Abstract
For wheelchair users, a common injury is a sitting‐acquired pressure ulcer (PU) which typically onsets near the interface between the ischial tuberosity (IT) and the overlying soft tissues. The risk of developing PUs can be reduced considerably if an adequate cushion is placed on the wheelchair in order to protect tissues from PUs by minimising interface mechanical loads between the body and cushion and also, exposure to internal soft tissue loads. In this work, we studied the biomechanical performances of an off‐loading (OL) cushion with limited adjustability, in comparison to a standard foam cushion and a fully adjustable air‐cell‐based (ACB) cushion. These different cushion design approaches were methodologically and quantitatively analysed and compared here using a finite element (FE) modelling framework. We determined the internal mechanical deformations, strains and stresses in soft tissues of the seated buttocks during symmetric sitting, in a specific anatomy of a person with a spinal cord injury that was acquired during sitting in an open, magnetic resonance imaging configuration. Our results have shown that strains and stresses in muscle, fat and skin tissues are orders of magnitude lower for the ACB cushion with respect to the standard foam and OL cushions. The OL cushion design has taken the approach of protecting at‐risk sites of the buttocks by transferring local internal tissue loads away from the ITs and towards the greater trochanters, at the price of increasing exposure to internal tissue loads at sites other than the ITs. The ACB cushion design, however, has taken a different approach, that is, immersion and envelopment of the entire buttocks structure, which is useful for minimising the exposure to internal tissue loads throughout the whole buttocks. Quantifying performances of wheelchair cushions using FE modelling provides insights into deep tissue loads, which is essential for informed decision‐making in developing sitting solutions for individuals at risk, as well as for patient groups.
Keywords: Deep tissue loads, Finite element modelling, Soft tissue, Support surfaces
Introduction
For wheelchair users, a common injury is a sitting‐acquired deep tissue injury (DTI), which is a specific form of a pressure ulcer (PU). A DTI onsets under intact skin, and in wheelchair users, it typically forms near the interface between the ischial tuberosity (IT) and the overlying soft tissues. Any form of a serious PU and DTIs in particular can strongly impact the health status, self‐esteem and quality of life of the affected individual, and can also be life threatening as a potential cause of sepsis and osteomyelitis when the skin breaks down. Patients with a spinal cord injury (SCI) are a particularly vulnerable population for developing PUs due to the chronic co‐morbidities and lack of protective sensory perception that these persons experience 1, 2. In terms of health care budgets, PUs often feed a costly vicious cycle of repeated hospitalisations and home care needs. Annual PUs treatment costs in the US are $9·1 to $11·6 billion dollars annually, and the total costs for treating PUs in SCI patients alone exceeds 1–2 billion US$ a year 1, 3, 4, 5, 6, 7. Given all these devastating consequences and the enormous financial burden, the consensus nowadays is that PU prevention is where health resources need to be invested in. Characterising a good wheelchair cushion which provides long‐lasting protection to the tissues of patients, that is, both their skin and their sub‐dermal tissues, is a key component of such efforts.
Indeed, it is known for decades that the risk of developing sitting‐acquired PUs can be reduced considerably by using a soft but thick enough cushion on the wheelchair. Such a cushion would act to protect from PUs by minimising the interface mechanical loads between the body and the cushion but also, the exposure to sustained, internal soft tissue loads 8, 9, 10. Pressure mats are often used in rehabilitation facilities worldwide to measure the interfacial pressures between the patient's body and the cushion placed on the wheelchair, and the information is then being used for selection or fitting or customising a cushion. Nevertheless, interface pressure data are not providing any meaningful information regarding the internal tissue loads such as the internal tissue strains and stresses, and so, these pressure data are useless in assessing the quality of protection provided by a certain cushion for the sub‐dermal tissues, that is in minimising exposure to mechanical loads there. Finite element (FE) computer modelling, in contrast, is a powerful tool in evaluating internal tissue loads and how these can be managed using adequate support surfaces, and specifically, how such tissue loads are being affected by the design specifications and features of a wheelchair cushion 1, 9, 11. In published work, FE modelling was used to investigate the biomechanical performances of standard foam cushions (typically made of uniform materials) 11, 12, 13, 14, 15, 16, 17, however, most of the more complex‐structured cushions were not investigated by means of the FE method yet, excluding our own work which reported the interactions of the seated buttocks in a SCI condition, with an air‐cell‐based (ACB) cushion technology 9.
One important approach for wheelchair cushion design which has not been studied computationally is the off‐loading (OL) approach, where there is a certain region in the cushion that is deliberately not supporting/transferring bodyweight loads, for example because there is space or ‘hole’ or a very soft material at a localised site, rather than a continuum of the cushion materials there. In OL cushions for wheelchair users, the off‐loaded sites are typically just under the ITs. While OL the ITs definitely protects the soft tissues right under the ITs from sustained tissue deformations and mechanical stresses, it is clear from basic principles of physics and mechanics that the bodyweight loads still need to be transferred from the body to the cushion, which means that there are greater loads developing in other sites. The physiological and biomechanical consequences of that were highlighted, though in an extreme situation, in a recent publication of our group which focused on tissue viability when sitting on the toilet for long times. We have experimentally and computationally studied factors, such as tissue perfusion, oxygenation and mechanical strains and stresses in sub‐dermal tissues on non‐cushion and cushioned toilet seats 18. The aforementioned work has shown that prolonged toilet sitting overloads the supported periphery of the buttocks and interrupts with perfusion and oxygenation of the buttocks tissues there 18. These new, published findings motivated questioning of what are the consequences of OL a certain anatomical site in other, adjacent sites which now need to carry and transfer the loads that have been taken‐off the off‐loaded site. For this purpose, as evident from our recent toilet sitting work, 18 FE modelling is an ideal research tool.
Hence, to compare the mechanical state of tissues on an OL cushion with reasonable, practical alternative solutions for tissue protection, we chose standard foam cushions (which is the entry level in cushion technology) and the ACB cushion which we have characterised extensively in the past, and which has been thoroughly investigated using FE modelling as well 9. To this end, these three different cushion design approaches – foam, OL and ACB – were methodologically and quantitatively analysed and compared using a FE modelling framework that has been developed in our group for such evaluation of cushions. For the first time, we have computationally evaluated the OL technology, now highlighting not only the pros in OL an anatomical site which is theoretically at risk, but also the cons of essentially transferring the risk elsewhere. Our present findings are critical for the rehabilitation community in making informed decisions regarding when to off‐load, and what would be the potential consequences of prescribing an OL cushion, though it also needs to be said that in some cases (particularly when there is an active wound and functionality needs to be maintained), OL may be a good approach.
Methods
The FE modelling is a well‐established, computational technique for evaluating internal mechanical loads (e.g. deformations, strains and stresses) in a complex structure, typically containing multiple materials that altogether form irregular shapes. By virtually dividing such complex structures into numerous small elements – each having a regular and simple shape (e.g. bricks or pyramids), the equations of force and stress equilibrium are solved by the computer in each element. Considering the mechanical behaviour of each material component, information regarding the loads obtained for a certain element is then transferred and used to solve the state of loads in neighbouring elements, eventually facilitating mapping of the magnitudes, distributions and intensities of the mechanical loads in the entire structure. In this work, an FE modelling framework has been developed for providing insights about the principles and modes of action of wheelchair cushion technologies that take two remarkably different approaches to tissue protection: ACB versus OL.
Geometry
A single‐coronal, magnetic resonance imaging (MRI) scan of the buttocks was used to generate an anatomically realistic slice model of the left buttock through the ScanIP module of the Simpleware® 19 segmentation software package. The MRI data were acquired from a male subject with an SCI (age: 21 years; weight 90 kg, injury level 13; scanned 12 months post the injury), using a scan protocol that is described in our previous work 13, 20. In order to generate the undeformed model geometry of the left buttock, a non‐weight‐bearing, single‐coronal MRI slice (the anatomy of subject #5 from the Linder‐Ganz et al. 13 study; Figure 1, left frame) was loaded into the ScanIP module of Simpleware® 19 and segmented to the following tissue components: subcutaneous fat, skin, gluteus maximus muscle and IT bone (Figure 1, right frame). The slice thickness of the buttocks model was determined to be identical to the slice thickness of the MRI, 4 mm in the Y‐axis. The above anatomy of the buttocks was studied while interacting with standard foam, OL and ACB cushions to compare the internal tissue loads across these three cushion technologies.
Figure 1.
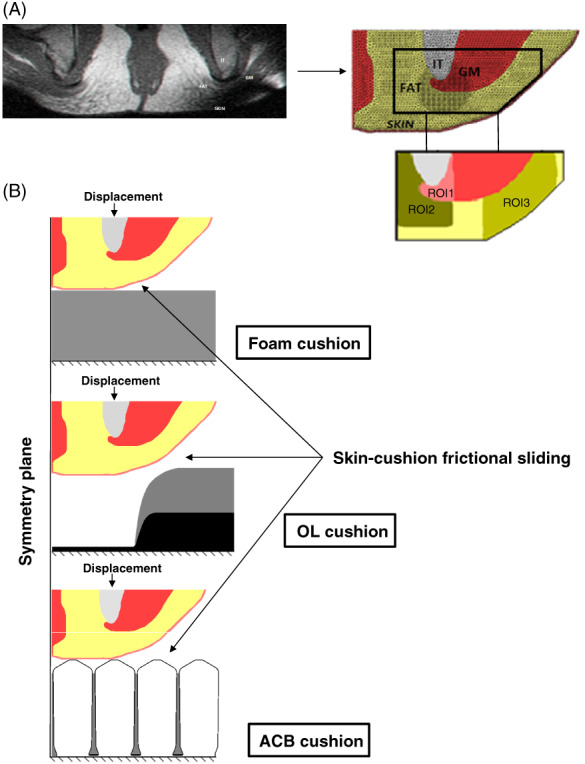
Computational model of the left buttock of a patient with a spinal cord injury (SCI patient #5 in Linder‐Ganz et al. 13): (A) MRI slice (left frame) used to generate three‐dimensional model of the left buttock and its mesh (right frame) with three regions of interest (ROIs) defined in the anatomy. F, fat tissue, IT, ischial tuberosity, G, gluteus muscle. (B) Configurations of the finite element models with boundary and loading conditions for the different cushion conditions: foam (upper frame), off‐loading (OL) (mid frame) and air‐cell‐based (ACB) (lower frame) cushions.
FE modelling
Mechanical properties of the model components
The constitutive laws and mechanical properties of all the tissue types included in the modelling were adopted from the literature. Specifically, the IT bone was modelled as a linear elastic isotropic material 21. Fat, muscle and skin tissues were assumed to be nearly incompressible, non‐linear, isotropic materials with a Poisson's ratio of 0·495. The Neo‐Hookean material model 22 was used to describe their non‐linear large deformation behaviour, using the strain energy density W function:
where G 0 is the instantaneous shear modulus, λi are the principal stretch ratios, K is the bulk modulus and J = det(F), where F is the deformation gradient tensor. The values that have been set for these parameters per each tissue type are detailed in Table 1.
Table 1.
Mechanical properties of the model components and mesh elements of the finite element models
| Model component | Shear modulus (kPa) | Bulk modulus (kPa) | Elastic modulus (kPa) | Poisson's ratio | Number of mesh elements | |
|---|---|---|---|---|---|---|
| Fat* | 0·286 | 28·5 | – | 0·495 | 28 879 | |
| Muscle* | 7·1 | 707·6 | – | 0·495 | 5144 | |
| Skin† | 31·9 | 3179·37 | – | 0·495 | 8984 | |
| Bone* | – | – | 7 × 106 | 0·3 | 2170 | |
| Foam cushion | – | – | 15·3 | 0·3 | 9000 | |
| OL cushion | Top foam | – | – | 15·3 | 0·3 | 18 308 |
| Bottom foam | – | – | 287·7 | 0·4 | 17 ,220 | |
| ACB cushion‡ | Cells | – | – | 100 | 0·3 | 245 ,235 |
| Gaps | – | – | 0·002 | 0·1 | 150 658 |
For mechanically characterising the OL cushion we have tested commercial products, and have followed the ASTM D3574 standard of a compressive force deflection test in order to obtain the elastic properties of the foam components as specified in Table 1. The Poisson's ratios were set as 0·3 and 0·4 for the top and bottom foam, respectively, based on literatures 23, 24. We have then assigned the same elastic modulus of the top foam in the OL to the standard foam cushion in the modelling, to allow direct comparisons of internal tissue loads in the buttocks when seated on these two foam‐made cushions. The ACB cushion, which was also included in the comparison, was modelled as described in our previous work 9; the mechanical properties of the air cells that were used in the modelling are listed in Table 1 as well.
Boundary and loading conditions
We simulated the downward displacement of the IT from the non‐weight‐bearing configuration, where the soft tissues in the buttocks are undeformed, to the full weight‐bearing configuration when seated on each cushion type (Figure 1). The downward displacement was prescribed on the superior surface of the IT for all model variants. For the foam cushion variant, the displacement that was applied to the IT was calibrated to produce the same extent of bone displacement that was measured empirically – by comparing the non‐weight‐bearing and weight‐bearing seated MRI studies of the same subject whose anatomy was used for the present modelling (Figure 1, upper frame). For the OL cushion variant employing the same anatomy, we have verified that the final distance between the skin surface and the base of the cushion was at least 15 mm, to avoid potential contact between the buttocks and the foam base of the OL cushion, as per guidelines for using these OL products. The same displacement of the IT that has been applied for the foam cushion variant (Figure 1, mid frame) met this condition. The downward displacement for the ACB cushion was adopted from the literature 9, with final distance of 32 mm between the skin and the base of the cushion (Figure 1, lower frame).
Contacts between all tissue types were set as ‘tie’. The contact between the skin and the cushions was set as frictional sliding, with a friction coefficient of 0·4. The inferior surfaces of the cushions were fixed for all rotations and translations, and the front and back planes of the buttocks and cushions were fixed in the perpendicular direction. We defined the symmetry condition at the medial plane, and therefore, the medial surfaces of the buttocks and the cushions were fixed for motion in the lateral direction (Figure 1). In the ACB cushion model a frictionless rigid wall was placed near the lateral air cell at 10° to its vertical wall to constrain lateral translations of the air cells, as the cover of this cushion type would act to do in real life 9.
Numerical method
The geometries were all meshed using the Scan‐IP module of Simpleware.® 19 The model of the left buttock included 45 177 four‐node, linear, tetrahedral elements assigned to the different tissues, with specific refinements to the fat and muscle tissues near the IT and to the entire skin tissue (Figure 1). The foam cushion model consisted of 9000 eight‐node, linear, hexandron elements. The OL cushion included a total of 35 528 four‐node, linear, tetrahedral mesh elements. The ACB cushion contained 395 893 four‐node, linear tetrahedrons (Table 1).
The FE simulations were all set up and pre‐processed using PreView (Ver. 1.20.4, University of Utah, Salt Lake City, Utah, USA), analysed using the Pardiso linear solver of FEBio (Ver. 2.6.3, University of Utah, Salt Lake City, Utah, USA) in its structural mechanics mode, and post‐processed using PostView (Ver. 1.10.3) 25. The runtime of each model variant with either foam or OL cushion was between 5 and 10 min, however the runtime of the ACB cushion model variant was considerably longer (due to the complexity of the collapse pattern of the air cells), between 7 and 10 h, using a 64‐bit Windows with a CPU comprising Intel® Xeon® CPU E5‐2620 2 GHz (two processors) and 64 GB RAM.
Outcome measures
We compared effective strain and stress distributions developed in the soft tissues of the left buttock when seated on foam, OL and ACB cushions. Furthermore, we determined and compared average strain and stress values between all the model variants in the three regions of interest (ROIs) defined in Figure 1. In order to determine the envelopment provided by these cushion technologies, we calculated the percentage of the skin surface that is in contact with the cushion at simulated full weight‐bearing (α), per each cushion type.
Results
We calculated the effective strain and stress distributions in the soft tissues of the left buttock when seated on the standard foam, OL and ACB cushions. Stress concentrations appeared in muscle tissue adjacent to the IT and strain concentrations were shown in fat near the IT and gluteus muscle for all the model variants. The immersion and envelopment were considerably greater for the ACB cushion due to the larger contact surface between the skin and this type of cushion. Specifically, α for the ACB cushion was approximately 93%, but only 60 and 36% for the standard foam and OL cushions, respectively. Thus, peak strain and stress values were four orders of magnitude lower for the ACB cushion, with respect to the standard foam and OL cushions (Figures 2 and 3).
Figure 2.
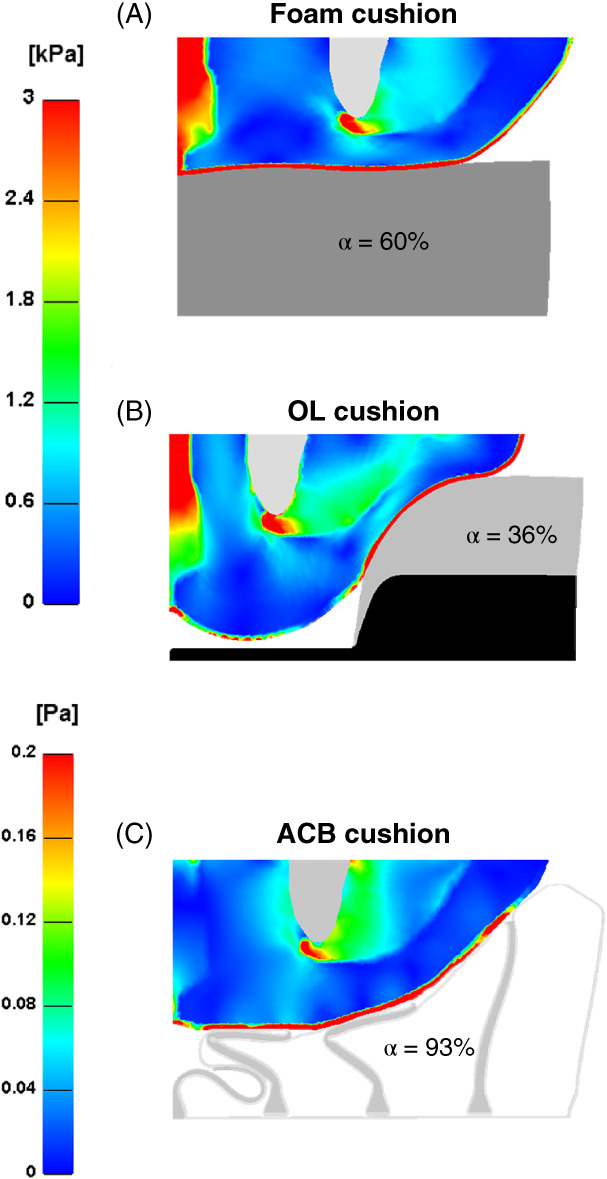
Effective stress distributions developed in the soft tissues of the left buttock when seated on: (A) foam, (B) off‐loading (OL) and (C) air‐cell‐based (ACB) cushions. The parameter α equals skin surface in full contact with the cushion divided by the entire skin surface.
Figure 3.
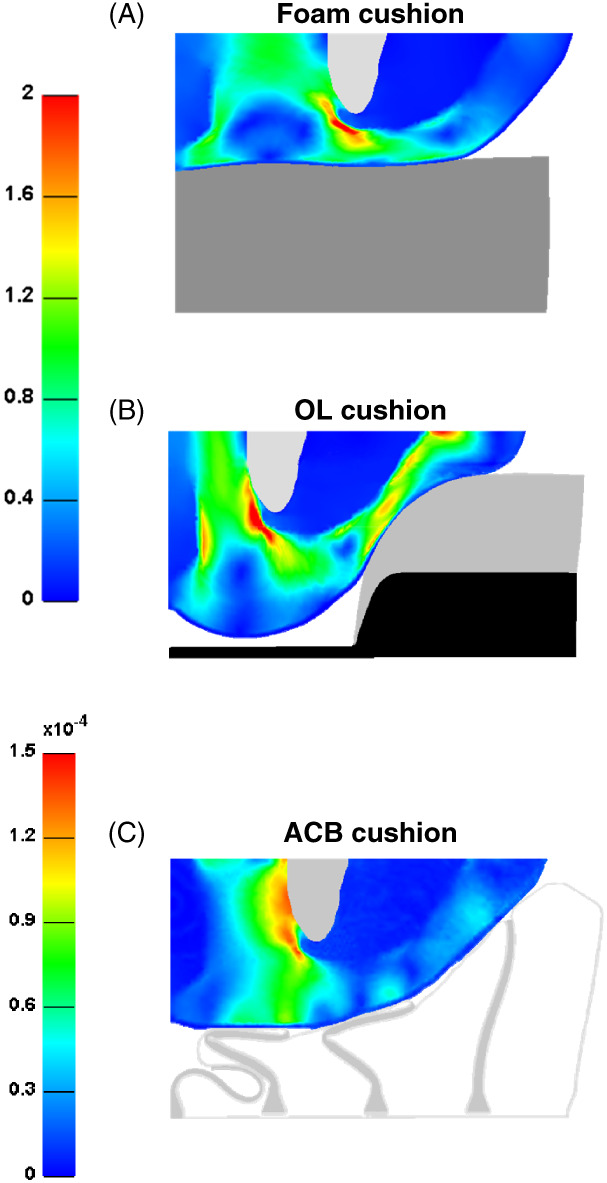
Effective strain distributions developed in the soft tissues of the left buttock when seated on: (A) foam, (B) off‐loading (OL) and (C) air‐cell‐based (ACB) cushions.
We further compared average strain and stress values across all the model variants in the three ROIs. Consistent with the data described above, the effective adjustability and greater immersion of the body contours in the ACB cushion resulted in superior internal load distributions in tissues, and accordingly, the resulting average tissue strain and stress values were four orders of magnitude lower for the ACB cushion for all the ROIs, as shown in Figure 4. The average stress values in ROI1 were one order of magnitude greater for all the model variants, compared to the other two ROIs.
Figure 4.
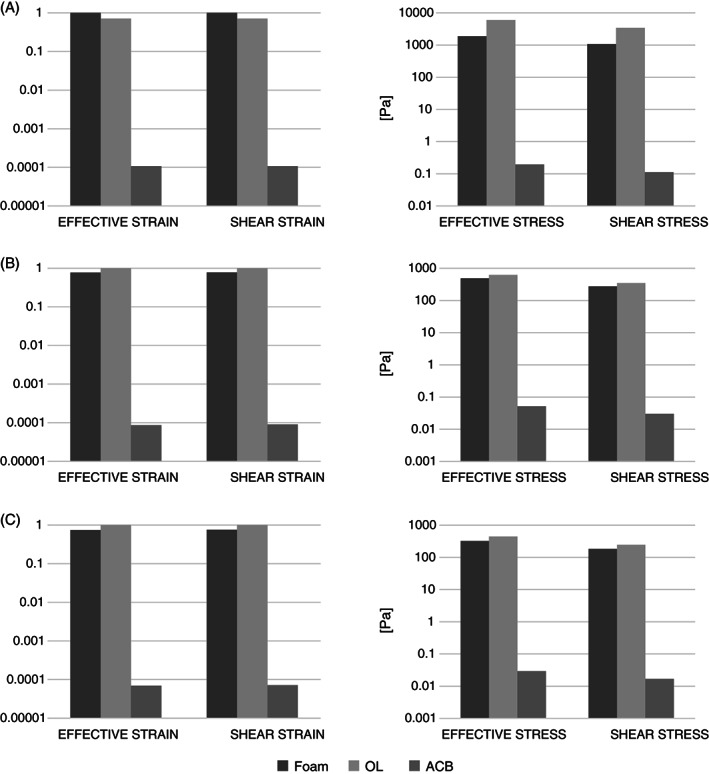
Comparison of average strain (left column) and stress (right column) values between the foam, off‐loading (OL) and air‐cell‐based (ACB) cushions in the three regions of interest (ROIs): (A) ROI1, (B) ROI2, (C) ROI3.
Comparing tissue loads when the body is seated on the OL versus the standard foam cushions resulted the same order of magnitude of average strains and stresses in the three ROIs. Specifically, average effective tissue strain values for the OL cushion were slightly lower in ROI1 (OL: 9%, foam: 13%), but greater in ROI2 (OL: 100%, foam: 77%) and ROI3 (OL: 70%, foam: 52%), with respect to the foam (Figure 4).
Discussion
PUs are one of the most challenging problems for wheelchair users. Their consequence can be death, or a severe loss of functionality and quality of life. The manpower and financial resources invested in treating PUs are a major burden on health services and health care budgets at any medical setting, including home care, and for all patient populations. Hence, prevention of PU is the key strategy. There is a large variety of commercially available cushion designs that are aimed at reducing the risk for PUs, but no objective, standard and quantitative criteria exist yet, to determine which sitting solution is adequate for protecting the tissues of individuals, particularly the subcutaneous tissues, from a pressure injury. Historically, cushion manufacturers have looked at how thickness and stiffness of cushions made of uniform materials, especially foams, affect interfacial pressures between the patient's body and the cushion, and have used pressure mats to measure these data 14. It was found that the sitting pressures decrease with an increase in the thickness of the cushion; however, there is a point where excessive thickness could lead to asymmetrical posture and weight‐transfer to the chair, that is the stability of the body on the cushion decreases, which then leads to asymmetrical interface pressure patterns as a manifestation of this condition 1, 14. While internal tissue strains and stresses cannot be measured using pressure mats, computational modelling for simulating these tissue loads and quantifying how they are shared, and the pattern by which they are transferred within body and tissue structures, can be extremely powerful in evaluating the effects of cushion designs on deep tissues.
In this study, we used FE modelling to compare biomechanical performances of a standard foam, OL and ACB cushions in minimising the exposure to sustained tissue deformations and stresses – as a measure of their efficacy in protecting against PUs and DTI in particular. The present results have shown that strains and stresses in muscle, fat and skin tissues are orders of magnitude lower for the ACB cushion with respect to the standard foam and OL cushions, which confirms our previous findings in this regard 9. The ACB cushion design facilitates immersion and envelopment of the buttocks in the cushion, which is highly effective for minimising internal tissue loads, particularly strain and stress concentrations near the ITs (Figures 2 and 3)9.
Tissue viability clearly depends not only on the magnitude of mechanical loads but also on the time of exposure to these loads 1, 26. Since the ACB cushion provides greater immersion and envelopment with respect to standard foam and OL cushions, it appears that theoretically, the ACB cushion technology could provide a longer safe‐sitting time 27, 28. With that being said, it is also important to note that it is not feasible to define the specific safe‐sitting time for the individual on any of these cushions, as tissue mechanical properties, injury thresholds, tissue repair capacities and chronic medical conditions vary considerably across individuals. The present modelling can, however, shed light on the OL technology which has not been investigated before by computational means, excluding the recent paper from our group on toilet seat cushions 18 which is in fact an extreme form of OL of the soft tissues of the buttocks. Theoretically, OL the centre of the seated buttocks using an OL cushion should protect the tissues directly above the OL site, superficially and deep within. Nonetheless, at the same time, and as shown in our aforementioned toilet seat work 18, the OL site in the cushion imposes elevated shear deformations and stresses on skin and deep tissues at the perimeter of the OL site (Figures 2 and 3).
Specifically, when comparing tissue loads when the body is seated on standard foam versus OL cushions, it was initially expected that the OL cushion would have resulted in reducing peak tissue loads, particularly under the IT bones and at the surrounding soft tissues. Nevertheless, the strain and stress concentrations when sitting on the OL cushion were actually in the same order of magnitude as those for the standard foam cushion, which is again due to the lateral shear at the perimeter of the OL site, as the body loads need to shift to, and transfer through the narrow non‐off‐loaded surfaces.
Computer modelling, by definition, involves assumptions and simplifications. For example, fully three‐dimensional modelling would probably provide additional information regarding tissue deformations on the three cushion types, but such information is not straight‐forward to analyse and interpret. Likewise, in order to facilitate direct comparisons, we did not consider anatomical variants, have omitted some anatomical details and did not analyse asymmetrical sitting, which would all require additional studies.
In summary, we showed here that an ACB cushion provides better envelopment of the entire buttocks structure with respect to standard foam and OL cushions. Surprisingly, an OL cushion did not demonstrate a compelling reduction of internal tissue loads underneath the ITs compared to a standard foam cushion, which may be unforeseen at first but actually, can be well expected when examining the biomechanics of sitting on non‐continuous surfaces. Specifically, the present work emphasises that OL comes with a substantial price of increasing tissue loads around the OL site, particularly shear deformations and stresses, which is the result of one of the fundamentals of physics and mechanics: equilibrium of forces. The same body forces need to be transferred via smaller contact areas when the body is seated on an OL cushion, which then generates high stress values and large stress concentrations at around these narrow support sites. Our recently published work on tissue mechanics in the buttocks when sitting on the toilet is very relevant in this regard 18, as sitting on the toilet is perhaps the extreme form of OL the centre of the buttocks. Clinicians would sometimes prescribe an OL cushion to either high‐risk patients or patients with an existing PU on their buttocks, to off‐load the sites at risk or the wound (and hence allow tissue repair). OL an existing PU may also have benefits in better management of wound exudates and the microclimate of the wound‐bed. While appreciating that sometimes there is no other choice but to off‐load a wounded region, one should always bear in mind that when OL one body site, the body forces and tissue deformations and stresses actually shift to other sites, and increase the risk for tissue injury in those other sites. The data here should contribute to the debate of whether OL is good for patients at risk (as opposed to the alternative support surface technologies) or whether it should only be used in cases of existing PUs, and even then, with caution to not generate new injuries. In conclusion, for an OL cushion the same body loads still need to be transferred to the wheelchair, however given that a certain region, the OL region, cannot transfer forces, more forces are transferred around the OL site, which create stress concentrations at the boundaries of the OL site, and in particular shear stress concentrations. The OL technology therefore needs to be used with great cautiousness, and perhaps be limited to cases where there is no other alternative such as when an existing wound needs to be treated – at the price of risking other, non‐injured tissue sites.
Acknowledgement
This research work was supported by Ms Kara Kopplin at Permobil Group (Belleville, IL, USA). The authors declare no conflict of interest.
References
- 1. Gefen A. The biomechanics of sitting‐acquired pressure ulcers in patients with spinal cord injury or lesions. Int Wound J 2007;4:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kruger EA, Pires M, Ngann Y, Sterling M, Rubayi S. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med 2013;36:572–85.24090179 [Google Scholar]
- 3. Miller H, Delozier J. Cost implications of the pressure ulcer treatment guideline. Columbia: Center for Health Policy Studies. (Contract No. 282‐91‐0070. Sponsored by the Agency for Health Care Policy and Research); 1994. [Google Scholar]
- 4. Bauer K, Rock K, Nazzal M, Olivia Jones O, Qu W. Pressure ulcers in the United States' inpatient population from 2008 to 2012: results of a retrospective nationwide study. Ostomy Wound Manage 2016;62:30–8. [PubMed] [Google Scholar]
- 5. Duncan KD. Preventing pressure ulcers: the goal is zero. Jt Comm J Qual Patient Saf 2007;33:605–610. [DOI] [PubMed] [Google Scholar]
- 6. Lyder CH, Ayello EA. Pressure Ulcers: A Patient Safety Issue. In: Hughes RG, editor. Patient safety and quality: an evidence based handbook for nurses. Rockville: Agency for Healthcare Research and Quality (US), 2008. [PubMed] [Google Scholar]
- 7. National Center for Injury Prevention and Control Centers for Disease Control and Prevention . Injury fact book of the Center for Disease Control and Prevention (CDC). Atlanta: The National Center for Injury Prevention and Control, 2001–2002, 2001. [Google Scholar]
- 8. Levy A, Kopplin K, Gefen A. Simulations of skin and subcutaneous tissue loading in the buttocks while regaining weight‐bearing after a push‐up in wheelchair users. J Mech Behav Biomed Mater 2013;28:436–47. [DOI] [PubMed] [Google Scholar]
- 9. Levy A, Kopplin K, Gefen A. An air‐cell‐based cushion for pressure ulcer protection remarkably reduces tissue stresses in the seated buttocks with respect to foams: finite element studies. J Tissue Viability 2014;23:13–23. [DOI] [PubMed] [Google Scholar]
- 10.The European and US National Pressure Ulcer Advisory Panels (EPUAP and NPUAP), and the Pan Pacific Pressure Injury Alliance (PPPIA). International Guidelines; 2014. URL http://www.epuap.org/guidelines/ [accessed on 8 August 2017]
- 11. Makhsous M, Lim D, Hendrix R, Bankard J, Rymer WZ, Lin F. Finite element analysis for evaluation of pressure ulcer on the buttock: development and validation. IEEE Trans Neural Syst Rehabil Eng 2007;15:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linder‐Ganz E, Gefen A. Stress analyses coupled with damage laws to determine biomechanical risk factors for deep tissue injury during sitting. J Biomech Eng 2009;131:011003. [DOI] [PubMed] [Google Scholar]
- 13. Linder‐Ganz E, Shabshin N, Itzchak Y, Yizhar Z, Siev‐Ner I, Gefen A. Strains and stresses in sub‐dermal tissues of the buttocks are greater in paraplegics than in healthy during sitting. J Biomech 2008;41:567–80. [DOI] [PubMed] [Google Scholar]
- 14. Ragan R, Kernozek TW, Bidar M, Matheson JW. Seat‐interface pressures on various thicknesses of foam wheelchair cushions: a finite modeling approach. Arch Phys Med Rehabil 2002;83:872–5. [DOI] [PubMed] [Google Scholar]
- 15. Raghavan P, Raza WA, Ahmed YS, Chamberlain MA. Prevalence of pressure sores in a community sample of spinal injury patients. Clin Rehabil 2003;17:879–84. [DOI] [PubMed] [Google Scholar]
- 16. Todd BA, Thacker JG. Three‐dimensional computer model of the human buttocks, in vivo. J Rehabil Res Dev 1994;31:111–9. [PubMed] [Google Scholar]
- 17. Verver MM, van Hoof J, Oomens CW, Wismans JS, Baaijens FP. A finite element model of the human buttocks for prediction of seat pressure distributions. Comput Methods Biomech Biomed Eng 2004;7:193–203. [DOI] [PubMed] [Google Scholar]
- 18. Lustig M, Levy A, Kopplin K, Ovadia‐Blechman Z, Gefen A. Beware of the toilet: the risk for a deep tissue injury during toilet sitting. J Tissue Viability 2017. https://doi:10.1016/j.jtv.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 19. Simpleware Ltd . ScanIP, +FE, +NURBS and + CAD Reference Guide version 5.1; 2012. URL http://www.simpleware.com/software/ [accessed on 8 August 2017]
- 20. Linder‐Ganz E, Shabahin N, Itzchak Y, Gefen A. Assessment of mechanical conditions in sub‐dermal tissues during sitting: a combined experimental‐MRI and finite element approach. J Biomech 2007;40:1443–54. [DOI] [PubMed] [Google Scholar]
- 21. Elsner JJ, Gefen A. Is obesity a risk factor for deep tissue injury in patients with spinal cord injury? J Biomech 2008;41:3322–31. [DOI] [PubMed] [Google Scholar]
- 22.The Musculoskeletal Research Laboratories at the University of Utah. FEBio: finite element for biomechanics, theory manual version 2.5; 2016. URL http://mrl.sci.utah.edu/software/febio [accessed on 8 August 2017]
- 23. Widdle RD Jr, Bajaj AK, Davies P. Measurement of the Poisson's ratio of flexible polyurethane foam and its influence on a uniaxial compression model. Int J Eng Sci 2008;46:31–49. [Google Scholar]
- 24. Nitta K, Yamana M. In: Juan De V, editor. ISBN:978‐953‐51‐0187‐1. Poisson's ratio and mechanical nonlinearity under tensile deformation in crystalline polymers, rheology. Croatia: InTechOpen. 2012. [Google Scholar]
- 25. Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: finite elements for biomechanics. J Biomech Eng 2012;134(1). doi: 10.1115/1.4005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reswick JB, Rogers JE. Experience at Rancho Los Amigos Hospital with devices and techniques to prevent pressure sores. In: Bedsore biomechanics. London, UK: Macmillan, 1976:301–14. [Google Scholar]
- 27. Gefen A. How much time does it take to get a pressure ulcer? Integrated evidence from human, animal, and in vitro studies. Ostomy Wound Manage 2008;54:26–8, 30–5. [PubMed] [Google Scholar]
- 28. Shabshin N, Zoizner G, Herman A, Ougortsin V, Gefen A. Use of weight‐bearing MRI for evaluating wheelchair cushions based on internal soft‐tissue deformations under ischial tuberosities. J Rehabil Res Dev 2010;47:31–42. [DOI] [PubMed] [Google Scholar]
- 29. Sopher R, Nixon J, Gorecki C, Gefen A. Exposure to internal muscle tissue loads under the ischial tuberosities during sitting is elevated at abnormally high or low body mass indices. J Biomech 2010;43:280–6. [DOI] [PubMed] [Google Scholar]


