Abstract
Patients who are stationary endure prolonged soft tissue distortions and deformations at contact areas between their body and the support surface, which may lead to the onset of pressure ulcers (PUs) over time. A novel technology for patient positioning employs innovation in materials science, specifically viscoelastic materials with shape memory properties that compose the Z‐Flo™ head positioner (Mölnlycke Health Care, Gothenburg, Sweden). Head positioners are generally known to reduce the occurrence of PUs in scalp tissues and the ears, but quantitative assessments of their biomechanical efficacy are missing in the literature. To determine potential differences in mechanical loads formed in the soft tissues of the back of the head while in contact with 2 head positioner types, Z‐Flo vs flat medical foam, we developed 2 comparable finite element model configurations, both including the same 3‐dimensional adult head. For both model variants, stresses in skin and fat peaked at the occiput. The skin at the back of the resting head is subjected to greater stress values with respect to fat; however, the Z‐Flo positioner reduced the exposure of both skin and fat tissues to elevated stresses considerably (by a factor of 3) compared to the medical foam support. We found the Z‐Flo device effective in reducing tissue loads at the surface of the head as well as internally in scalp tissues, with a particular strength in reducing internal tissue shear. The Z‐Flo device achieves this protective quality through highly effective immersion and envelopment of the back of the head, generated in the process of manual moulding of the device in preparation for use. Additional protection is achieved through the viscoelastic response of the filling material of this positioner, which relaxes promptly and considerably under the weight of the head (by more than 2‐fold within approximately 1 s) as opposed to the elastic recoil of the foam that pushes back on scalp tissues.
Keywords: deep tissue injury, finite element modelling, repositioning, soft tissues, support surfaces
1. INTRODUCTION
Pressure ulcers (PUs) are localised injuries resulting primarily from sustained soft tissue deformations.1 Patients who are stationary, whether paralysed or under anaesthesia, endure prolonged pressures and shear loads at contact areas between their body and the support surface and sustained deformations in their weight‐bearing soft tissues, which leads to the onset of PUs (also called pressure injuries) over time.2, 3 The most common injury sites are the back of the head and ears, the shoulders, the elbows, the lower back and buttocks, the hips, the inner knees and the heels.4
As PUs may have devastating consequences on health status and body image and are difficult and costly to treat, efforts are focused on developing effective PU prevention (PUP) strategies.5 Current clinical practice guidelines outline several measures that should be taken for PUP, including risk assessment; routine skin inspections; regular repositioning; appropriate use of adequate support surfaces; incontinence management; education of patients, families and staff members; topical skin protection; and nutritional interventions.1 Repositioning is defined as the movement of patients from 1 position to another in an effort to alleviate or redistribute any pressure exerted on the body tissues.6 A typical question in this regard is how often 1 needs to reposition patients on a certain support surface. This question becomes more complicated in operation rooms (ORs) because it is difficult, dangerous and sometimes impossible to reposition during surgery, and various limitations may exist with respect to body posture and the ability to move patients.7, 8 Common positioning techniques that nursing staff are employing often include the use of items that are not specifically designed for the purpose of therapeutic positioning, such as rolled towels and blankets that flatten and will not maintain a set position and may also heat up.8 Head positioning is required for maintaining midline and/or the chin upwards, for access to the airways in the OR or for tracheal care in intensive care units (ICU), as well as for distribution of bodyweight loads under the head.
A novel technology for head positioning, including in ORs and ICUs, employs innovation in materials science, specifically viscoelastic materials with shape memory properties, which compose the Mölnlycke Z‐Flo™ Fluidized Positioner (Mölnlycke Health Care, Gothenburg, Sweden). The nursing team shapes each positioner manually, moulding it into the desired position for each patient according to their individual needs in the OR, ICU or elsewhere. The positioner can be folded away from the ears and can also be moulded away from existing PUs if needed. The Z‐Flo device will retain its shape, and hence, the patient will maintain their position even over a long surgery. When repositioning is required, the nurse can simply remould the positioner into the new chosen shape needed to support the patient in his or her altered position, and there will be no elastic response that promotes return of the positioner to its previous shape. The positioner can be used to either redistribute the bodyweight forces over a greater surface area, through immersion and envelopment, or help offloading bony prominences and the ears while maintaining a neutral body alignment. Based on clinical experience, positioning devices that adapt or adjust to the contours of the body are generally known to reduce the occurrence of PUs,9 but a quantitative assessment of the specific effectiveness of the Z‐Flo device in alleviating internal and surface tissue loads is missing in the literature. Finite element (FE) modelling is a powerful bioengineering research method that facilitates evaluation of internal as well as surface (skin) tissue loads and further allows the isolation of the influence of biomechanical characteristics from other potential risk factors, for example, impaired circulation or tissue repair capacities. Here, we present a novel FE model of a realistic adult head, rested on the Z‐Flo head positioner vs on a standard medical foam, to determine the relative protective efficacy of the new positioner technology in protecting the head from PUs.
2. METHODS
To determine potential differences in mechanical loads formed in the soft tissues of the back of the head while in contact with the 2 head positioner types, Z‐Flo vs foam, we developed 2 comparable FE model configurations, both including the same adult head (Figures 1 and 2). That head model was built using the visible human (male) project® image database.10 Tissues in each transverse slice of the head were segmented and then unified to create a 3‐dimensional (3D) computational model using the Scan‐IP module of the Simpleware® segmentation software package.11 Although the anatomical details of the brain, sinuses, optic nerves and other soft tissue structures that are contained within the skull have been included in the head modelling for completeness, they do not influence scalp tissue loads other than applying gravitational (tissue weight) forces, and hence, we do not provide information regarding their mechanical behaviour hereafter. The dimensions of the head were 16.5 cm ear to ear and 21.5 cm occiput to forehead, and its weight was 5 kg, which is representative of an adult male head.12
Figure 1.
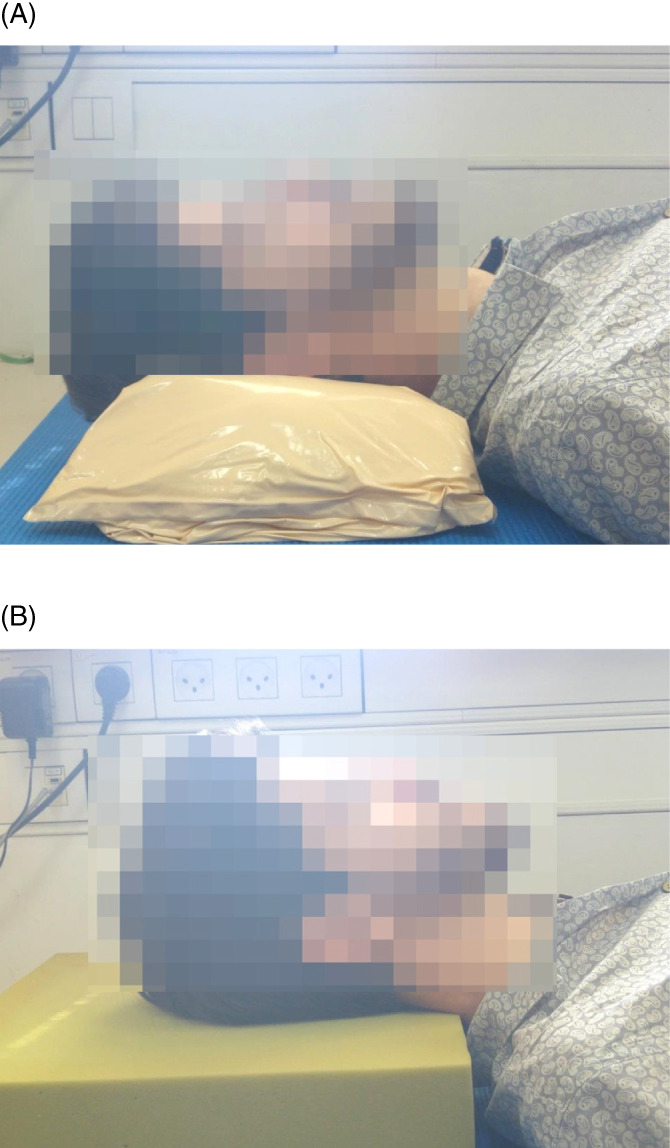
Real‐life head rest positions using the (a) Z‐Flo head positioner (Mölnlycke health care) and (b) a standard medical foam support
Figure 2.
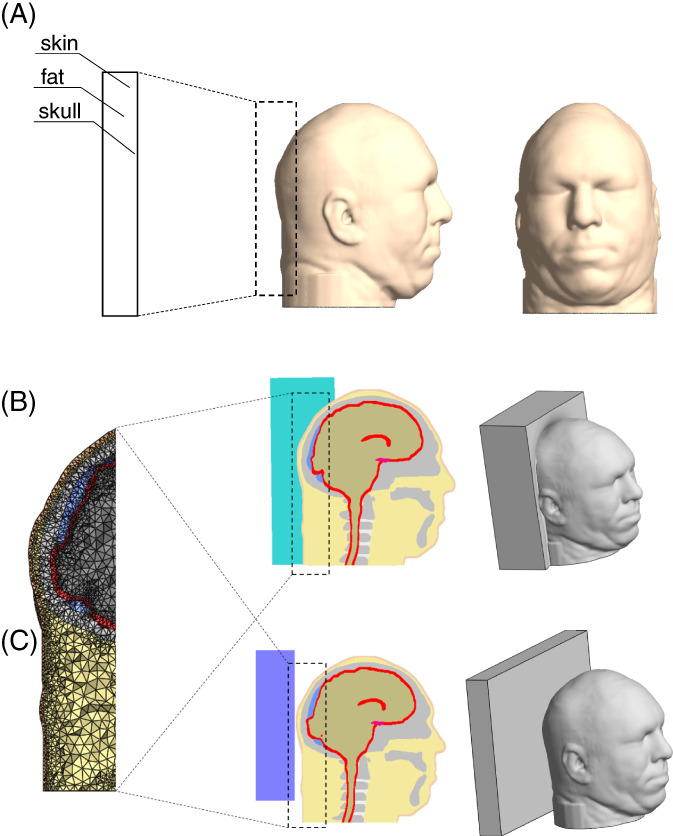
Model geometry: (a) the head model in frontal (right frame) and side (middle frame) views and magnification of tissues at the back of the head (left frame); (b) the head and Z‐Flo head positioner model: three‐dimensional (3D) view (right frame), midsagittal view (middle frame) and a magnification of the finite element mesh (left frame); (c) the head and medical foam support (reference) model: 3D view (right frame), midsagittal view (middle frame) and a magnification of the mesh (left frame)
In the reference model variant, the head was resting on a flat, thick (4 cm) medical foam support. In the other model variant, the head was resting on the Z‐Flo head positioner after simulating manual moulding to conform to the shape of the back of the head, as in clinical practice according to product guidelines. The reference (foam support) model geometry was built using Preview, the FEBio pre‐processing software, whereas the Z‐Flo head positioner model was created using the Scan‐IP module of Simpleware due to its greater geometrical complexity.
The mechanical properties of the relevant tissues of the head—the skin and fat of the scalp and bone tissue of the skull—were adopted from the literature. Specifically, both skin and fat were represented using the Mooney‐Rivlin constitutive model:
| (1) |
where I 1 and I 2 are the first and second invariants of the right Cauchy‐Green deformation tensor, respectively, and J is the determinant of the deformation gradient tensor. The relevant mechanical properties of scalp tissues are specified in Table 1, separately for the skin and fat components. The bone tissue of the skull was assumed to be isotropic and linear elastic. An elastic modulus of 63 kPa and Poisson's ratio of 0.3 were assigned to the reference (medical foam) positioner.18
Table 1.
Mechanical properties and element data of the finite element model variants
In order to obtain the viscoelastic mechanical properties of the Z‐Flo filling, 3 different laboratory experiments were conducted: (1) the material was studied with an unconfined compression test at a crosshead speed of 5 mm/minute in order to obtain the hyperelastic instantaneous behaviour; (2) the material was also tested with a confined compression test for obtaining the bulk modulus; and (3) finally, the material was tested with a stress relaxation, ramp‐and‐hold indentation test (3.6‐s ramp phase at 1 mm/s and 1.5‐s ramp phase at 3 mm/s and then hold for 60 s) to obtain the temporal viscoelastic characteristics. The aforementioned unconfined compression and stress relaxation tests have been conducted using an electromechanical testing machine that is specific for testing medical materials (Instron model 3345; Instron Co., City, High Wycombe, United Kingdom). After repeating each of the above experiment types six times, we fitted a stress‐strain curve to the instantaneous compressive response using ABAQUS (Dassault Systems, Boston, MA) to assign the material parameters of the strain energy density (SED) function. Consistent parameters for the instantaneous response (stress‐strain curve) of the Z‐Flo filling material were obtained when using the Ogden SED function:
| (2) |
where λ1,λ2,λ3 are the principal stretch ratios, and μp and αp are material constants. We then analysed the stress relaxation test data using Matlab (MathWorks, Natick, MA) to obtain the time constants and other material parameters of the Prony series, which describe how the shear stiffness of the Z‐Flo filling material decays over time when statically loaded with a constant (head) weight:
| (3) |
where Gi, τi, are tissue‐specific material constants, specified in Table 2.
Table 2.
Mechanical properties of the Z‐Flo head positioner
| μi (MPa) | αi | Gi (MPa) | τi [s] | K (MPa) |
|---|---|---|---|---|
| μ 1 = −6.321 | α1 = 2.000 | G1 = 0.15080 | τ1 = 1.038 | 0.600 |
| μ 2 = 4.047 | α2 = 4.000 | G2 = 0.02287 | τ2 = 0.720 | |
| μ 3 = 2.285 | α3 = −2.000 | G3 = −0.17130 | τ3 = 1.00 | |
| Gl = 0.00177 |
The boundary conditions in the FE modelling were chosen to simulate weight bearing of the head on the positioners during supine lying as occurs, for example, in the OR. The inferior surfaces of both the foam and Z‐Flo head positioner were fixed for all translations and rotations (Figure 2). We applied displacement of the head towards the positioner, which resulted in a reaction force of 4 kg for both model variants.
The aforementioned model geometries, excluding the foam support, were meshed using the Scan‐IP module of Simpleware. The foam support was meshed using the Preview module of FEBio, (version 1.18, University of Utah, Salt Lake City, Utah)20, 21 which was also used for setting up all the FE simulations. All elements were of the tetrahedral type; numbers of elements in each model component are specified in Table 1. The simulations were solved using the Pardiso FE solver (version 2.5) and post‐processed using PostView (version 1.9.1) which are both FEBio modules. The runtimes of the simulations were 45 and 16 h for the foam support and Z‐Flo head positioners, respectively, using a 64‐bit Windows 7‐based workstation with a CPU comprising Intel Xeon E5645 2.40 GHz (2 processors) and 64 GB RAM.
As outcome measures that evaluate tissue exposure to mechanical loads, we determined the effective and shear (Cauchy) stresses in skin and fat tissues separately for each tissue type. The values and volumetric distributions of intensities of these outcome measures within the scalp tissues were then quantitatively compared between the medical foam support and Z‐Flo head positioner.
3. RESULTS
A comparison of the distributions of effective and maximal shear stresses that develop in the soft tissues at the back of the head on the Z‐Flo head positioner vs the medical foam support are shown in Figures 3, 4, 5. For both model variants, stresses in skin and fat peaked at the occiput (Figure 3). The average and peak effective stresses at the soft tissues covering the occipital region when using the Z‐Flo vs the medical foam are detailed in Table 3. The skin at the back of the resting head is subjected to greater stress values with respect to fat; however, the Z‐Flo positioner reduced the exposure of both skin and fat tissues to elevated stresses considerably, compared to the medical foam support (Table 3, Figures 3, 4, 5). It is further shown that across all stress levels, the Z‐Flo head positioner substantially decreased the volumetric exposure of skin tissue to stresses with respect to the extent of decrease achieved by means of the medical foam (Figure 6). For fat tissue, the Z‐Flo has been shown to be mostly effective in reducing the volumetric exposure to stresses at the high stress domain.
Figure 3.
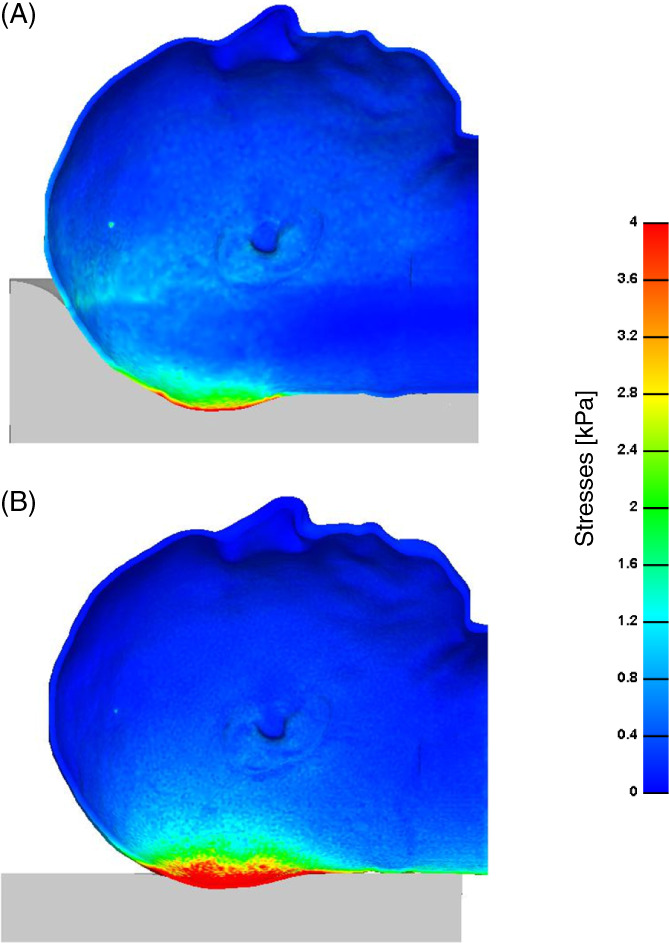
Effective stresses on the skin through a cross‐sectional view: The model variants of the (a) head rested on the Z‐Flo head positioner and (b) head rested on the medical foam support (reference model)
Figure 4.
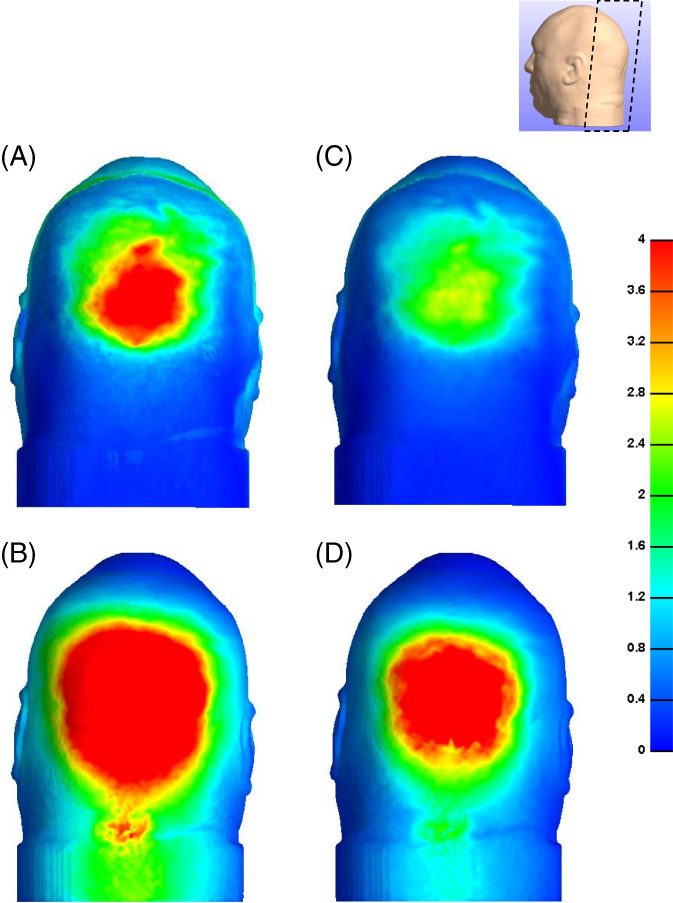
Effective (left column) and shear (right column) stresses on the skin: (a, c) head rested on the Z‐Flo head positioner and (b, d) head rested on the medical foam support (reference model)
Figure 5.
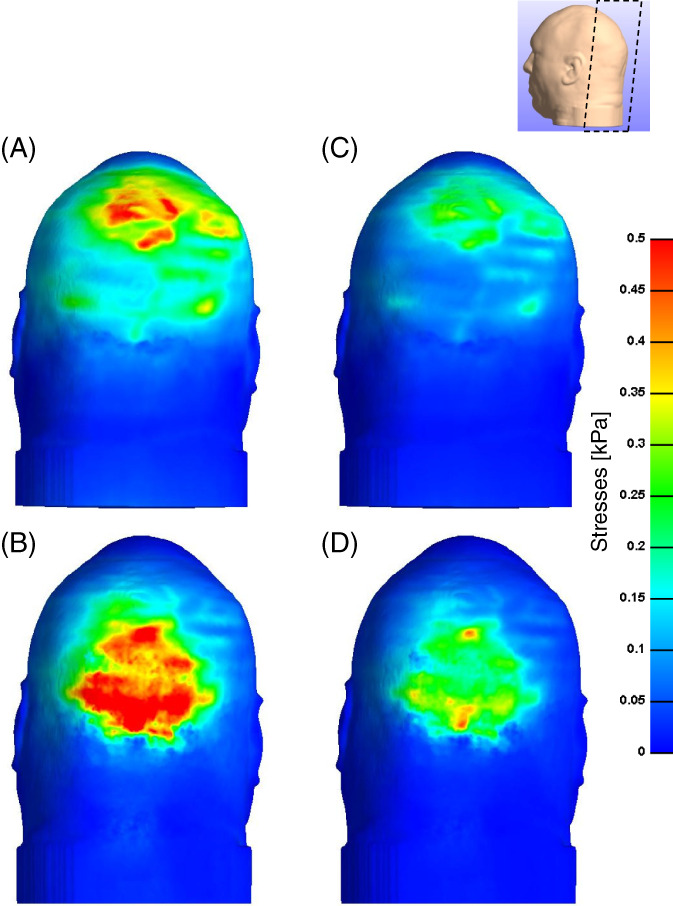
Effective (left column) and shear (right column) stresses in the subcutaneous fat: (a, c) head rested on the Z‐Flo head positioner and (b, d) head rested on the medical foam support
Table 3.
Outcome measures of the finite element simulations
| Head support | Average effective stress [kPa] | Peak effective stress [kPa] | ||
|---|---|---|---|---|
| Skin | Fat | Skin | Fat | |
| Z‐Flo head positioner | 231. | .0880 | 6.3 | 0.63 |
| Medical foam | 344. | 0.26 | 17.9 | 1.49 |
Figure 6.
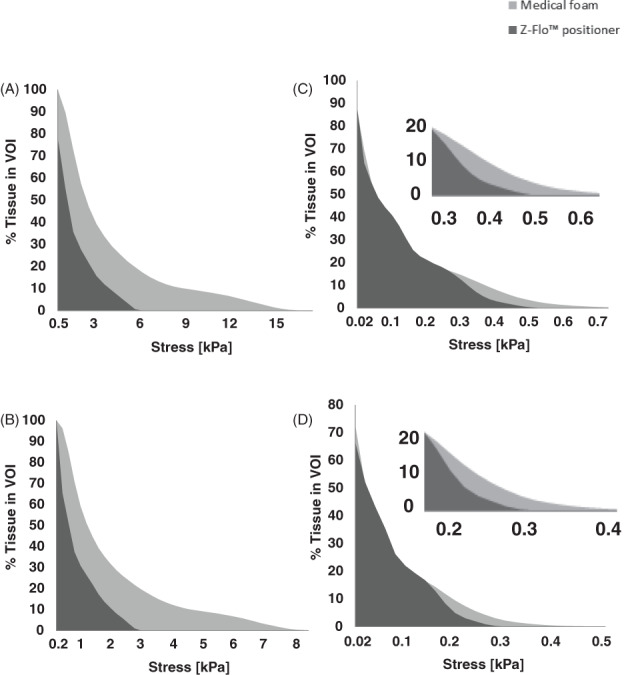
Cumulative percentage of skin (left column) and subcutaneous fat (right column) exposures to (a, c) effective and (b, d) shear stress levels when the head is rested on the Z‐Flo head positioner vs on a medical foam support
4. DISCUSSION
The Z‐Flo head positioner has been designed to distribute the mechanical loads applied by the weight of the head while maintaining adequate head and neck alignment. This device distributes and alleviates scalp tissue loads and reduces stress concentrations in tissues, thus lowering the risk of developing occipital PUs, for example, in ORs. The positioner can be easily shaped to a desired position for a given patient and medical condition, and if repositioning is further required, it can be promptly remoulded into a different shape as needed.
In the present study, we compared the state of mechanical loads in scalp tissues enveloping the occipital region of the skull when using the Z‐Flo head positioner vs a standard medical foam support for an adult patient lying supine. We used computational FE modelling, a well‐established scientific approach for comparing biomechanical efficacies of medical devices and surgical techniques in the wound care arena,18, 22, 23, 24 particularly with regards to distributions of mechanical loads in tissues, which cannot be measured directly. Our data demonstrated that the Z‐Flo positioner indeed alleviates tissue stresses, on the skin as well as internally and subcutaneously, and to a considerably greater extent compared to flat medical foam. The Z‐Flo specifically reduces shear stresses on the surface of the head as well as in soft tissues internally (Figures 4, 5, 6), which by itself provides important protective value in PUP.
There are 2 distinct physical principles by which the Z‐Flo device achieves its efficacy in reducing tissue loads and stress concentrations. The first is that even in real‐life conditions, in clinical practice, the Z‐Flo is able to provide an (nearly) ideal immersion and envelopment through the process of manually moulding the device in preparation for positioning. The resulting 3D surface matching between the head and the Z‐Flo, which utilises the memory shape feature of the filling material in the device, facilitates maximal contact area between the head and the positioner, which in turn minimises tissue loads, particularly in shear. Adequate immersion and envelopment is the most fundamental feature of a good support surface as demonstrated in our many published studies of wheelchair cushions.24, 25 The second physical principle that contributes to the protective efficacy of the Z‐Flo device is the strong viscoelastic response of its filling material, which relaxes by more than 2‐fold within approximately 1 s (as per our experimental data reported in Table 2). This is in contrast to the foam material, which is elastic in nature and hence pushes back on the scalp tissues when subjected to the weight of the head. The good envelopment of the structure of the Z‐Flo positioner and the high viscoelasticity of its filling material act synergistically to minimise tissue distortion and loads, which results in the efficacy of the Z‐Flo positioner in PUP.
Traditional head positioners that are commonly used for PUP to protect the occipital region include off‐loading foam rings (or foam wedges) and donut‐shaped gel‐made positioners. Foam structures are known to lose stiffness and shape over time, and gel donut‐shaped positioners induce shear stress concentrations in skin and sub‐dermal tissues at the perimeters of the off‐loaded occiput,19 similarly to off‐loading wheelchair cushions25 or toilet seats.26 The strengths of the Z‐Flo positioner are in providing nearly ideal envelopment, which is superior to off‐loading in terms of exposure to internal tissue loads,25, 26 and in its dominant viscoelastic shape memory material filling properties that are durable.
As with any modelling work, there are inherent limitations here that are associated primarily with the assumptions and the omissions made. First, the mechanical properties of the tissues are adopted from animal studies because of a lack of specific literature describing human scalp tissue properties under sustained, quasi‐static loading conditions as relevant to PU research. Furthermore, the head anatomy and the biomechanical properties of tissues represent healthy conditions and do not account for pathologies such as existing wounds, scars, abnormally fragile skin, which is characteristic of an end‐of‐life stage, or to inherited diseases such as epidermolysis bullosa. It is also worthwhile noting that the present study cannot be directly extrapolated to paediatrics because neonate or toddler heads are not simply miniature adult heads with respect to both the anatomy of the head and the mechanical properties of the relevant tissues. In other words, additional modelling work is needed to investigate the issue of head positioning in pre‐term infants, neonates and toddlers.
In conclusion, we have developed a novel modelling framework for evaluating head positioners for use in ORs and in other relevant medical facilities, focusing here on supine‐lying adult patients. We have utilised our modelling to evaluate the new Z‐Flo head positioner technology that offers shape memory and a strong viscoelastic filling material behaviour with respect to a flat medical foam support (as a reference case as the analysis of efficacy in PUP is, by definition, a relative one). We found the Z‐Flo device effective in reducing tissue loads at the surface of the head as well as internally in scalp tissues, with a particular strength in reducing internal tissue shear. The Z‐Flo device achieves this protective quality through highly effective immersion and envelopment of the back of the head, generated—thanks to the large contact surface area between the back of the head and the Z‐Flo device—through the process of manual moulding of the device in preparation for use. Additional protection is achieved through the viscoelastic response of the filling material of this positioner, which relaxes promptly and considerably under the weight of the head, as opposed to the elastic recoil of the foam that pushes back on the scalp tissues. Adequate immersion and envelopment are well‐established characteristics of good support surfaces, and their importance in minimising the exposure to tissue loads and hence in PUP has been demonstrated repeatedly in the literature with respect to wheelchair cushions. As this current work demonstrates, the same physical principles explained above apply to head support/positioning technology as well.
ACKNOWLEDGEMENTS
We thank Ms. Natalie Benayahu for her technical contribution to the characterization of material properties reported in this work.
Katzengold R, Gefen A. What makes a good head positioner for preventing occipital pressure ulcers. Int Wound J. 2018;15:243–249. 10.1111/iwj.12857
Funding information Mölnlycke Health Care, Gothenburg, Sweden, Grant/Award number: The study was supported by an unrestricted educational grant from Mölnlycke Health Care
REFERENCES
- 1. European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Ulcer Advisory Panel (NPUAP) and the Pan‐Pacific Alliance (PPA) International Guidelines ‘Aetiology’ chapter; 2014.
- 2. Gefen A. The biomechanics of sitting‐acquired pressure ulcers in patients with spinal cord injury or lesions. Int Wound J. 2007;4(3):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manorama A, Meyer R, Wiseman R, Bush TR. Quantifying the effects of external shear loads on arterial and venous blood flow: implications for pressure ulcer development. Clin Biomech (Bristol, Avon). 2013;28(5):574–578. [DOI] [PubMed] [Google Scholar]
- 4. Giuglea C, Marinescu S, Florescu IP, Jecan C. Pressure sores‐a constant problem for plegic patients and a permanent challenge for plastic surgery. J Med Life. 2010;3(2):149–153. [PMC free article] [PubMed] [Google Scholar]
- 5. Lyder CH, Ayello EA. Chapter 12: Pressure ulcers: a patient safety issue. In: Hughes RG, ed. Patient Safety and Quality: An Evidence‐Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 6. Gillespie BM, Chaboyer WP, McInnes E, Kent B, Whitty JA. Thalib L. Cochrane Database Syst Rev. 2014;4:CD009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spruce L, Van Wicklin SA. Back to basics: positioning the patient. AORN J. 2014. Sep;100(3):298–305. [DOI] [PubMed] [Google Scholar]
- 8. Waters T, Short M, Lloyd J, et al. AORN ergonomic tool 2: positioning and repositioning the supine patient on the OR bed. AORN J. 2011. Apr;93(4):445–449. [DOI] [PubMed] [Google Scholar]
- 9. Brennan MR, Laconti D, Gilchrist R. Using conformational positioning to reduce hospital‐acquired pressure ulcers. J Nurs Care Qual. Apr‐Jun 2014;29(2):182–187. [DOI] [PubMed] [Google Scholar]
- 10. Visible Human Project Gallery , U.S. National Library of Medicine.
- 11. Simpleware® Ltd . ScanIP, +FE, +NURBS and +CAD Reference Guide ver. 5.1; 2012. http://www.simpleware.com/software/
- 12. Goldstein JP. The effect of motorcycle helmet use on the probability of fatality and the severity of head and neck injuries a latent variable framework. Eval Rev. 1986;10(3):355–375. [Google Scholar]
- 13. Linder‐Ganz E, Shabahin N, Itzchak Y, Gefen A. Assessment of mechanical conditions in sub‐dermal tissues during sitting: a combined experimental‐MRI and finite element approach. J Biomech. 2007;40(7):1443–1454. [DOI] [PubMed] [Google Scholar]
- 14. Sopher R, Nixon J, Gorecki C, Gefen A. Exposure to internal muscle tissue loads under the ischial tuberosities during sitting is elevated at abnormally high or low body mass indices. J Biomech. 2010;43(2):280e6. [DOI] [PubMed] [Google Scholar]
- 15. Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng. 2007;129(6):924–930. [DOI] [PubMed] [Google Scholar]
- 16. Moore DF, Jérusalem A, Nyein M, Noels L, Jaffee MS, Radovitzky RA. Computational biology‐ modeling of primary blast effects on the central nervous system. Neuroimage. 2009. Aug;47(Suppl 2):T10–T20. [DOI] [PubMed] [Google Scholar]
- 17. Greaves CY, Gadala M, Oxland TR. A three‐dimensional finite element model of the cervical spine with spinal cord: an investigation of three injury mechanisms. Ann Biomed Eng. 2008;36(3):396–405. [DOI] [PubMed] [Google Scholar]
- 18. Levy A, Frank MB, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability. 2015;24(1):1–11. [DOI] [PubMed] [Google Scholar]
- 19. Levy A, Kopplin K, Gefen A. Device‐related pressure ulcers from a biomechanical perspective. J Tissue Viability. 2017;26(1):57–68. [DOI] [PubMed] [Google Scholar]
- 20. Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: finite elements for biomechanics. J Biomech Eng. 2012;134(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. FEBio , Finite element for biomechanics, theory manual ver. 1.5; 2012. http://mrl.sci.utah.edu/software/febio.
- 22. Levy A, Gefen A. Computer modeling studies to assess whether a prophylactic dressing reduces the risk for deep tissue injury in the heels of supine patients with diabetes. Ostomy Wound Manage. 2016;62(4):42–52. [PubMed] [Google Scholar]
- 23. Katzengold R, Topaz M, Gefen A. Tissue loads applied by a novel medical device for closing large wounds. J Tissue Viability. 2016;25(1):32–40. [DOI] [PubMed] [Google Scholar]
- 24. Levy A, Kopplin K, Gefen AA. Computer modeling study to evaluate the potential effect of air cell‐based cushions on the tissues of bariatric and diabetic patients. Ostomy Wound Manage. 2016. Jan;62(1):22–30. [PubMed] [Google Scholar]
- 25. Peko Cohen L, Gefen A. Deep tissue loads in the seated buttocks on an off‐loading wheelchair cushion versus air‐cell‐based and foam cushions: Finite element studies. Int Wound J. 2017; in press (available online), 10.1111/iwj.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lustig M, Levy A, Kopplin K, Ovadia‐Blechman Z, Gefen A. Beware of the toilet: The risk for a deep tissue injury during toilet sitting. J Tissue Viability. 2017; in press (available online), 10.1016/j.jtv.2017.04.005. [DOI] [PubMed] [Google Scholar]


