Abstract
To conduct a systematic review and meta‐analysis on the effects of electrical stimulation therapy (EST) on healing pressure ulcers in individuals with spinal cord injury (SCI). CINAHL, The Cochrane Library, PubMed, SCOPUS, EMBASE, Nursing & Allied Health and Dissertation & Theses databases were searched for relevant English language articles from the date of inception to 31 January 2014. Separate searches were conducted in Google Scholar and academic journals specialised in wound care. Two reviewers independently assessed study eligibility. Studies were included if EST was used to treat pressure ulcers in individuals with SCI. A total of 599 articles were screened, and 15 studies met the inclusion criteria. A meta‐analysis with five studies demonstrated that EST significantly decreased the ulcer size by 1·32%/day [95% confidence interval (CI): 0·58–2·05, P < 0·001] compared to standard wound care (SWC) or sham EST. Another meta‐analysis conducted with four studies showed that EST increased the risk of wound healing by 1·55 times compared with standard wound care or sham EST (95% CI: 1·12 to 2·15, P < 0·0001). Because of the wide array of outcome measures across studies, a single meta‐analysis could not be conducted. EST appears to be an effective adjunctive therapy to accelerate and increase pressure ulcer closure in individuals with SCI.
Keywords: Electrical stimulation therapy, Meta‐analysis, Pressure ulcers, Spinal cord injuries, Systematic review
Introduction
Persons with spinal cord injury (SCI) experience a number of secondary health complications over their lifetime, with pressure ulcers being the most common. Despite the numerous prevention and management recommendations available 1, 2, it has been estimated that 31–40% of individuals with SCI develop pressure ulcers in a given year 3, 4, 5 and up to 85% of them develop at least one pressure ulcer over the course of their lifetime 6. In addition to having a significant impact on the health care system, pressure ulcers are also associated with serious health implications for individuals with SCI including reductions in physical activity levels, social participation and overall quality of life 7.
Current recommendations for managing pressure ulcers involve standard wound care (SWC), which includes practices such as debridement, dressing, nutrition counselling and physical and occupational therapy 8. However, pressure ulcers may not always respond positively to SWC alone. Electrical stimulation therapy (EST) is an adjunctive approach to SWC that delivers low levels of electric current to the wound bed to increase the rate of wound healing 9. It has been suggested that EST accelerates wound healing by mimicking the natural electrical current of the skin when it is injured 9. EST has been shown to affect most phases of wound healing including inflammation, proliferation and remodelling 9. In vitro studies have demonstrated that EST can: induce fibroblastic activity by increasing DNA and protein synthesis 10, as well as calcium influx 11; promote migration and activation of macrophages 12; and promote myofibroblast transdifferentiation 13. Furthermore, in vivo studies have demonstrated that EST can: enhance angiogenesis by increasing capillary density, improve tissue oxygenation 14 through increased blood flow 15, 16 and improve tissue tensile strength 17, 18 by increasing collagen deposition 19. In addition, researchers have suggested that EST has both bacteriostatic and bactericidal effects on microbes that commonly infect wounds 20, 21, 22.
Earlier, systematic reviews 23, 24 and meta‐analyses 25, 26, 27 have been carried out to examine the healing potential of EST in various types of chronic wounds. The three meta‐analyses 25, 26, 27, which each included data from a different group of publications, concluded that the application of EST as an adjunctive therapy for treating ulcers accelerates the rate of wound healing and promotes wound closure compared with SWC alone. Results from two systematic reviews 23, 24 suggest there are quite a number of randomised controlled trials that have examined the effect of EST on healing of pressure ulcers. However, there have been no meta‐analyses that combine the results and estimates of the pooled effect of EST on pressure ulcers. Furthermore, results from published meta‐analyses involving a variety of wound types may not be transferable to the SCI population. Recent research has shown that the levels of various proteins including growth factors, cytokines and enzymes are quite different in pressure ulcers in people with SCI compared with those without SCI 28. Specifically, levels of pro‐inflammatory cytokines such as IL‐G and IL‐2, and cellular adhesion molecules (i.e. ICAM‐1), are significantly higher in individuals with SCI 29, 30, while concentrations of tissue inhibitor of metalloproteinases (TIMPs), matrix metalloproteinases (MMPs) and wound fluid proteins are significantly lower in individuals with SCI compared with those without SCI 28. Therefore, because of the differences in blood serum and wound biochemistry, it may be the case that the response to EST in pressure ulcer healing in individuals with SCI varies from that in the general population.
The purpose of this study was to conduct a systematic review and meta‐analysis to determine the effectiveness of EST on the healing of pressure ulcers in individuals with SCI, specifically, in comparison with control groups. We also aimed to understand the patients' ability to adhere to the treatment schedule, the implications of EST on the quality of life and pain relief in this population and the economic impact on the health care system.
Methods
Types of studies
This systematic review included only studies where EST was applied to individuals with SCI and pressure ulcers. Clinical controlled trials comparing EST intervention (alone or in conjunction with SWC) with sham EST or SWC alone were examined for inclusion in the meta‐analysis, while non‐controlled trials (i.e. case studies) were described narratively. A summary of the inclusion and exclusion criteria used to select the studies included for the review is presented in Table 1.
Table 1.
Inclusion and exclusion criteria of the study
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
EST, electrical stimulation therapy; SCI, spinal cord injury.
Types of participants
Studies with men and women above the age of 18 years wherein at least 50% of participants had with acute or chronic SCI and the presence of pressure ulcers were considered for inclusion in the review. Pressure ulcers were defined as open, full‐thickness skin lesions that were likely caused by pressure or other external forces, such as friction or shear.
Types of interventions
Studies that involved conductively coupled EST, which is applied using two or more surface electrodes placed on the wound bed or in the surrounding area, were included in this study. There were four distinct EST modalities examined:
Low intensity direct current (LIDC) involves applying continuous, unidirectional flow of current of low intensity (<1 mA) for at least one second 9.
Monophasic pulsed current (MPC) involves brief pulses of unidirectional flow of current followed by a finite off period 9. Common MPC waveforms include rectangular and twin‐peaked [e.g. high‐voltage pulsed current (HVPC)].
Biphasic pulsed current (BPC) consists of brief pulses of bidirectional current that has either a symmetric or asymmetrical biphasic waveform. In symmetric BPC, the bidirectional pulsed current is equal and balanced, while asymmetric BPC produces a bidirectional current that is unequal and may or may not be balanced. Balanced asymmetric BPC has no net positive or negative charge, while unbalanced asymmetric BPC creates a net positive or negative charge over time 9.
Microcurrent (MC) includes MPC or BPC that provides current at a subsensory level.
All variations of frequency, amplitude and duration of EST were included. Control conditions in the studies included sham EST (i.e. power source disconnected, leads cut) or SWC. Studies where subjects were assigned to receive different EST protocols were also included by either totalling the number of healed ulcers or by taking the healing rate of the EST protocol with the largest sample of pressure ulcers.
Outcome measures
The primary outcome that was considered was wound healing, operationally defined as rate of change in ulcer size, absolute change in wound size and number of wounds completely healed post EST. Secondary outcomes including EST‐related adverse events, quality of life, pain relief, economic analysis and EST compliance were collated and summarised narratively.
Data sources
Electronic searches were performed by one author using the following databases: CINAHL (Cumulative Index to Nursing and Applied Health Literature, 1981); The Cochrane Library (2009); Dissertation & Theses (1861); EMBASE (1947); Proquest – Nursing & Allied Health (1988); PubMed (1946) and SCOPUS (1966) from their date of availability through to 31 January 2014. The following keywords were used in each database: spinal cord injury, spinal cord injuries, spinal cord, tetraplegia, quadriplegia, paraplegia, pressure ulcer, pressure sore, bedsore, decubitus ulcer, wound healing, electrical stimulation therapy, direct current, alternating current, galvanic current, monophasic current, biphasic current, pulsed current. Articles not available via the university library, interlibrary loan system or published in a language other than English were excluded. An experienced librarian was consulted to develop an appropriate search strategy for each database.
Separate searches (electronic and manual) were conducted in journals specialised in wounds including Advances in Wound Care (1988), Advances in Skin and Wound Care (2010), International Wound Journal (2004), Ostomy Wound Management (2001), Wounds: A Compendium of Clinical Research and Practice (1990) and Wound Repair and Regeneration (1993) from their date of availability through to 4 February 2014.
The reference lists of pertinent review articles and eligible studies were searched for additional relevant studies that were missed in the database search. Grey literature (i.e. articles not published through journals) was identified through Google Scholar (http://scholar.google.ca) and speaking with local experts in the field.
Study selection
Two reviewers screened the titles and abstracts (citations) independently. A paired consensus process was used to select relevant citations. Disagreements between reviewers were discussed until consensus was achieved.
Full articles were retrieved from all relevant citations and for those that were unclear or had missing abstracts from the database citation. Full‐text articles were then reviewed and assessed for potential inclusion in the study. Paired consensus was repeated to confirm article eligibility. Any disagreement between the two reviewers was resolved through discussion.
Data extraction
One reviewer reviewed each article and extracted data using a standard data extraction sheet. The information extracted from each study included study design, participant characteristics (i.e. age, sex and SCI characteristics), pressure ulcer characteristics (i.e. stage, location and duration of ulcer), description of EST protocol used (i.e. type, waveform, pulse frequency, intensity, duration, polarity and electrode placement), treatment schedule (i.e. frequency and total treatment time) and primary and secondary outcomes. Crossover data were not examined. A second author checked the accuracy of all the data extracted.
Methodological quality assessment
The methodological quality of the clinical controlled trials was assessed using the PEDro (Physiotherapy Evidence Database) scale. The PEDro scale is based on a Delphi list developed by Verhagen et al. 31 and consists of 11 items. The first item on the PEDro scale (eligibility criteria) assesses external validity via a ‘yes’ or ‘no’ response, and the next 10 items assess the internal validity of a trial. Items 2 through 11 are scored out of 1, resulting in a total possible score of 10. The PEDro scale has demonstrated high construct validity 32 and good inter‐rater reliability 33. One reviewer independently assessed the methodological quality of each study.
Data synthesis
All data were analysed using Comprehensive Meta‐Analysis 2.0 (CMA; Biostat, NJ). Pooled analysis was conducted for trials that expressed healing as a rate [percent per day (%/day)] and number of pressure ulcers healed. No computations of raw data were performed for any of the trials. Dichotomous outcomes were expressed as risk ratio (RR) with 95% confidence interval (CI). RR was interpreted as follows: RR < 1, risk of healing is lower in EST group; RR = 1, risk of healing is same between EST and control groups; and RR > 1, risk of healing is greater in the EST group. Hedge's g was used to interpret the effect size of continuous variables. Hedge's g is a variation of Cohen's d that corrects for bias caused as a result of sample size 34. The criteria used for Cohen's d 35 were used to interpret Hedge's g: small, 0·2; moderate, 0·5; and large, 0·8. Treatment effect was significant if P < 0·05. Heterogeneity between the studies was determined using I 2 and P‐value; I 2 exceeding 50% was used as the threshold to identify significant heterogeneity. A fixed effects model was used when the threshold for heterogeneity was not reached, and a random effects model was used when the threshold for heterogeneity was exceeded 36. Funnel plots and Orwin's fail‐safe N test were to be used to assess publication bias if more than 10 trials were included in the meta‐analysis 37, although this was not possible because of the small number of trials in each pooled analysis.
Results
Results of the systematic review
A total of 599 citations including title and abstract were identified using electronic databases (n = 498) and secondary sources (n = 101), of which 507 citations were excluded. Full text was reviewed for 49 articles; 34 were subsequently excluded. Reasons for exclusion were study participants were not individuals with SCI (n = 13), the articles were conference abstracts (i.e. full‐text not available; n = 8), studies where <50% of participants had SCI (n = 5) or pressure ulcers (n = 1), there were no wound healing outcomes reported (n = 5) or EST was delivered using indwelling electrodes (n = 2). A PRISMA flow diagram is presented in Figure 1.
Figure 1.
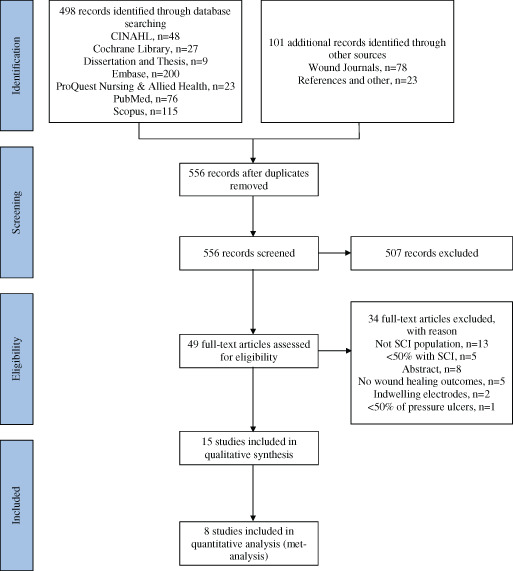
Study flow chart (PRISMA).
Inclusion criteria were met by 15 studies (Tables 2 and 3); six were randomised controlled clinical trials (RCTs) 38, 39, 40, 41, 42, 43, three prospective controlled trials 44, 45, 46, four case studies 47, 48, 49, 50 and two retrospective controlled trials 51, 52. More than 500 persons with SCI were included in these reports, in which 214 patients were included in a large retrospective analysis 52. Six of the published reports were generated from a group of researchers from Slovenia. It appears that the same data are presented in one of the publications 46, and it is not clear if there is any overlap in the data used within the two retrospective analyses 51, 52. Across all these studies, regardless of study design, authors consistently concluded that EST accelerated wound healing rate and/or the incidence of complete wound closure.
Table 2.
Summary of the clinical controlled studies included in the meta‐analysis
| Author, year, country | Study design; treatment; control | Number of patients; incomplete/complete (I/C); DOI | Number of pressure ulcers; duration of ulcers (DOU); Stage (NPUAP) | Electrode placement; EST waveform; stimulus parameter; treatment schedule | Results | Study conclusion | |
|---|---|---|---|---|---|---|---|
| Control | ES | ||||||
| Baker et al. 38, USA |
RCT TRT1: Asym BPC TRT2: Sym BPC CON1: MC CON2: Sham MC |
Asym BPC, n = 20 Sym BPC, n = 21 MC, n = 20 Sham MC, n = 19 I/C: 19/52 DOI: 1–420 months |
Asym BPC, n = 67 Sym BPC, n = 58 MC, n = 42 Sham MC, n = 25 DOU: 2–1095 days Stage: n/a |
Electrodes applied on opposite sides on the wound edge, active electrode more proximal Asym BPC: rectangular, 100 microseconds, 50 Hz, 7:7 on/off ratio Intensity: submotor Sym BPC: rectangular, 300 microseconds, 50 Hz, 7:7 on/off ratio Intensity: submotor MC: rectangular, 10 microseconds, 1 Hz, 4 mA Intensity: subsensory 30 minutes × 3/day × 5 days/weeks until healed |
MC: 23·3 ± 4·8%/week, 18 healed Sham MC: 32·7 ± 7·0%/week, 6 healed |
Asym BPC: 36·4 ± 6·2%/week, 35 healed Sym BPC: 29·7 ± 5·1%/week, 33 healed |
WHR significantly faster in Asym BPC versus MC and sham MC |
| Barczak et al. 44, Germany |
PCT TRT: MPC CON: Sham MPC |
MPC, n = 10 Sham MCP, n = 14 I/C: 6/18 DOI: n/a |
MPC, n = 16 Sham MPC, n = 17 DOU: <1 to >6 months Stage: 3–5 according to Daniel |
Active electrode applied directly over wound MPC: rectangular, 140 microseconds, 38 mA Intensity: n/a 60 minutes/day × 7 days/weeks × 4 weeks |
1·1 ± 0·96%/day, 65% of initial WSA | 2·0 ± 1·47%/day, 38% of initial wound size | WHR significantly faster in EST versus CON |
| Griffin et al. 39, USA |
RCT TRT: HVPC CON: Sham HVPC |
HVPC, n = 8 Sham HVPC, n = 9 I/C: 3/14 DOI: 3–1820 weeks |
HVPC, n = 8 Sham HVPC, n = 9 DOU: 1–116 weeks Stage: II–IV |
Active electrode applied directly over wound, dispersive applied on intact skin HVPC: 75 microseconds, 100 Hz, 500 μA Intensity: subsensory 60 minutes/day × 7 day/week × 2·9 weeks |
52% ↓ in WSA, 2 healed | 80% ↓ in WSA, 3 healed | Significant ↓ in WSA in EST versus CON |
| Houghton et al. 40, Canada |
RCT TRT: HVPC CON: SWC |
HVCP, n = 16 SWC, n = 18 I/C: n/a DOI: 1–51 years |
HVCP, n = 16 SWC, n = 18 DOU: 0·3–20 years Stage: II–IV or unstageable |
Active electrode applied directly over wound, dispersive applied on intact skin (n = 11); electrodes applied on opposite sides of wound edge (n = 2); electroconductive sock (n = 3) HVPC: 50 microseconds, 10–100 Hz Intensity: submotor 240 minutes/day × 7 day/week × 13 weeks |
36 ± 61% ↓ in WSA, 5 healed | 70 ± 25% ↓ in WSA, 6 healed | Significant ↓ in WSA in EST versus CON |
| Jerčinonić et al. 41, Slovenia |
RCT TRT: Asym BPC CON: SWC |
Asym BPC, n = 42 SWC, n = 31 I/C: n/a DOI: 32 ± 60 months |
Asym BPC, n = 61 SWC, n = 48 DOU: 143·5 ± 229·2 days Stage: n/a |
Electrodes applied on opposite sides on the wound edge Asym BPC: balanced, 250 microseconds, 40 Hz, 4:4 on/off ratio Intensity: motor 120 minutes/day × 5 days/week × 4 weeks |
2·7 ± 3·6%/day | 5·7 ± 7·1%/day | WHR significantly faster in EST versus CON |
| Karba et al. 45, Slovenia |
PCT TRT1: LIDC+ TRT2: LIDC+/− CON: Sham LIDC+/− |
LIDC+, n = 16 LIDC+/−, n = 18 Sham LIDC+/−, n = 16 I/C: n/a DOI: n/a |
LIDC+, n = 16 LIDC+/−, n = 18 Sham LIDC+/−, n = 16 DOU: n/a Stage: III–IV |
LIDC+: Active electrode applied directly over wound, dispersive applied on intact skin, 0·6 mA Intensity: subsensory LIDC+/−: Electrodes applied on opposite sides of wound edge, 0·6 mA Intensity: subsensory 120 minutes/day × 7 days/week until healed |
4·2 ± 1·1%/day |
LIDC+: 7·4 ± 1·6%/day LIDC+/−: 4·8 ± 1·5%/day |
WHR significantly faster in LIDC+ versus CON |
| Karba et al. 42, Slovenia |
RCT TRT: Asym BPC CON: Sham BPC |
Aysm BPC, n = 6 Sham BPC, n = 6 I/C: n/a DOI: n/a |
Aysm BPC, n = 6 Sham BPC, n = 6 DOU: n/a Stage: n/a |
Electrodes applied on opposite sides of wound edge Asym BPC: balanced, 250 microseconds, 4:4 on/off ratio Intensity: motor 120 minutes/day × 7 days/week until healed |
−0·66 ± 1·16%/day, 0 healed | 7·13 ± 1·46%/day, 6 healed | WHR significantly faster in EST versus CON |
| Stefanovska et al. 51, Slovenia |
Retrospective CT TRT1: Asym BPC TRT2: LIDC CON: SWC |
Number of patients not specified I/C: n/a DOI: 31·0 ± 59·7 months |
Asym BPC, n = 82 LIDC, n = 18 SWC, n = 50 DOU: 207·9 ± 482·14 days Stage: n/a |
Electrodes applied on opposite sides of wound edge Asym BPC: balanced, 250 microseconds, 40Hz, 4:4 on/off ratio Intensity: submotor LIDC: 600 μA 120 minutes/day × 7 days/week × 4 weeks |
2·21 ± 3·27%/day |
Asym BPC: 5·43 ± 4·40%/day LIDC: 3·11 ± 3·83%/day |
WHR significantly faster in Asym BPC versus SWC and LIDC |
Study design: CS, case study or case studies; CT, controlled trial; PCT, prospective controlled trial; RCS, retrospective case series; RCT, randomised controlled trial.
Treatment: Asym BPC, asymmetrical biphasic pulsed current; Sym BPC, symmetrical BPC; HVPC, high voltage pulsed current; LIDC, low intensity direct current; MPC, monophasic pulsed current.
Results: WHR, wound healing rate; WSA, wound surface area.
n/a, Not available.
Table 3.
Summary of the clinical studies not included in the meta‐analysis
| Author, year, country | Study design; treatment; control | Number of patients; incomplete/ complete (I/C); DOI | Number of pressure ulcers; Duration of ulcers (DOU); Stage (NPUAP) | Electrode placement; EST waveform; stimulus parameter; treatment schedule | Results | Study conclusion | |
|---|---|---|---|---|---|---|---|
| Control | ES | ||||||
| Adegoke and Badmos 43, Nigeria |
RCT TRT: MPC CON: Sham MPC |
MPC, n = 3 Sham MPC, n = 3 I/C: n/a DOI: n/a |
MPC, n = 3 Sham MPC, n = 3 DOU: 10 ± 2·0 weeks Stage: IV |
Active electrode applied directly over wound, dispersive applied on intact skin MPC: 30Hz, 2:1 on/off ratio Intensity: submotor 45 minutes/day × 3/week × 4 weeks |
2·6 ± 0·0% ↓ in WSA | 22·2 ± 1·4% ↓ in WSA | Significant ↓ in WSA in EST versus CON |
| Allen and Houghton 47, Canada |
CS TRT: MPC |
MPC, n = 1 I/C: 0/1 DOI: 30 years |
MPC, n = 1 DOU: n/a Stage: III |
Active electrode applied directly over wound, dispersive applied on intact skin MPC: rectangular, 140 microseconds, 128 Hz Intensity: n/a 30 minutes/day × 7 days/week × 12 weeks |
n/a | 19·85%/week | EST facilitates wound healing |
|
Chalker 49, USA |
CS TRT: BPC |
BPC, n = 1 I/C: n/a DOI: n/a |
BPC, n = 1 DOU: 4 weeks Stage: III |
Hand‐held electrode applied directly over wound BPC: not specified Intensity: n/a 60 minutes/day × 7 days/week until healed |
n/a | Ulcer healed | EST facilitates wound healing |
| Cukjati et al. 52, Slovenia |
Retrospective CT TRT1: BPC TRT2: LIDC CON1: Sham LIDC CON2: SWC |
214 patients (71·7% SCI) I/C: n/a DOI: 1–69 months |
BPC, n = 181 LIDC, n = 42 Sham LIDC, n = 23 SWC, n = 54 DOU: 2–22 mos Stage: I–IV |
BPC: Electrodes applied on opposite sides on the wound edge, 250 microseconds, 40 Hz, 4:4 on/off ratio Intensity: motor LIDC: Electrodes applied on opposite sides on the wound edge or active electrode applied over wound, four dispersive electrodes applied on edge around wound, 0·6 mA Intensity: subsensory 120 minutes/day × 7 days/week × 60 weeks |
Sham LIDC: 0·162 mm/day SWC: 0·145 mm/day |
BPC: 0·190 mm/day LIDC: 0·168 mm/day |
WHR significantly faster in BPC versus CON |
| Pollack et al. 48, USA |
CS TRT: MPC |
MPC, n = 1 I/C: n/a DOI: 10 years |
MPC, n = 1 DOU: 23 months Stage: IV |
Electrodes applied over bilateral gluteus, hamstring, and quadriceps muscles MPC: rectangular, 400 microseconds, 60Hz Intensity: n/a 0·37–2·23 minutes × 2/week × 28 weeks |
n/a | Ulcer healed | EST facilitates wound healing |
| Recio et al. 50, USA |
RCS TRT: HVPC |
HVPC, n = 3 I/C: 1/3 DOI: n/a |
HVPC, n = 3 DOU: 11–14 months Stage: III–IV |
Active electrode applied directly over wound, dispersive applied on intact skin HVPC: 10 microseconds, 100 Hz Intensity: submotor 60 minutes/day × 3–5/week until healed |
n/a | 95·58–100% ↓ in WSA | EST facilitates wound healing |
| Trontelj et al. 46, Slovenia |
PCT TRT: Asym BPC CON: SWC |
Asym BPC, n = 63 SWC, n = 43 I/C: n/a DOI: n/a |
Asym BPC, n = 63 SWC, n = 43 DOU: n/a Stage: n/a |
Electrodes applied on opposite sides on the wound edge Asym BPC: balanced, 1·25 milliseconds, 40 Hz, 4:4 on/off ratio Intensity: motor 120 minutes/day × 7 days/week until healed |
2·60 ± 2·59%/day | 4·89 ± 3·80%/day | WHR significantly faster in EST versus CON |
Study design: CS, case study or case studies; CT, controlled trial; PCT, prospective controlled trial; RCS, retrospective case series; RCT, randomised controlled trial.
Treatment: Asym BPC, asymmetrical biphasic pulsed current; Sym BPC, symmetrical BPC; HVPC, high voltage pulsed current; LIDC, low intensity direct current; MPC, monophasic pulsed current.
Results: WHR, wound healing rate; WSA, wound surface area.
EST, electrical stimulation therapy; n/a, not available.
Methodological quality of the controlled studies
The overall methodological quality of the controlled trials was low 33. The scores ranged from 3 to 8 with a mean PEDro score of 5·2 out of a total possible score of 10 (Table 4). Six studies stated that subjects were randomly allocated to groups 38, 39, 40, 41, 42, 43, whereas three did not 44, 45, 46, and two studies collated data from a retrospective analysis Only two studies 40, 43 included a description of how allocation was concealed 51, 52. Blinding of participants, therapists and assessors was performed in only one study 39, and partially in two others 44, 45. None of the studies included an explicit statement of the use of intention‐to‐treat analysis; however, five studies did not report any participant dropout or loss to follow‐up 40, 42, 46, 51, 52.
Table 4.
Methodological quality scores of the clinical controlled trials using the Physiotherapy Evidence Database (PEDro) scale
| Study, author, year | Items in PEDro scale | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Y/N) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score | |
| Adegoke and Badmos 43 | Y | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 5 |
| Baker et al. 38 | N | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Barczak et al. 44 | Y | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Cukjati et al. 52 | Y | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Griffin et al. 39 | Y | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 7 |
| Houghton et al. 40 | Y | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Jerčinović et al. 41 | Y | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 4 |
| Karba et al. 45 | Y | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 4 |
| Karba et al. 42 | N | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Stefanovska et al. 51 | N | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Trontelj et al. 46 | N | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Average score | 5·2 | |||||||||||
1, Eligibility; 2, random allocation; 3, concealed allocation; 4, baseline similarity; 5, blinding of subjects; 6, blinding of therapists; 7, blinding of all assessors; 8, measures of key outcomes from more than 85% of subjects; 9, intention‐to‐treat analysis; 10, between‐group statistical comparison; 11, point measures of variability; Y, yes; N, no; 1, item present; 0, item absent.
In general, the controlled trials had small sample sizes. Baker et al. 38 reported the largest sample; however, several recurrent or multiple ulcers were randomised to different groups.
Characteristics of the studies included in the meta‐analysis
Of the 15 studies, eight had data available to calculate effect sizes and were included in the meta‐analysis. One RCT 43 and one retrospective controlled trial 52 were excluded from the meta‐analysis because the healing outcomes could not be pooled with the other trials. One additional controlled trial 48 was excluded from the meta‐analysis because similar data were reported in previous publications 51, 52. Details of the patients, treatment schedule, EST parameters including electrode placement and waveform and results are presented in Table 2. The meta‐analyses included more than 302 participants; one trial did not report the number of participants involved 51. A total of 360 pressure ulcers were treated with EST, while 237 received either sham EST or SWC. Four studies used BPC 38, 41, 42, 51, three used MPC 39, 40, 44, two used LIDC 45, 51 and only one used MC 38. There was considerable diversity in EST stimulus parameters (i.e. waveform, intensity, polarity), electrode placement (i.e. applied directly over the wound versus across the wound on intact skin) and treatment schedules. Treatment schedules for these studies ranged from 60 to 240 minutes/day, 5–7 days/week, for 2·9–13 weeks or until healed.
Multiple outcome measures were examined between and within the studies. Five studies expressed wound healing as a rate either in percent decrease per day 41, 42, 44, 45, 51 or percent decrease per week 38, while four studies calculated the total number of ulcers healed over the study period 38, 39, 40, 42. Overall percentage change in wound surface area was also expressed in two studies 39, 40.
Daily percent decrease in pressure ulcer size
Two RCTs 41, 42, two prospective controlled trials 44, 45 and one retrospective control trial 51 were used to evaluate the effects of EST on the rate of healing (i.e. daily percent decrease) of pressure ulcers in persons with SCI. A significant large overall effect size was found in favour of EST when examining the daily percent decrease in pressure ulcer size compared with SWC or sham EST (Hedge's g = 1·32, 95% CI: 0·58–2·05, P < 0·001, Figure 2). Significant heterogeneity was evident across these trials (I 2 = 85·3%, P < 0·0001).
Figure 2.
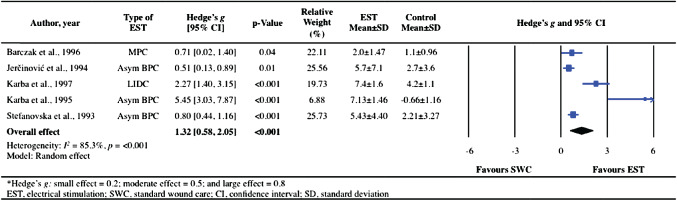
Forest plot illustrating the effect of electrical stimulation therapy (EST) on healing rate expressed as percent per day compared with standard wound care or sham EST.
Two other studies including one retrospective control trial 52 and one prospective control trial 46 evaluated the effect of EST on the rate of healing (Table 3). Cukjati et al. 52 treated 300 wounds with either asymmetric BPC, LIDC, sham or SWC. After 60 weeks of 30–120 minutes/day, 7 days/week, those treated with BPC healed (0·190 mm/day) significantly faster than those treated with LIDC (0·168 mm/day), sham (0·162 mm/day) and conservative therapy (0·145 mm/day). Similarly, Trontelj et al. 46 found a significantly faster healing rate in pressure ulcers treated with asymmetric BPC (4·89 ± 3·80 %/day) compared with SWC (2·60 ± 2·59 %/day) when electrodes were applied on opposite sides of the wound edge. This study was excluded from the meta‐analysis because the data were replicated in later publications 41, 51.
Overall risk of healing
Four RCTs 38, 39, 40, 42 evaluated the effects of EST on pressure ulcer healing (i.e. number of healed events). Healing of a pressure ulcer with EST was found to be 1·55 (95% CI: 1·12–2·15, P = 0·01) times more likely than that with SWC or sham EST (Figure 3). The data were homogeneous (I 2 = 0%, P = 0·47).
Figure 3.
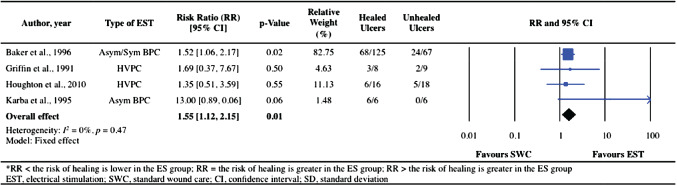
Forest plot illustrating the effect of electrical stimulation therapy (EST) on healed events compared with standard wound care or sham EST.
Other healing outcome measures
Three RCTs 39, 40, 43 expressed wound healing as an overall percent decrease in wound surface area (Tables 2 and 3); however, because of limited available data within the studies, a meta‐analysis could not be conducted. Houghton et al. 40 found that pressure ulcers receiving HVPC with 20‐minute cycles of 100 Hz, 10 Hz and no stimulation for 8 hours/day, 7 days/week for 3 months, had an overall percent decrease in wound surface area of 70 ± 25%, which was statistically higher than that in the SWC group (36 ± 61%). Comparably, Adegoke and Badmos 43 found a noticeable percent decrease in wound surface area in the group provided with ‘interrupted direct current’ (22·2%) compared with the sham group (2·5%). In another RCT by Griffin et al. 39, eight males with SCI received HVPC stimulation at 100 Hz, 500 μA for 60 mintes/day for 20 days, while nine males received sham stimulation. Although, all stage II pressure ulcers healed regardless of treatment type, those receiving EST showed a significant percent decrease in wound surface area of stage III and IV pressure ulcers compared with the sham group. The median percent decrease in wound surface area from pre‐treatment was 80% in the HVPC group and 52% in the sham group.
Secondary outcome measures
Only one 40 of the 15 studies reported adverse events related to EST treatment, which were minor (i.e. skin irritation) and were quickly resolved.
None of the 15 studies reported on or measured the effect of EST in alleviating pain related to the pressure ulcer. Similarly, no studies assessed the impact of treating the pressure ulcer with EST on participants' quality of life.
One case study 47 assessed the economic costs associated with using EST for the treatment of pressure ulcers. Allen and Houghton 47 reported that the total cost to deliver EST in a community‐based programme for 12 weeks was $1477·46 (Canadian dollars).
Compliance with the use of EST was addressed in two 38, 40 of the 15 trials. Houghton et al. 40 indicated that mean duration of EST was reported to be 3·0 ± 1·5 hours/day, which was approximately half of the recommended treatment time. Baker et al. 38 reported that 80% of the subjects were at least ‘semi‐compliant’ (i.e. performed half the recommended treatment time).
Discussion
This systematic review and meta‐analysis suggests that EST is an effective adjunctive therapy for treating pressure ulcers in individuals with SCI. The results of our meta‐analysis have shown that EST decreases pressure ulcer size at a rate of 1·32%/day and increases pressure ulcer closure by 1·55 times more than with SWC alone or sham EST. These results are based on eight controlled clinical trials involving 597 pressure ulcers. The overall PEDro score for the clinical controlled trials was 5·2. This level of methodological quality is considered low.
High heterogeneity across some of the studies included in the meta‐analysis was evident. This can be explained by the lack of standardised method of measuring pressure ulcer size, the considerable variations in type of EST and parameters used (i.e. waveform, frequency, intensity, polarity), the varying methods of delivering EST (i.e. the placement of electrodes) and the different treatment schedules. This was seen in studies with both large 38, 41, 51 and small sample sizes 39, 40, 42, 44, 45. The meta‐analysis also included both randomised and non‐randomised studies, which could have also contributed to the high heterogeneity. As a result of the heterogeneity between these studies, we used a more conservative model known as the random effects model. With this more conservative analysis, EST was still superior to SWC and sham EST in treating pressure ulcers in individuals with SCI.
The lower heterogeneity associated with the studies included in the meta‐analysis of the number of healed ulcers is not surprising given the protocol by Baker et al. 38. They compared four treatment groups: asymmetrical BPC, symmetrical BPC, microcurrent (MC) and sham MC. It should be noted that as the authors of this study 38 originally created MC to be a ‘stimulated control’; we decided to combine the healed events of the MC group with the sham group, and the healed events of the asymmetrical BPC group with the symmetrical BPC group. This is in line with multiple previous studies that have demonstrated that MC has little to no effect in decreasing the healing time of wounds 53, 54, 55. The study by Baker et al. 38 included a notable 192 pressure ulcers, which was a much larger sample size than other studies included in the meta‐analysis. This resulted in the study having a large relative weight within the meta‐analysis. Therefore, any negative effects associated with EST from smaller studies may be negated or masked. It should also be noted that several participants (42·5%) experienced recurrent or multiple pressure ulcers and each of these pressure ulcers was randomised to a particular group. This resulted in data from the same participant being recorded multiple times.
In addition, within this meta‐analysis, two trials 39, 40 reported the same numbers of pressure ulcers healed in both the EST and control groups. This was likely because of a high number of stage II pressure ulcers assigned to the control groups. In both trials, all of the stage II ulcers healed completely, regardless of group assignment, suggesting that SWC alone may be sufficient to heal these ulcers in individuals with SCI. These results are consistent with recent best practice guidelines that recommend the use of EST combined with SWC to manage more severe, stage III and IV, pressure ulcers 2.
The results of this meta‐analysis are comparable to previous meta‐analyses; Gardner et al. 25, Barnes et al. 27 and Koel and Houghton 26 all demonstrated positive effects of EST on wound healing. However, our meta‐analysis is unique in that we focused our findings on the effects of EST on pressure ulcers specifically in the SCI population. The previous meta‐analyses included studies with different wound aetiologies beyond pressure ulcers and different patient populations. We included both randomised and non‐randomised control trials and did not attempt to standardise the outcome measures as performed in the past 25. Our meta‐analysis, which pooled studies expressing the daily percent decrease in pressure ulcer size, included four additional studies (one RCT, one retrospective controlled trial and two prospective controlled trials), which were not included in previous meta‐analyses 27. Barnes et al. 27 included only one RCT in their meta‐analysis that looked at the effects of EST on mean daily percentage change in ulcer size compared with SWC. Pressure ulcers were stratified by location to obtain the treatment effect of EST, which may have concealed the true strength of EST on healing pressure ulcers.
In the meta‐analysis by Koel and Houghton 26, they found that unidirectional current flow was a more promising mode of EST delivery than bidirectional current flow. Baker et al. 38 found similar results showing that asymmetrical BPC is superior to symmetrical BPC. Although, both these currents flow in a bidirectional manner, the asymmetrical current has a net unidirectional flow. When we compared the results of unidirectional and bidirectional EST in these studies involving pressure ulcers and SCI, we actually found greater healing effects with bidirectional EST compared with unidirectional EST. Therefore, healing outcomes are likely dependent on many other variables, such as the different EST protocols in the included studies. Based on the aforementioned findings, the optimal stimulus parameters for EST remain to be determined.
Secondary outcome measures
There were no trials that measured pain relief and/or quality of life as an outcome measure in relation to EST treatment. Adverse events and compliance with EST were reported inconsistently; the majority of studies did not undertake a systematic approach to evaluating or recording adverse events and compliance, as collected in the methodological section. Only one RCT addressed device‐related adverse events, including contact dermatitis associated with self‐adhesive electrodes, while two trials 38, 40 discovered that individuals with SCI were frequently unable to adhere to prescribed EST protocols.
Economic comparisons between SWC and EST were also scarce with only one case study suggesting that EST might be a cost‐effective treatment 47. While there have been other studies 56, 57 reporting similar findings in individuals with SCI with pressure ulcers, these articles did not meet our search criteria. Given the cost‐savings associated with using EST to improve healing rates in pressure ulcers, it is interesting that EST is not being used earlier during management. In Canada, for example, this therapy is either not provided or a last resort following other adjunctive therapies 58, while in the USA, EST is covered only if other adjunctive therapies were unsuccessful.
Methodological quality of the trials
The majority of the trials included in the meta‐analysis had a quality score (out of 10) of 5 or less 38, 41, 42, 44, 45, 51. Lack of description about the method of randomisation and allocation concealment, blinding and intention‐to‐treat analysis was the most common weakness in the trials. However, the low quality 33 across studies was not unexpected as the majority of trials were conducted prior to 2000, before most of the tools to assess methodological quality were developed. Unlike pharmacological trials, blinding of the participants and therapists to a treatment protocol is problematic because it produces visible muscle contractures and/or sensory stimulation. Only two studies used blinded assessor; however, it could be argued that wound size measures and determining that a wound has closed are objective outcomes free of assessor bias.
Study limitations
There are a number of limitations in this review. There were a relatively limited number of studies that met the inclusion criteria and the sample size of pressure ulcers and participants was small in each study. In addition, a wide array of healing outcome measures was used between the studies, which prevented the data from all studies from being pooled into one meta‐analysis.
There was considerable variation in the EST intervention used including stimulus parameters (waveform, intensity, frequency and polarity) and treatment scheduling. This likely contributed to the significant heterogeneity between included studies when estimating the overall rate of pressure ulcer healing. However, from a clinical standpoint, these consistent positive results regardless of specific EST features could also suggest that a variety of EST paradigms, which ultimately deliver similar electrical charge to subcutaneous tissues, can stimulate physiological responses to healing. Furthermore, matching the EST treatment paradigm to the patient's preferences may be a more prudent approach rather than devising a single optimal EST treatment protocol for all to use.
Another limitation was the use of the PEDro scale for assessing methodological quality for both randomised and non‐randomised controlled trials. Although the PEDro was designed specifically for the sake of comparing RCTs, we also used it to evaluate non‐RCTs. In this meta‐analysis, we believed that it was imperative that the same scale be used for accurate comparisons of quality across trials.
Publication bias was also a concern; it is well known that studies with insignificant results are less likely to be published. Unfortunately, we were unable to assess publication bias because of the small number of studies (i.e. <10 studies) 37. We attempted to minimise the bias by searching grey literature and conducting a paired consensus process for study selection and data extraction.
Conclusion
In conclusion, this systematic review found a total of 15 published reports that evaluated the effect of EST on healing of pressure ulcers in people with SCI. Eight controlled clinical trials (n = 274 patients) included in the meta‐analysis found a significant overall effect favouring EST over SWC or sham EST. These results are generalisable to the majority of individuals with SCI as the controlled and uncontrolled studies were conducted in multiple countries and included a wide array of participant characteristics. Unfortunately, because of low methodological quality and high heterogeneity across some of the findings, the findings must be interpreted carefully.
This review also suggests that several types of EST and various treatment schedules can be applied to enhance pressure ulcer healing, allowing regulated health care providers to adapt to different EST programmes depending on a patient's needs. However, conclusions cannot be made regarding the optimal EST treatment protocol for healing pressure ulcers.
Researchers should attempt to investigate the ideal treatment protocol for treating pressure ulcers in individuals with SCI. In addition, future studies should address device‐related adverse events, compliance rates and cost‐effectiveness of EST compared with SWC, and the implications of EST for relieving pressure ulcer pain and improving the overall quality of life.
Acknowledgements
We would like to thank Marisa Surmacz for her assistance with the literature search. One of the authors of this meta‐analysis was a principal investigator of a study included in the meta‐analysis.
References
- 1. Consortium for spinal cord medicine . Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health‐care professionals, 2nd edn. Washington, D.C: Paralyzed Veterans of America, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Houghton PE, Campbell KE, CPG Panel . Canadian best practice guidelines for the prevention and management of pressure ulcers in people with spinal cord injury. A resource handbook for clinicians [Internet]. URL http://onf.org/system/attachments/168/original/Pressure_Ulcers_Best_Practice_Guideline_Final_web4.pdf [accessed 20 February 2015].
- 3. Garber SL, Rintala DH, Hart KA, Fuhrer MJ. Pressure ulcer risk in spinal cord injury: predictors of ulcer status over 3 years. Arch Phys Med Rehabil 2000;81:465–71. [DOI] [PubMed] [Google Scholar]
- 4. Saunders LL, Krause JS, Acuna J. Association of race, socioeconomic status, and health care access with pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 2012;93:972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith BM, Guihan M, LaVela SL, Garber SL. Factors predicting pressure ulcers in veterans with spinal cord injuries. Am J Phys Med Rehabil 2008;87:750–7. [DOI] [PubMed] [Google Scholar]
- 6. Niazi ZB, Salzberg CA, Byrne DW, Viehbeck M. Recurrence of initial pressure ulcer in persons with spinal cord injuries. Adv Wound Care 1997;10:38–42. [PubMed] [Google Scholar]
- 7. Lala D, Dumont FS, Leblond J, Houghton PE, Noreau L. The impact of pressure ulcers on individuals living with a spinal cord injury. Arch Phys Med Rehabil 2014;95:2312–9. [DOI] [PubMed] [Google Scholar]
- 8. Registered Nurses' Association of Ontario . Risk assessment and prevention of pressure ulcers (Revised). Toronto: Registered Nurses' Association of Ontario, 2011. [Google Scholar]
- 9. Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005;4:23–44. [DOI] [PubMed] [Google Scholar]
- 10. Bourguignon GJ, Bourguignon LY. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1987;1:398–402. [DOI] [PubMed] [Google Scholar]
- 11. Bourguignon GJ, Jy W, Bourguignon LY. Electric stimulation of human fibroblasts causes an increase in Ca2+ influx and the exposure of additional insulin receptors. J Cell Physiol 1989;140:379–85. [DOI] [PubMed] [Google Scholar]
- 12. Cho MR, Thatte HS, Lee RC, Golan DE. Integrin‐dependent human macrophage migration induced by oscillatory electrical stimulation. Ann Biomed Eng 2000;28:234–43. [DOI] [PubMed] [Google Scholar]
- 13. Rouabhia M, Park H, Meng S, Derbali H, Zhang Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One 2013;8:e71660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solis LR, Gyawali S, Seres P, Curtis CA, Chong SL, Thompson RB, Mushahwar VK. Effects of intermittent electrical stimulation on superficial pressure, tissue oxygenation, and discomfort levels for the prevention of deep tissue injury. Ann Biomed Eng 2011;39:649–63. [DOI] [PubMed] [Google Scholar]
- 15. Petrofsky J, Hinds CM, Batt J, Prowse M, Suh HJ. The interrelationships between electrical stimulation, the environment surrounding the vascular endothelial cells of the skin, and the role of nitric oxide in mediating the blood flow response to electrical stimulation. Med Sci Monit 2007;13:CR391–7. [PubMed] [Google Scholar]
- 16. Ud‐Din S, Perry D, Giddings P, Colthurst J, Zaman K, Cotton S, Whiteside S, Morris J, Bayat A. Electrical stimulation increases blood flow and haemoglobin levels in acute cutaneous wounds without affecting wound closure time: evidenced by non‐invasive assessment of temporal biopsy wounds in human volunteers. Exp Dermatol 2012;21:758–64. [DOI] [PubMed] [Google Scholar]
- 17. Stromberg BV. Effects of electrical currents on wound contraction. Ann Plast Surg 1988;21:121–3. [DOI] [PubMed] [Google Scholar]
- 18. Brown M, Gogia PP. Effects of high voltage stimulation on cutaneous wound healing in rabbits. Phys Ther 1987;67:662–7. [DOI] [PubMed] [Google Scholar]
- 19. Thawer HA, Houghton PE. Effects of electrical stimulation on the histological properties of wounds in diabetic mice. Wound Repair Regen 2001;9:107–15. [DOI] [PubMed] [Google Scholar]
- 20. Rowley BA, McKenna JM, Chase GR, Wolcott LE. The influence of electrical current on an infecting microorganism in wounds. Ann N Y Acad Sci 1974;238:543–51. [DOI] [PubMed] [Google Scholar]
- 21. Kincaid CB, Lavoie KH. Inhibition of bacterial growth in vitro following stimulation with high voltage, monophasic, pulsed current. Phys Ther 1989;69:651–5. [DOI] [PubMed] [Google Scholar]
- 22. Szuminsky NJ, Albers AC, Unger P, Eddy JG. Effect of narrow, pulsed high voltages on bacterial viability. Phys Ther 1994;74:660–7. [DOI] [PubMed] [Google Scholar]
- 23. Kawasaki L, Mushahwar VK, Ho C, Dukelow SP, Chan LLH, Chan KM. The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: a systematic review. Wound Repair Regen 2014;22:161–73. [DOI] [PubMed] [Google Scholar]
- 24. Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, Aubut J‐AL. A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 2009;90:213–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta‐analysis. Wound Repair Regen 1999;7:495–503. [DOI] [PubMed] [Google Scholar]
- 26. Koel G, Houghton PE. Electrostimulation: current status, strength of evidence guidelines, and meta‐analysis. Adv Wound Care 2014;3:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnes R, Shahin Y, Gohil R, Chetter I. Electrical stimulation vs. standard care for chronic ulcer healing: a systematic review and meta‐analysis of randomised controlled trials. Eur J Clin Invest 2014;44:429–40. [DOI] [PubMed] [Google Scholar]
- 28. Edsberg LE, Wyffels JT, Ogrin R, Craven C, Houghton P. A pilot study evaluating protein abundance in pressure ulcer fluid from people with and without spinal cord injury. J Spinal Cord Med 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Segal JL, Gonzales E, Yousefi S, Jamshidipour L, Brunnemann SR. Circulating levels of IL‐2R, ICAM‐1, and IL‐6 in spinal cord injuries. Arch Phys Med Rehabil 1997;78:44–7. [DOI] [PubMed] [Google Scholar]
- 30. Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 2007;88:1384–93. [DOI] [PubMed] [Google Scholar]
- 31. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235–41. [DOI] [PubMed] [Google Scholar]
- 32. De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009;55:129–33. [DOI] [PubMed] [Google Scholar]
- 33. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–21. [PubMed] [Google Scholar]
- 34. Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Chapter 4: effect sizes based on means. Introduction to meta‐analysis. Chichester: John Wiley & Sons, Ltd, 2009:21–32. [Google Scholar]
- 35. Cohen J. Statistical power analysis for the behavioural sciences (Revised). New York: Academic Press, 1977. [Google Scholar]
- 36. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 38. Baker LL, Rubayi S, Villar F, Demuth SK. Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Repair Regen 1996;4:21–8. [DOI] [PubMed] [Google Scholar]
- 39. Griffin JW, Tooms RE, Mendius RA, Clifft JK, Vander Zwaag R, el‐Zeky F. Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Phys Ther 1991;71:433–42; discussion 442–4. [DOI] [PubMed] [Google Scholar]
- 40. Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter PJ, Woodbury GM. Electrical stimulation therapy increases rate of healing of pressure ulcers in community‐dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010;91:669–78. [DOI] [PubMed] [Google Scholar]
- 41. Jerčinonić A, Karba R, Vodovnic L, Stefanovska A, Kroselj P, Turk R. Low frequency pulsed current and pressure ulcer healing. IEEE Trans Rehabil Eng 1994;2:225–33. [Google Scholar]
- 42. Karba R, Benko H, Savrin R, Vodovnik L. Combination of occlusive dressings and electrical stimulation in pressure ulcer treatment. Med Sci Res 1995;23:671–3. [Google Scholar]
- 43. Adegoke BO, Badmos KA. Acceleration of pressure ulcer healing in spinal cord injured patients using interrupted direct current. Afr J Med Med Sci 2001;30:195–7. [PubMed] [Google Scholar]
- 44. Barczak M, Kluger P, Kluger J, Bäuerle J, Puhl W. Therapeutic effectiveness of electric stimulation in paraplegic patients with pressure sores. PhD thesis of M. Barczak submitted to Prof. Dr. W. Puhl at the Medical School of the University of Ulm, Germany, 2001.
- 45. Karba R, Semrov D, Vodovnik L, Benko H, Savrin R. DC electrical stimulation for chronic wound healing enhancement. Part 1. Clinical study and determination of electrical field distribution in the numerical wound model. Bioelectrochem Bioenerg 1997;43:265–70. [Google Scholar]
- 46. Trontelj K, Karaba R, Vodovnik L, Savrin R, Strukelj MP. Treatment of chronic wounds by low frequency pulsed electrical current. J Tissue Viability 1994;4:105–9. [Google Scholar]
- 47. Allen J, Houghton PE. A case study for electrical stimulation on a stage III pressure ulcer. Wound Care Canada 2004;2:34–6. [Google Scholar]
- 48. Pollack SF, Ragnarsson KT, Dijkers M, Djikers M. The effect of electrically induced lower extremity ergometry on an ischial pressure ulcer: a case study. J Spinal Cord Med 2004;27:143–7. [DOI] [PubMed] [Google Scholar]
- 49. Chalker M. Healing of decubitus ulcers of patients in neuro‐kinesthetic program with the Electro‐Acuscope 80. Crit Care Update 1983;10:50–2. [PubMed] [Google Scholar]
- 50. Recio AC, Felter CE, Schneider AC, McDonald JW. High‐voltage electrical stimulation for the management of stage III and IV pressure ulcers among adults with spinal cord injury: demonstration of its utility for recalcitrant wounds below the level of injury. J Spinal Cord Med 2012;35:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stefanovska A, Vodovnik L, Benko H, Turk R. Treatment of chronic wounds by means of electric and electromagnetic fields. Part 2. Value of FES parameters for pressure sore treatment. Med Biol Eng Comput 1993;31:213–20. [DOI] [PubMed] [Google Scholar]
- 52. Cukjati D, Robnik‐Sikonja M, Rebersek S, Kononenko I, Miklavcic D. Prognostic factors in the prediction of chronic wound healing by electrical stimulation. Med Biol Eng Comput 2001;39:542–50. [DOI] [PubMed] [Google Scholar]
- 53. Malin EW, Galin CM, Lairet KF, Huzar TF, Williams JF, Renz EM, Wolf SE, Cancio LC. Silver‐coated nylon dressing plus active DC microcurrent for healing of autogenous skin donor sites. Ann Plast Surg 2013;71:481–4. [DOI] [PubMed] [Google Scholar]
- 54. Ullah M. A study to detect the efficacy of micro‐current electrical therapy on decubitus wound. J Med Sci 2007;7:1320–4. [Google Scholar]
- 55. Baker LL, Chambers R, DeMuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 1997;20:405–12. [DOI] [PubMed] [Google Scholar]
- 56. Mittmann N, Chan BC, Craven BC, Isogai PK, Houghton PE. Evaluation of the cost‐effectiveness of electrical stimulation therapy for pressure ulcers in spinal cord injury. Arch Phys Med Rehabil 2011;92:866–72. [DOI] [PubMed] [Google Scholar]
- 57. Chan BC, Nanwa N, Mittmann N, Bryant D, Coyte PC, Houghton PE. The average cost of pressure ulcer management in a community dwelling spinal cord injury population. Int Wound J 2013;10:431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Craven BC, Verrier M, Balioussis C, Wolfe DL, Hsieh JT, Noonan VK, Rasheed A, Cherban E. Rehabilitation environmental scan atlas: capturing capacity in Canadian SCI rehabilitation. Rick Hansen Institute: Vancouver, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


