 d |
| Patients who are pregnant or breast feeding | |
| Patients who are taking medications that are considered immune system modulators that could affect graft incorporation | |
| Patients taking a Cox‐2 inhibitor | |
| Patients with wounds healing greater than 20% during the screening period |
2.2. Randomisation
Randomisation used a block size of 10. In practical terms, this meant that there were 5 sheets of paper with an SOC assignment and 5 with a dHACA assignment. Each sheet of paper was placed in an opaque envelope that was sealed, and the envelopes were then shuffled by the study coordinator and labelled 1 through 10, this process being observed by the principal investigator and study staff. After repeating the process 8 times, the envelopes were distributed to individual sites. Site investigators were blinded to the randomisation method and treatment assignment, thus fulfilling allocation concealment.
2.3. dHACA
Patients with study wounds randomised to dHACA received aseptically processed, dehydrated amnion and chorion (AmnioBand Membrane, MTF). Each graft was gifted to investigators for use in the trial in a variety of size‐ and shape‐specific forms, from 1‐cm diameter disks to 4 × 6 cm2 rectangles, to minimise wastage.
The index wound was outlined on the chosen graft, and a photograph was taken to document the size and portion of the unused graft (wastage). The graft was then cut to match the wound size with a 15‐blade scalpel, pie‐crusted to no greater than 1.5:1.0 ratio if needed, rinsed with sterile saline, and placed over the wound site, ensuring that it was consistently covered and adhered to the entire wound surface.
2.4. Other treatments and patient withdrawal criteria
Post‐randomisation, patients were treated for 12 weeks unless the index wound closed or the patient was withdrawn from the trial. At each weekly visit, vital signs were taken and blood glucose levels measured using an Accu‐Chek test. Per protocol, patients found to be in poor metabolic control were referred to their primary care physician or endocrinologist to ensure adequate diabetes management. It should be noted that no patients were withdrawn from the study because of glucose‐related issues.
Regardless of allocation assignment, all index wounds were cleansed weekly with sterile normal saline solution, debrided if needed, photographed, and their surface area measured. Suspicion of infection required anaerobic and aerobic cultures obtained via wound swabs and appropriate systemic antibiotic treatment until the infection was clinically resolved. If infection (and its treatment) interfered with scheduled visits or barred dHACA application, the patient was withdrawn from the trial and considered a treatment failure.
Index wounds in the SOC group were dressed daily with collagen alginate (Fibracol, Acelity, San Antonio, Texas) by patients or their caregivers 6 days a week at home and once a week by the site investigator, whereas the dHACAs were covered with a non‐adherent dressing, Adaptic Touch (Acelity), topped with a moisture‐retentive dressing (hydrogel bolster) and a padded 3‐layer dressing, Dynaflex (Acelity), or equivalent. dHACA was applied weekly during the study period until complete epithelialisation, the study was completed, or the patient was withdrawn from the study.
Percentage area reduction (PAR) was calculated for the index wound 6 weeks after randomisation. If the DFU had not been reduced in area by at least 50%, the patient was withdrawn from the study to pursue other treatment options.13
2.5. Healing validation
Index wounds were determined by the site investigator as healed if complete (100%) epithelialisation occurred without drainage and need for dressing. A follow‐up visit was scheduled 1 week after initial wound closure to assess durable closure. The principal investigator was responsible for approving protocol pathway decisions regarding wound closure or individual patient continuation in the study based on photographic review. Healing validation was adjudicated by an independent panel of physicians blinded to patient study group assignments, as well as being blinded to the principal investigator's assessment; they included a vascular surgeon, 2 plastic surgeons, a general surgeon, and a podiatrist. The panel reviewed decisions made by site investigators regarding patient enrolment, healing, and study continuation. Patients were fitted for and dispensed diabetic shoes and moulded insoles, provided complimentary by the sponsor at study exit.
2.6. Study outcomes
The primary study endpoint was percentage of wounds healed at 6 weeks between the 2 treatment groups. Secondary endpoints included percentage of wounds healed at 12 weeks, time to heal within 6 and 12 weeks, number of applications, wastage, and cost to closure for healed wounds in the dHACA group. To characterise the appropriateness of dHACA size offerings, wastage for each healed wound was determined. The percentage of the area of the dHACA that was discarded and the mean percentage waste were calculated for the group. When disks of dHACA were used, the calculation assumed a square wound within the dHACA circle for the application. dHACA costs for each wound were calculated by summing the costs of the dHACA applications from all visits using the published fee.
2.7. Sample size calculations and statistical analysis
Group sample sizes of 40 in Group 1 and 40 in Group 2 were sufficient to achieve an 80% power to detect a difference of 0.3 between the group proportions. The proportion in Group 1 (the treatment group) was assumed to be 0.3 under the null hypothesis and 0.6 under the alternative hypothesis. The proportion in Group 2 (the control group) was 0.3. The test statistic used was the 2‐sided Z test with pooled variance. The significance level of the test was targeted at 0.05, and the significance level actually achieved by this design was 0.0484.
The intent‐to‐treat (ITT) and safety populations comprised randomised patients who received at least 1 treatment. All analyses used the ITT approach. The last observation carried forward (LOCF) principle was used with regard to missing observations. Study variables were summarised as means and ±SDs for continuous variables, as well as medians for non‐normal data. Categorical variables were presented as counts and proportions or percentages. Although statistical testing between treatment groups at baseline is not recommended as logical according to CONSORT guidelines,14 such testing was carried out to examine the success of randomisation. For categorical variables, χ 2 or Fisher's exact tests were performed, and for continuous variables, independent t tests or Mann–Whitney tests were used (depending on variable normality) to test for statistical differences. A Kaplan–Meier analysis was conducted to compare time to heal within 6 or 12 weeks between the 2 treatment groups. A Cox regression analysis was carried out to analyse time to heal with 12 weeks, adjusting for all available covariates that were significant or marginally statistically significant (P < .1). Covariates were entered into 1 block with stepwise elimination of non‐significant covariates. The proportional hazards assumption was tested in the final model using each significant covariate multiplied by a time factor (T_Cov). Log linearity with regard to the log of hazard function was assessed for model covariates by graphically plotting computed Martingale residuals on the y axis against the omitted covariate on the x axis.
To adjust for the family‐wise error rate (FWER), P‐values were reported using the Hochberg step‐up procedure. Adjusted 2‐sided P values <.05 were considered significant. PASW 25 (IBM, Chicago, Illinois) was used to perform all statistical testing.
PAR for the index wound at 6 or 12 weeks was calculated as ([A I − A XW]/A I)*100, where A I is the area of the index wound at randomisation, and A XW is the area at 6 or 12 weeks.
3. RESULTS
Of the 95 patients screened, 80 met the screening criteria and were randomised to dHACA + SOC (n = 40) or SOC alone (n = 40) (Figure 2). At the 6‐week point, 12 patients from the SOC group and 2 patients from the dHACA plus SOC group were withdrawn from the study because their wounds failed to reduce in area by at least 50% and were therefore considered study failures. While all wounds remained closed 1 week after initial closure in the dHACA plus SOC group, 2 wounds in the SOC group reopened after initial wound closure.
Figure 2.
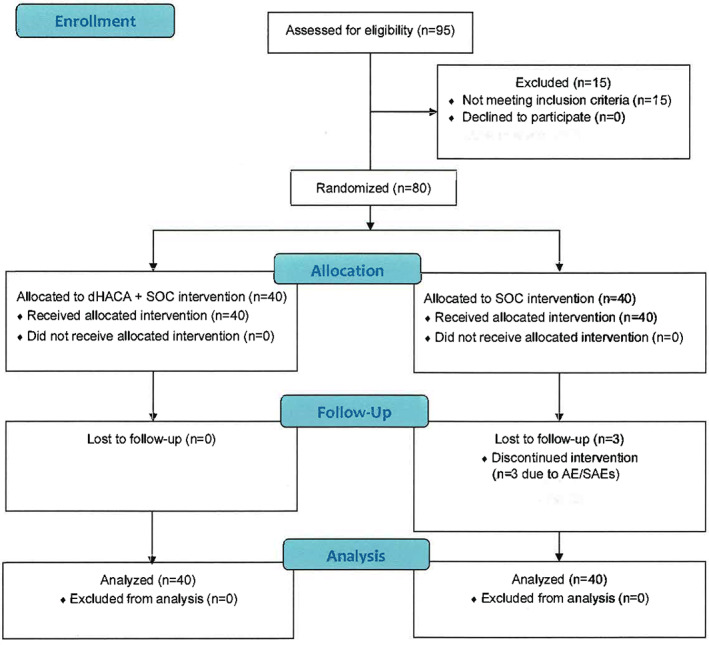
Flow chart of trial participants
Both groups were well matched with regard to patient and wound‐related parameters, with only gender being marginally statistically significant (Table 2).
Table 2.
Wound‐ and patient‐related variables between groups at randomisation. Continuous variables are reported as means and SDs and categorical variables as number (n) and percentage (%)
| Variable | dHACA | SOC | P |
|---|---|---|---|
| Age (y) | 60.1 (11.77) | 61.0 (10.66) | .71 |
| Race | |||
| Caucasian | 38 (95) | 37 (93) | .60 |
| African American | 2 (5) | 2 (5) | |
| Hispanic | 0 (0) | 1 (2) | |
| Gender | |||
| Male | 23 (58) | 31 (78) | .056 |
| Female | 17 (42) | 9 (22) | |
| Body Mass Index (BMI) | 34.0 (9.30) | 34.5 (9.42) | .83 |
| Smoker | 4 (10) | 3 (8) | 1.0 |
| Drinks alcohol | 8 (20) | 8 (20) | 1.0 |
| HbA1c | 7.6 (1.47) | 7.9 (1.48) | .36 |
| Creatinine | 1.1 (0.44) | 1.1 (0.40) | .52 |
| Initial wound area (cm2) | 2.1 (1.46); median: 1.6 | 3.1 (3.58); median: 1.8 | .15 |
| Wound plantar surface | 29 (73) | 30 (10) | .80 |
| Wound location | |||
| Toe | 10 (25) | 9 (23) | .69 |
| Forefoot | 16 (40) | 14 (35) | |
| Midfoot | 12 (30) | 12 (30) | |
| Heel/ankle/hindfoot | 2 (5) | 5 (12) | |
dHACA, dehydrated human amnion and chorion allograft; SOC, standard of care.
Average of HbA1c values (beginning and end of study).
At 6 weeks, with regard to the primary endpoint of complete wound healing, 68% (27/40) of the dHACA plus SOC‐treated DFUs had healed compared with 8 of 40 (20%) with SOC alone (P = 1.9 × 10−5) (Figure 3). At 12 weeks, 85% of the DFUs in the dHACA plus SOC group had healed (34/40) compared with 33% (13/40) in the SOC group (P = 6.0 × 10−6) (Figure 4). At 6 weeks, mean time‐to‐heal for the dHACA plus SOC group was 29.2 days (95% CI: 25.1‐33.4) compared with 39.5 days (95% CI: 37.4‐41.5) for the SOC group (P = 1.6 × 10−5). At 12 weeks, the difference in mean time to heal between the groups had lengthened: dHACA plus SOC: 37.0 days (95% CI: 29.5‐44.4); SOC: 67.3 days (95% CI: 59.0 ‐79.6; P = 6.0 × 10−6). The median time to heal within 12 weeks for the dHACA plus SOC cohort was 35.0 days (95% CI: 26.5‐43.5).
Figure 3.
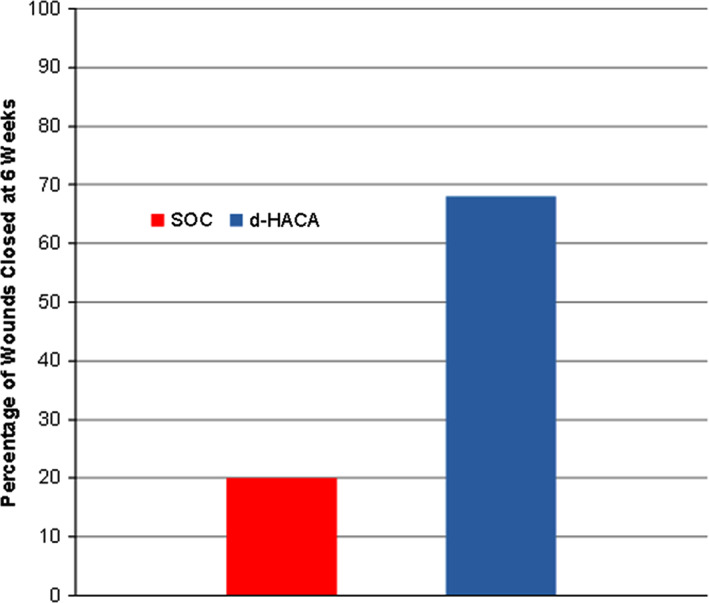
Bar graph showing complete wound healing at 6 weeks for the dehydrated human amnion and chorion allograft (dHACA) and standard of care (SOC) groups (P = 1.9 × 10−5)
Figure 4.
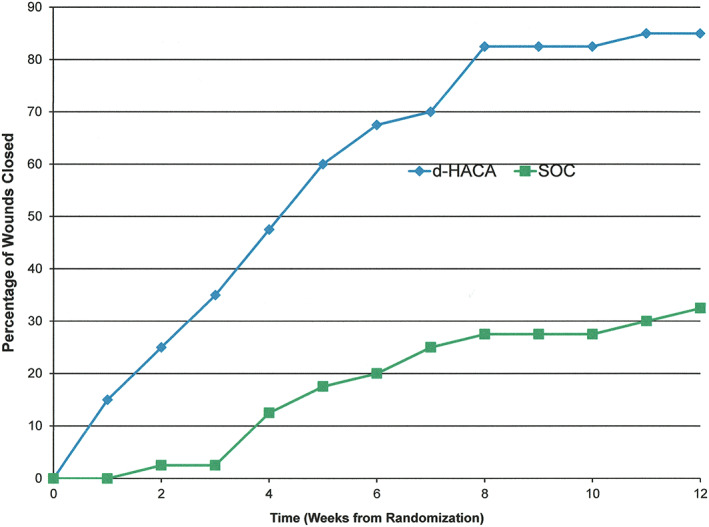
Plotted graph showing complete wound healing at 12 weeks for the dehydrated human amnion and chorion allograft (dHACA) and standard of care (SOC) group (P = 6.0 × 10−6)
The final Cox regression model at 12 weeks only included treatment and initial wound area as significant covariates. While the proportional hazards assumption was met for both covariates, the initial area did show a break point at about ~5 cm2 with regard to log linearity. The hazard ratio (HR) for dHACA plus SOC treatment was 4.25 (95%CI: 0.44‐0.79; P = 2.5 × 10−5) compared with SOC as treatment after controlling for initial wound area (Figure 5). (The HR for initial area was 0.59 [95%CI: 0.44‐0.79]; P = 4.5 × 10−4.)
Figure 5.
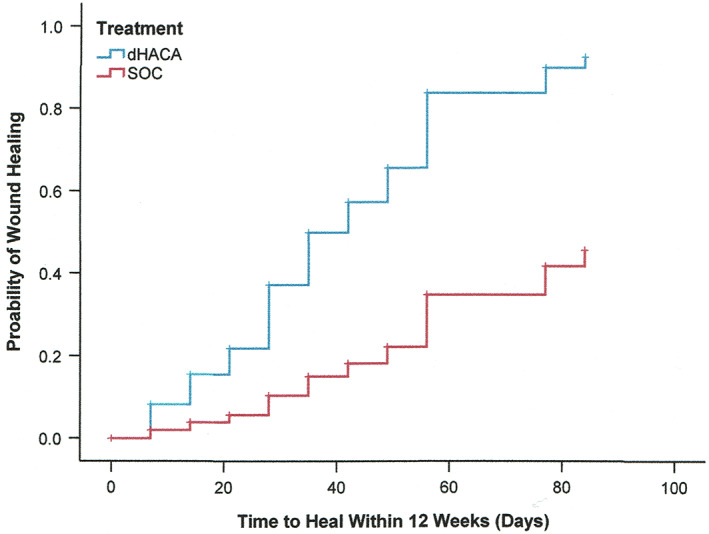
Kaplan–Meier plot of time to heal within 12 weeks by treatment group, after controlling for initial area of wounds (P = 2.2 × 10−5)
At 12 weeks, mean PAR for the SOC group was 45.6 (SD: 74.2) compared with 90.3 (SD: 13.65) for the dHACA plus SOC group (P = 6.0 × 10−6) (Figure 6).
Figure 6.
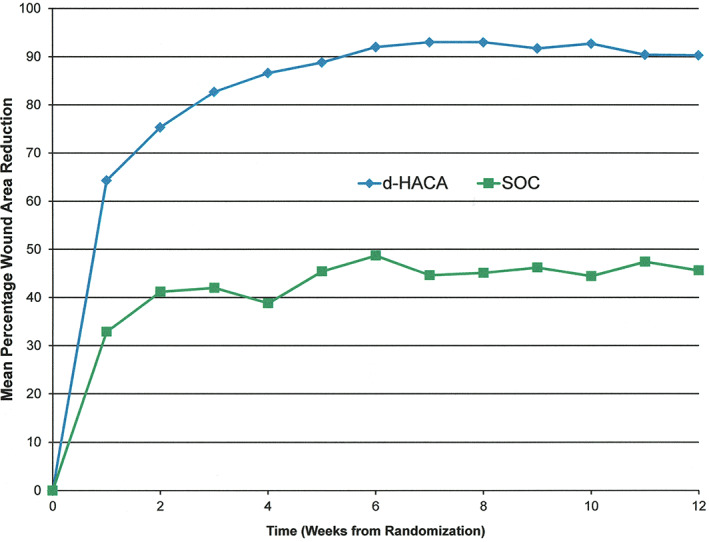
Weekly percentage wound area reduction up to week 12 (intent‐to‐treat basis) by treatment group (P = 6 × 10−6)
At 6 weeks, the mean number of grafts used per wound for the dHACA plus SOC group was 3.7 (SD: 1.97), the mean cost of graft to heal wounds was $1701 (SD: $1261; n = 27), and the mean percentage wastage was 34.4 (SD: 18.8). At 12 weeks, the mean number of grafts used per healed wound was 4.0 (SD: 2.56), the mean cost of graft to heal a wound was $1771 (SD: $1375; n = 34), and the mean percentage wastage was 35.3 (SD: 18.8).
Eleven adverse events (AEs) occurred, with 3 in the dHACA plus SOC group (8%) and 8 in the SOC group (20%). All AEs involved localised pedal infections initially treated with antibiotics. There were a total of 4 serious adverse events (SAEs), with 1 in the dHACA plus SOC group (3%) and 3 in the SOC (8%). The SAEs involving foot infections all required hospitalisation, and the majority progressed to osteomyelitis and were treated with IV antibiotics and OR debridement as necessary. There were no adverse events found to be graft related.
The number needed to treat (NNT) at 12 weeks was 1.9 (95% CI: 1.4‐2.9).
4. DISCUSSION
The results of the full clinical trial in which 80 patients were enrolled mirrored the interim results.11 Clinical outcomes in terms of proportion of wounds healed, time to heal, and PAR at both 6 and 12 weeks were all statistically superior for the dHACA plus SOC group compared with the SOC group. After adjustment for initial wound area, wounds treated with dHACA and SOC were more than 4 times as likely to heal within 12 weeks as wounds treated with SOC alone. Mean and median time to heal for the dHACA group within 12 weeks (37/35 days) was faster than the majority of other cellular and/or tissue‐based products (CTPs) studied in other RCTs,15, 16, 17, 18, 19, 20, 21, 22, 23, 24 indicating that it may be 1 of the most effective CTPs available.
To that end, in reviewing previous published studies of similar size populations. the 85% wound healing of dHACA in this 12‐week trial stands out as being clearly superior. For example, Reyzelman et al. demonstrated, in their 86‐patient multicentre trial, a healing rate of 69.6% with their allograft (Graftjacket, Acelity),20 and Niezgoda et al. reported a 49% healing rate in their 76‐patient trial, with their CTP made from small intestine submucosa SIS (OASIS Wound Matrix, Smith and Nephew, Kingston, United Kingdom).23
By 12 weeks, the mean number of dHACA applications in the full trial (4.0) was almost identical compared with our interim results (3.8),11 with similar mean percentage wastage figures (35.3 vs 40.0). Our costs for healed dHACA‐treated wounds over 12 weeks were slightly higher in the full trial ($1771) compared with the interim results ($1400); however, the mean starting wound size was also slightly higher in the full cohort. When evaluating cost to closure in the literature for similar allograft and CTP studies, dHACA shows the lowest cost to closure over 12 weeks at $1771 per healed wound even when compared with a similar dehydrated amnion and chorion allograft (dHACM; Epifix, Mimedx, Marietta, Georgia) for which cost to closure at 12 weeks was $2798 per wound.16 Low tissue wastage (mean 35%) confirms the appropriateness of dHACA graft sizes available to patients and maximisation of the donor tissue.
The study strengths of the full trial include more robust results than the interim findings, with a larger sample size and higher statistical power for clinical outcomes, as well as pragmatic SOC that provided healing outcomes within an expected range, satisfactory allocation concealment, ITT analysis, appropriate adjustment for multiple statistical testing, and reporting according to CONSORT guidelines. Our main study limitation was withdrawing patients at 6 weeks rather than continuing through 12 weeks of treatment if clinicians judged that their wounds were not sufficiently responding to treatment in order to ensure patient safety and permit other treatment pathways.13
Future trials of dHACA should consider a comparative arm using an advanced skin substitute for greater evidence and may even permit wounds of greater severity or depth. Patients with a higher proportion of serious comorbidities may also be considered in order to enhance representation of a more “real‐world” scenario.
In conclusion, our full clinical trial results have confirmed outcomes reported in published interim data, especially on cost to closure and number of applications, and in including additional patients’ data, establish that dHACA in conjunction with SOC results in improved likelihood of and accelerated healing rate at 12 weeks in DFUs.
DiDomenico LA, Orgill DP, Galiano RD, et al. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: A prospective, randomised, multi‐centre clinical trial in 80 patients. Int Wound J. 2018;15:950–957. 10.1111/iwj.12954
Funding information Musculoskeletal Transplant Foundation, Grant/Award Number: 002
REFERENCES
- 1. Nube V, Frank G, White J, et al. Hard‐to‐heal diabetes‐related foot ulcers: current challenges and future prospects. Chronic Wound Care Manage Res. 2016;3:133‐146. [Google Scholar]
- 2. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33:1493‐1498. [DOI] [PubMed] [Google Scholar]
- 3. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe: baseline results from the Eurodiale study. Diabetologia. 2007;50:18‐25. [DOI] [PubMed] [Google Scholar]
- 4. Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J Vasc Surg. 2014;59:220‐234. e1–2. [DOI] [PubMed] [Google Scholar]
- 5. Iversen MM, Tell GS, Riise T, et al. History of foot ulcer increases mortality among individuals with diabetes: ten‐year follow‐up of the Nord‐Trøndelag health study, Norway. Diabetes Care. 2009;32:2193‐2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong DG, Boulton AJ, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367‐2375. [DOI] [PubMed] [Google Scholar]
- 7. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27‐32. [DOI] [PubMed] [Google Scholar]
- 8. Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituing skin grafts: a preliminary report. JAMA. March 29, 1913;60(13):973‐974. [Google Scholar]
- 9. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. April 29, 2008;15:88‐99. [DOI] [PubMed] [Google Scholar]
- 10. Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. January 1, 2009;30(1):2‐10. [DOI] [PubMed] [Google Scholar]
- 11. DiDomenico LA, Orgill DP, Galiano RD, et al. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open. 2016;4:e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo KY, Sibbald RG. A cross‐sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage. 2009;55:40‐48. [PubMed] [Google Scholar]
- 13. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care. 2003;26:1879‐1882. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;340:e1‐e37. [DOI] [PubMed] [Google Scholar]
- 15. Zelen CM, Orgill DP, Serena T, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. 2017;14:307‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi‐Centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13:272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cazzell SM, Lange DL, Dickerson JE Jr, Slade HB. The management of diabetic foot ulcers with porcine small intestine submucosa tri‐layer matrix: a randomized controlled trial. Adv Wound Care (New Rochelle). 2015;4:711‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23:891‐900. [DOI] [PubMed] [Google Scholar]
- 19. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi‐Centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11:554‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reyzelman A, Crews R, Moore J, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomized, multicentre study. Int Wound J. 2009;6(3):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290‐295. [DOI] [PubMed] [Google Scholar]
- 22. Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701‐1705. [DOI] [PubMed] [Google Scholar]
- 23. Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP, OASIS Diabetic Ulcer Study Group . Randomized clinical trial Comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5):258‐266. [DOI] [PubMed] [Google Scholar]
- 24. DiDomenico L, Emch KJ, Landsman AR, Landsman A. A prospective comparison of diabetic foot ulcers treated with either a cryopreserved skin allograft or a bioengineered skin substitute. Wounds. 2011;23(7):184‐189. [PubMed] [Google Scholar]


