Abstract
Combat injuries are associated with a high incidence of infection, and there is a continuing need for improved approaches to control infection and promote wound healing. Due to the possible local and systemic adverse effects of standard 1% cream formulation (Silvadene), we had previously developed a polyethylene glycol (PEGylated) fibrin hydrogel (FPEG)‐based wound dressing for the controlled delivery of silver sulfadiazine (SSD) entrapped in chitosan microspheres (CSM). In this study, we have evaluated the antimicrobial and wound healing efficacy of SSD‐CSM‐FPEG using a full‐thickness porcine wound infected with Pseudomonas aeruginosa. Infected wounds treated with a one‐time application of the SSD‐CSM‐FPEG wound dressing demonstrated significantly reduced bacterial bioburden over time (99·99% of reduction by day 11; P < 0·05) compared with all the other treatment groups. The epithelial thickness and granulation of the wound bed was significantly better on day 7 (150·9 ± 13·12 µm), when compared with other treatment groups. Overall, our findings demonstrate that the SSD‐CSM‐FPEG wound dressing effectively controls P. aeruginosa infection and promotes wound healing by providing a favourable environment that induces neovascularisation. Collectively, sustained release of SSD using fibrin hydrogel exhibited enhanced benefits when compared with the currently available SSD treatment, and this may have significant implications in the bacterial reduction of infected wounds in military and civilian populations.
Keywords: Chitosan microspheres, PEGylated fibrin hydrogel, Porcine infection wound model, Pseudomonas aeruginosa, Silver sulfadiazine
Introduction
One of the most frequent problems in combat‐related traumatic injury is infection, which requires immediate treatment to reduce the possibility of further complications 1, 2, 3. Despite control of bacterial contamination using improved materials, implants, clean room techniques and chemoprophylaxis, infection still remains a major problem in combat‐related skin wounds 4. Specifically, the pattern and prevalence of combat‐related injuries and the rate of infection have remained fairly consistent (∼30%) 4, 5, 6. Combat wounds are contaminated with Gram‐positive and/or Gram‐negative bacteria acquired either immediately after injury or through nosocomial transmission 7, 8. As a result, there is a prolonged inflammatory phase and production of a poor provisional matrix causing reduced vascularisation and matrix remodelling, resulting in delayed wound healing. One of the most aggressive and challenging microorganisms isolated from combat wounds is Pseudomonas aeruginosa 8. Once the bacteria starts secreting its extracellular polysaccharide matrix (EPS), it has the ability to establish a biofilm, which is protected within a glycocalyx. Through quorum sensing, these organisms can communicate with the residing microbial community and can regulate their phenotypes accordingly in response to their environment stimuli 9, 10. Therefore, a novel treatment regimen is essential to combat these organisms to not only avoid formation of biofilms but treat existing infections.
Use of conventional dry wound dressings often results in progressive dehydration followed by devitalisation and necrosis, providing a less optimal environment for healing; however, moist dressings that can stimulate healing may help reduce the incidence of wound infection. Various agents have been developed and promoted to control bacterial infection, and topical silver sulfadiazine (SSD) has been routinely used both clinically and experimentally 11, 12. It possesses a broad spectrum of activity against Gram‐positive and Gram‐negative bacteria, as well as fungal infections 13, 14, 15. SSD activity works by binding to the thiol groups found within many enzymes and disrupting their activity by forming new disulfide bonds within the secondary structure of the proteins. In addition, silver has a high propensity to ionise from SSD and later intercalates with bacterial DNA. Despite the beneficial qualities of SSD and its antimicrobial nature, SSD cream requires continuous reapplication (typically once or twice every day), which requires labour‐intensive and often painful dressing changes. The direct tissue application of SSD cream can also cause necrosis of surrounding tissue and may delay the normal wound‐healing process. In addition, the frequent application of SSD cream leads to the absorption of high levels of silver ions, leading to potentially toxic levels of silver in the body 16. Therefore, it is advantageous to optimise the delivery of SSD through controlled release so that the concentration of silver possesses antimicrobial activity without exceeding to toxic levels.
We have previously stated that silver can be delivered through microcarriers in a controlled manner at levels that control infection, with the amount of silver in the serum remaining well below toxic levels 17. Chitosan‐based drug delivery systems are a promising candidate for a wide range of drug delivery applications, with potentials for further clinical translation 18, 19, 20, 21. Chitosan is an excellent mucoadhesive polymer that integrates as an extracellular glycosaminoglycan component within the wound tissue.
In addition to providing an efficient medium for controlling infection in combat wounds, it is necessary to formulate a means to improve the healing by recruitment of host cells essential for healing. To overcome these problems, fibrin‐based hydrogels were developed for ease of application and stability in a wound 22, 23. The major concern of using fibrin‐based hydrogels as wound repair scaffolds is still; their relatively quick contraction, low mechanical stiffness (which limits durability) and their rapid degradation once placed at the wound site. We have recently shown that a polyethylene glycol‐modified fibrin hydrogel (PEGylated fibrin hydrogel) induces vasculogenesis both in vitro and in vivo 24, 25. The addition of extra cross‐linking between polyethylene glycol (PEGylated) fibrin (FPEG) during thrombin‐mediated fibrin polymerisation produces a highly hydrated hydrogel microenvironment, allowing cell seeding within the matrix. Combining antibacterial SSD and natural biological activity of fibrin has been shown to encourage tissue and blood vessel growth in the healing wound 26. Therefore, in this study, we have used FPEG hydrogel as a matrix substitute as well as a delivery device to control the release of SSD. We have incorporated SSD‐loaded chitosan microspheres (CSM) within the FPEG hydrogel and evaluated its efficiency in vivo using our previously developed porcine full‐thickness wound model 27, 28, 29. In general, FPEG hydrogel‐based dressing functions as a moist wound dressing and provides a hostile environment for positive wound healing to take place. The addition of an antibacterial factor is anticipated to primarily control infection and is further evaluated for its ability to induce healing response when applied to an infected wound.
Materials and methods
Preparation of CSM and SSD‐CSM
CSM and SDD‐CSM were prepared using our previous published protocol 26. Briefly, chitosan (3% solution in 0·5 M acetic acid, Sigma‐Aldrich, St. Louis, MO) was emulsified in an oil mixture of soya oil (Sigma‐Aldrich) and n‐octanol (Acros Organics, New Jersey, NY), with span 80 (Sigma‐Aldrich) as an emulsifier, using an overhead stirrer. Micelles of chitosan were slowly solidified using 1% w/v of KOH in n‐octanol. After ionically cross‐linking the chitosan micelles, the oil phase of the mixture was slowly decanted, and the CSM were recovered using acetone. Discrete microspheres were obtained by sonicating at 600Hz (Vibracell, Viewsonics, Newtown, CT) for 15 minutes under a constant amplitude of 42% with an intermittent on/off pulse of 9 seconds/4 seconds. Finally, the microspheres were dried in a vacuum desiccator. SSD (Sigma‐Aldrich)‐loaded microspheres were prepared by following similar procedure as mentioned above, in which 10 mg of SSD was added to aqueous phase of 100 mg chitosan and sonicated before the emulsification process. The morphological features of CSM and SSD‐CSM were assessed by using an Scanning Electron Microscope (SEM). For analysis, the specimens were sputter‐coated with gold–palladium (40–60%). The ultra‐structural features of CSM and SSD‐CSM were analysed with a JEOL JSM 5610 (JEOL, Peabody, MA) series SEM equipped with an electron optical system with a 0·5–30‐kV capacity electron gun and an electron detector.
Preparation of SSD‐CSM‐FPEG hydrogels
FPEG hydrogel was prepared as per our previous procedure 24, 30. Briefly, modified PEG (Succinimidyl glutarate‐modified polyethylene glycol; SG‐PEG‐SG, 3400 Da; NOF America Corporation, White Plains, NY) was added to fibrinogen (Sigma‐Aldrich) at concentration ratio of 1:10 in tris‐buffered saline (TBS), pH 7·8 and incubated for 20 minutes in a 5% CO2‐humidified incubator at 37 °C. This mixture constitutes a molar concentration ratio of 1:10, SG‐PEG‐SG:fibrinogen. An equal volume of thrombin (Sigma‐Aldrich) in 40 mM CaCl2 at a final concentration of 10 U/ml was added to the PEG–fibrinogen solution and incubated for 10 minutes at 37 °C to allow for complete polymerisation/gelation. The resulting hydrogels were rinsed with TBS (pH 7·8) before application. To prepare FPEG hydrogels incorporated with SSD‐CSM, SSD‐CSM (40 mg) was added to the SG‐PEG‐SG:fibrinogen solution after incubation followed by gelation with thrombin.
Full‐thickness infected porcine excision wound model
Three 3 female, pathogen‐free (SPF: Looper Farms, Granite Falls, NC) pigs weighing 35–40 kg (approximately 2–3 months old) were acclimated for 2 weeks prior to initiating all experiments. The animals were fed a basal diet ad libitum and housed individually in American Association for Accreditation of Laboratory Animal‐accredited animal facilities with controlled temperature (19–21 °C) and lights (12 h/12 h LD). During surgery and treatments, the animals were sedated with Telazol HCl, Xylazine, Atropine (I.M.) followed by endotracheal tube inhalation of an Isofluorane (Isothesia; Abbott Laboratories, Chicago, IL) and oxygen combination. The flank and back of the experimental animals were clipped with standard animal clippers on the first day of experimentation. The skin on both sides of each animal was prepared for wounding by washing with a non‐antibiotic soap (Neutrogena Soap Bar; Johnson and Johnson, Los Angeles, CA) and sterile water. The animals were then blotted dry with sterile gauze. Full‐thickness wounds were made on the paravertebral and thoracic area with a 10‐mm punch biopsy, and the wounds were separated from one another by 15 mm of unwounded skin. Immediately after punch biopsies were made and bleeding stopped, the wounds were inoculated with P. aeruginosa (PA 09–010, provided by US Army Institute of Surgical Research). The challenge inoculum suspension was prepared by scraping the overnight growth from a culture plate into 20 ml of normal saline. The inoculum was serially diluted to a concentration of 106 CFU/ml, and 25 µl of this suspension was applied directly into the individual wounds and immediately covered with a polyurethane film dressing (Tegaderm™ Transparent Dressing; 3 M Health Care, St. Paul, MN). All sites were secured with surgical tape and the entire animal loosely wrapped with Coban self‐adhesive elastic wrap (3 M, St. Paul, MN). The Tegaderm was left in place for 24 hours to allow for formation of a bacterial biofilm in the wounds.
Efficiency of SSD hydrogel‐based dressing
A total of 24 hours after infection with P. aeruginosa, the wounds were treated, and secondary Tegaderm dressings were re‐applied. The wounds were randomly assigned to the following groups: (A) CSM (40 mg), (B) SSD‐loaded CSM (SSD‐CSM, 40 mg), (C) FPEG hydrogel incorporated with 40 mg CSM, (CSM‐FPEG), (D) FPEG hydrogel incorporated with 40 mg of SSD‐CSM (SSD‐CSM‐FPEG), (E) Silvadene cream (positive Control) and (F) Tegaderm Polyurethane film dressing (negative control). Treatment groups A–D received a collagen sheet that was prepared from rat tail tendon as per previously published protocol 31. Briefly, type 1 collagen (5 mg/ml; Travigen, Gaithersburg, MD) from rat tail tendon was fibrillated by adjusting the pH to 6·8–7·0 using Dulbecco's PBS and 1 N NaOH. The fibrillated collagen (20 ml) was uniformly cast over horizontally placed polypropylene platforms of 10 × 10 × 2 cm3 dimensions. They were allowed to dry at a constant temperature of 34 °C until a thin sheet was obtained. The collagen sheet was then washed with distilled water twice, air‐dried and stored in a desiccator for further experimental purpose. All wounds were covered with Tegaderm to maintain a moist wound environment. The treatments and dressings were secured using surgical tape (Elasticon, New Brunswick, NJ) and Coban elastic wrap. The positive control group was treated with Silvadene cream, daily, through out the experiment.
Assessment of microbial load
The wounds were biopsied using a 6‐mm punch biopsy and by making an incisional wedge, which contained normal skin on each side, on days 5, 7 and 11 post‐treatment applications. Punch biopsies were taken from the centre of the wound, weighed and then immediately placed in 1 ml of all‐purpose neutralising solution. The sample was then combined with Neutralising Solution (containing tween 80, lecithin, sodium oleate, sodium thiosulfate, protease peptone and tryptone) and homogenised in a sterile homogenisation tube (Tenbroeck Tissue Grinder). Serial dilutions were made, and scrub solutions were quantified using the Spiral Plater System, which deposits a small defined volume (50 µl) of the bacterial suspension over the surface of a rotating culture plate. Pseudomonas Agar base with CN supplementation (containing cetrimide 100 mg and sodium nalidixate 7·5 mg) was used to specifically identify P. aeruginosa from the wounds. After plating, all samples were incubated aerobically for 24 hours at 37 °C. After the incubation period, the colonies on the plates were counted, and the CFU/g of wound tissue biopsies was calculated.
Histological assessment
Incisional wedge biopsies were placed in 10% neutral buffered formalin and then stained with haematoxylin and eosin (H&E). Paraffin sections were prepared and histomorphometrically analysed. The specimens were evaluated in a ‘blinded’ fashion through light microscopy and examined for the following elements to determine a potential treatment response: percent wound epithelialisation, new blood vessel formation (angiogenesis) and granulation tissue formation. Percent of wound epithelialisation was determined by measuring the length of the wound that was covered with new epithelium. Histological analysis of new blood vessels formation was carried out using a mean score measurement method: 1 = absent, 2 = mild, 3 = moderate, 4 = marked, 5 = exuberant. Granulation tissue formation was measured by the approximate amount of new granulation tissue formed (in the dermis) and graded by a trained pathologist as follows: 1 = ≤5%, 2 = 6–25%, 3 = 26–50%, 4 = 51–75%, 5 = 76–100%.
Statistical analysis
All graphical illustrations in this study are represented as mean ± SD (microbiology) or ± SEM (histology) and analysed for significance using an ANOVA of the mean Log CFU/g of the combined animal data. Variance within each group was compared individually and also against two, three and all groups combined together. Test for significance was performed with a confidence limit of 95 and 99%, that is, P < 0·05 and 0·01%, respectively, and was considered statistically significant.
Results
SSD‐CSM and FPEG hydrogel‐based wound dressing
We have previously shown the controlled release and delivery of SSD‐impregnated CSMs, and FPEG hydrogel is an effective, biocompatible, antibacterial tool in vitro 26. The CSMs (Figure 1A) and SSD‐loaded CSM (SSD‐CSM; Figure 1B) used in this study were in the size range of 125–180 µm, with 76·50 weight% of drug entrapment/mg of chitosan. For in vivo application, 750 µl FPEG hydrogels were prepared as previously described 26. In this study, we evaluated the efficiency of FPEG hydrogel incorporated with SSD‐CSM to control P. aeruginosa infection in a porcine full‐thickness wound model. Figures 1D and E are representative photographs showing the application of SSD‐CSM‐FPEG over an excision wound 24 hours post‐infection and an H&E‐stained histological section of biopsies taken at different days, respectively.
Figure 1.
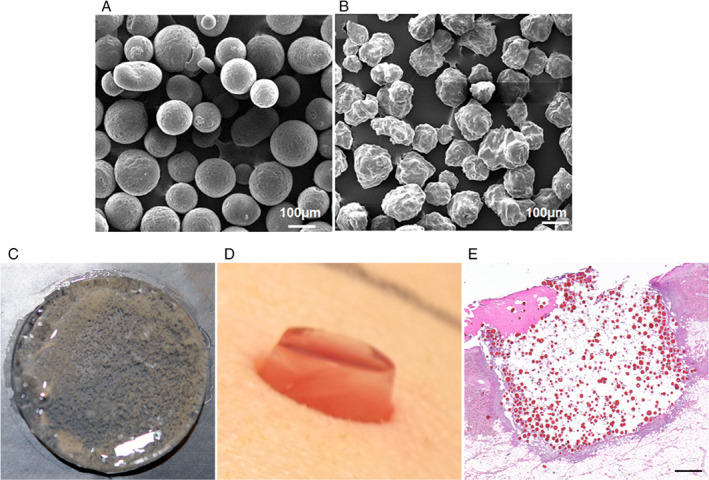
Silver sulfadiazine (SSD)‐loaded chitosan microspheres (SSD‐CSM) and PEGylated fibrin hydrogel‐based wound dressing. Scanning electron micrographs show (A) CSM with smooth surface before drug loading and (B) SSD‐CSM with rough surface due to crystalline nature of entrapped SSD. (C) A photomicrograph of PEGylated fibrin gel (FPEG) impregnated with SSD‐CSM. (D) Photograph showing application of FPEG‐SSD‐CSM over an excision wound 24 hours post‐inoculation. (E) Histological section stained with Haematoxylin and Eosin showing PEGylated fibrin gel, with SSD‐CSM filled within the wound (appears as red‐coloured particles). Scale bar in (E) = 20 µm.
SSD‐CSM‐FPEG decreases microbial load
In a rodent study (unpublished), we had observed that SSD‐CSM (40 mg) delivered using a FPEG hydrogel would effectively control P. aeruginosa (1 × 106 cfu) infection. Based on that study, 40 mg of SSD‐CSM was used for treatment in the current study. Microbiological assessment on day 5 of the wounds treated with SSD cream and SSD‐CSM‐FPEG treatment showed a better bacterial reduction than other groups (∼1–3 Log CFU/g). On day 7, all SSD groups (SSD‐CSM and SSD‐CSM‐FPEG) showed reductions in P. aeruginosa counts compared with other treatment groups. Interestingly, assessment of the SSD‐CSM‐FPEG hydrogel dressing showed a significant decrease in P. aeruginosa on day 11 in comparison with SSD and other treatment groups (Figure 2), with over a 5 Log reduction compared with Tegaderm control.
Figure 2.
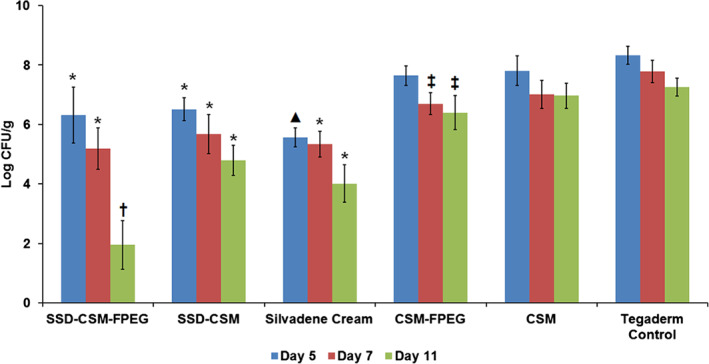
Reduction of Pseudomonas aeruginosa cfu observed in different treatment groups on various treatment days (5, 7, 11). *P < 0·05 compared with CSM‐FPEG, CSM and Tegaderm™ control groups; ▴P < 0·05 compared with CSM‐FPEG, SSD‐CSM, CSM and Tegaderm control groups; ‡P < 0·05 compared with Tegaderm control; †P < 0·05 compared with all other five treatment groups.
Wound healing
On days 5, 7 and 11, wounds from all treatment groups had similar rates of reepithelialisation except those treated with SSD‐CSM, which showed less epithelialisation than SSD‐CSM‐FPEG (day 7: P < 0·01), CSM alone (day 7: P < 0·05) and CSM‐FPEG (day 11: P < 0·05). The degree of white cell infiltrate was decreased in wounds treated with SSD‐CSM‐FPEG (days 7 and 11). Interestingly, these wounds also showed the greatest reduction in P. aeruginosa. Wounds from all treatment groups had similar amounts of granulation tissue (except those treated with SSD‐CSM, which had lower amounts on day 7). Histological images of a granulating wound bed stained with H&E shows that silvadene cream treatment initiated granulation earlier (day 5), whereas on the later days, specifically after day 7, the wounds treated with SSD‐CSM‐FPEG hydrogel exhibited improved granulation tissue formation. On day 11, SSD‐CSM‐FPEG exhibited the formation of distinct reticular dermis and underlying subcutaneous region with defined adipose tissue (Figure 3). Specifically on day 7, the SSD‐CSM‐FPEG had significantly more (P < 0·05; score = 4·88 ± 0·08) granulation tissue formation than the FPEG hydrogel without SSD (CSM‐FPEG group) (Figure 4). While no significant differences were found among any group at three time points, the SSD‐containing groups always obtained the highest score for granulation tissue formation. Importantly, the epithelial thickness was significantly better in the wound treated with SSD‐CSM‐FPEG hydrogel on day 7 (150·9 ± 13·12 µm, P < 0·05) when compared with all the other groups (Figure 5).
Figure 3.
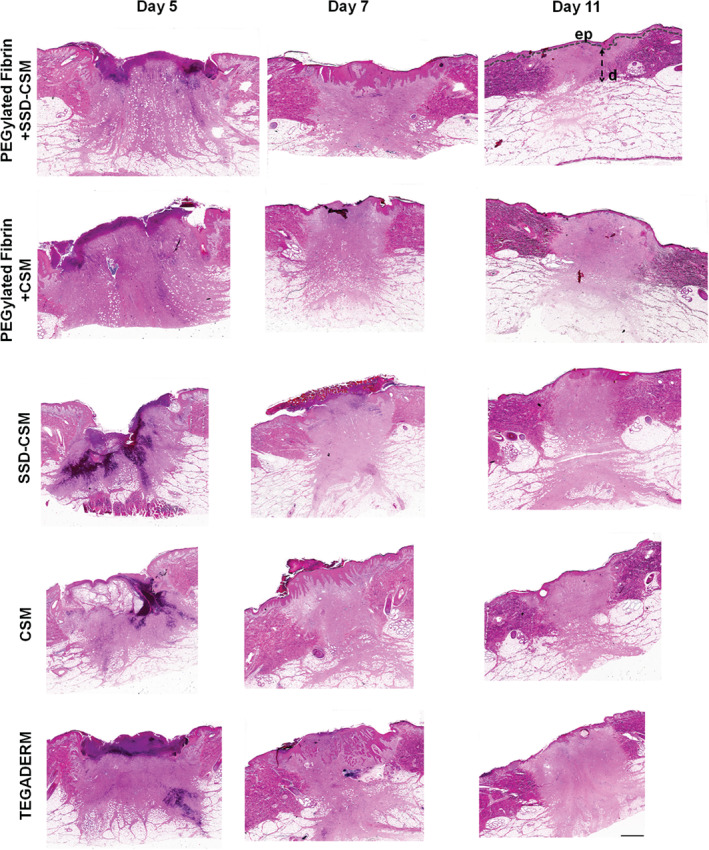
H&E‐stained sections of biopsies taken from wounds treated with SSD‐CSM‐FPEG, CSM‐FPEG, SSD‐CSM, CSM and Tegaderm™. FPEG‐SSD‐CSM group exhibited formation of distinct reticular dermis and underlying subcutaneous region with defined adipose tissue on day 11. ep, epidermis; d, dermis; all the images were taken at same magnification and scale bar = 20 µm.
Figure 4.
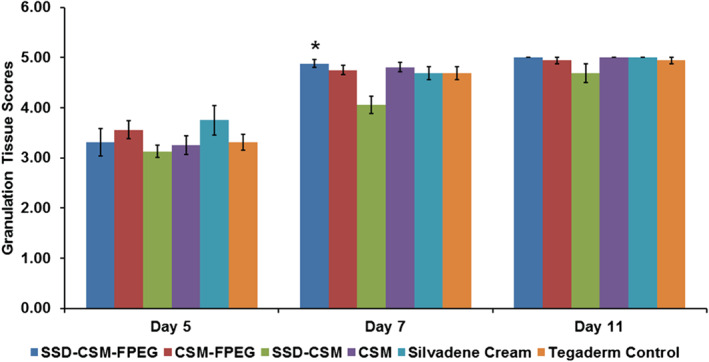
Comparative scores of granulating wound bed assessed on days 5, 7 and 11 in different treatment groups from histological sections stained with H&E. A statistically significant score was observed on day 7 when SSD‐CSM‐FPEG was compared with the SSD‐CSM group. *P < 0·05 compared with SSD‐CSM. Mean scores for granulation tissue formation: 0, <1%; 0·5, 1–10%; 1, 11–30%; 2, 31–50%; 3, 51–70%; 4, 71–90%; 5, >90%.
Figure 5.
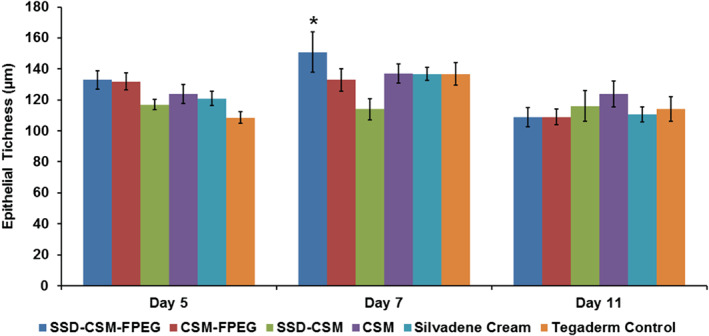
Determination of epithelial thickness from H&E wound biopsy sections. A significant difference was observed in the wound treated with SSD‐CSM‐FPEG on day 7 when compared with SSD‐CSM. *P < 0·05 compared with SSD‐CSM. Epithelial thickness was a measure of an average thickness of five points of newly formed epithelium in each treatment group on various assessment days (5, 7, 11).
FPEG hydrogel‐based wound dressing initiates early angiogenic response
Histological slides stained with H&E were scored by a trained pathologist (blinded). Angiogenesis within the wound bed was characterised and scored by newly formed capillary blood vessels with proliferating endothelial cells sprouting from adjacent existing blood vessels. Infected wounds treated with FPEG hydrogel‐based dressings (with or without SSD loaded CSM) had better and significant scores (3·88 ± 0·157 and 3·75 ± 0·164, respectively; P < 0·01) than the other groups (Figure 6). The wounds treated with silvadene cream and SSD‐CSM alone had lower scores than all other groups, but they were not statistically significant. On day 7, the FPEG hydrogel‐based groups, with or without SSD‐CSM, and SSD group showed better angiogenic response in comparison to Tegaderm alone; however, no statistically significant difference was found. Collectively, these results indicate that FPEG hydrogels were able to instigate an early angiogenic response.
Figure 6.
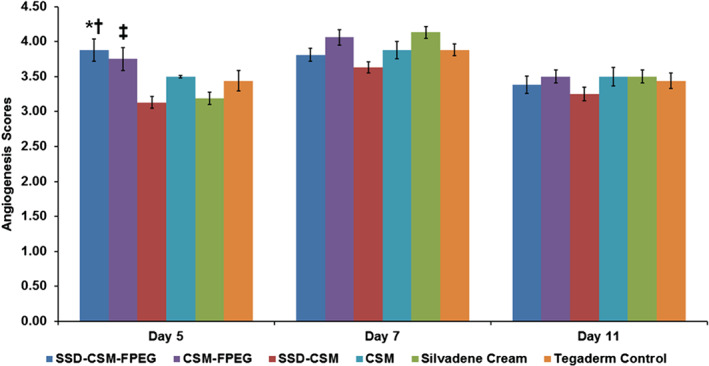
Angiogenesis scores determined from histological sections stained with H&E of all treatment groups on different assessment days (5, 7, 11). *P < 0·05 compared with all other treatment groups; †P < 0·01 compared with CSM‐FPEG group; ‡P < 0·05 compared with SSD‐CSM, CSM, Silvadene ccream and Tegaderm™ control groups. Mean score for angiogenesis: 1, absent; 2, mild; 3, moderate; 4, marked; 5, exuberant.
Discussion
Pathogenesis of bacterial contamination in skin wounds occurs due to extracellular virulence factors such as elastase, exotoxins and exozymes, which directly and indirectly influence the healing process 3. Wound contamination/infection is a major problem in combat injuries due to improvised explosive devices, which typically involves large skin loss, making them highly vulnerable to infection by pathogenic microorganisms like P. aeruginosa 2, 3. Although SSD creams and silver‐based dressings still remain the treatment of choice against bacterial infections, it has also been shown to impair the wound‐healing process 16. As a result, improved treatments are necessary. Therefore, we have developed a novel dressing in which SSD is delivered in CSM using an FPEG‐based hydrogel carrier to effectively reduce P. aeruginosa infection and also promote healing in a porcine full‐thickness wound model.
Numerous studies have utilised chitosan as a drug carrier, but there are limited studies on the use of chitosan in the form of microcarriers for wound‐healing application. We have previously shown that CSM act as a mucoadhesive polymer that provides an excellent cell adhesive surface 32. The cell adhesiveness is due to it being a cationic polysaccharide offering ionic sites for interactions with tissues and mucosal layers 33, 34. CSM in our study was developed by an ionic gelation process, and it offers polycationic sites that can potentially interact not only with negatively charged cell surfaces but also with anionic compounds and drugs. In this study, we have taken advantage of the polycationic sites of chitosan that interact with Ag+ ions present in SSD and form weak ionic bonds. The SEM image clearly shows the entrapment of SSD within CSM. The CSM had smooth surface, and following drug entrapment, the microspheres developed an irregular morphology because of the crystalline nature of entrapped SSD (Figure 1). In our previous studies, we have shown that release of SSD from CSM follows first‐order release kinetics within the required bactericidal concentrations to inhibit P. aeruginosa growth in vitro. Through this in vivo study, we show that SSD‐CSM‐based wound dressing can be used to control infection without affecting the normal healing process 26.
Persistent microbial infections of wounds impede vascularisation and epithelialisation, resulting in increased scarring 35. Active biopolymer‐based wound dressings, like hydrogel‐based systems, provide a moist wound environment, helping to optimise the healing process and decrease the number of dressing changes 36. Therefore, implementation of biological scaffolds that controls the release of SSD as well augment healing was considered important. Most of the current wound dressings are primarily designed to address only infection and do not provide an active platform to induce angiogenesis. Fibrin has been used clinically as an FDA‐approved haemostatic agent and as a sealant in a variety of clinical applications 37. In addition, fibrin hydrogels also have applications for promoting angiogenesis 38. We have developed a modified FPEG hydrogel that possesses desirable mechanical stability, supports the formation of a tubular network in combination with stem cells in vitro and significantly promotes blood vessel formation in vivo 25. These attributes of FPEG hydrogel prompted us to use it as an effective wound dressing.
Previously, we have shown that the FPEG hydrogels impregnated SSD‐CSM at a concentration of 50 µg/ml reduced inoculated bacterial load and promoted tube formation by adipose‐derived stem cells (ASC) in vitro. Furthermore, the morphology of the tube‐like structures formed was comparable to the hydrogels without SSD‐CSM. The tube‐like structures exhibited phenotypic characteristics of pericytes (NG‐2 and PDGFRβ positive), which are considered to be vital for further endothelial cell infiltration during neo‐vascularisation 26. Therefore, it is anticipated that when applied in vivo, SSD‐CSM‐FPEG hydrogels may act as an active platform to induce vasculogenesis in an infected wound environment. The results from our current study demonstrated significant reduction in bacterial load throughout the experiment (5, 7 and 11 days). Interestingly, the efficacy of SSD appeared to be optimised with the addition of SSD‐CSM to FPEG hydrogel as seen by the significant decrease in Pseudomonas infection levels on day 11, the final assessment day. A total of 5·31 ± 0·53 Log CFU/g (a 99·99% of reduction) of Pseudomonas was observed on day 11, compared with the untreated wounds. In addition, the SSD‐CSM‐FPEG group facilitated early angiogenesis, based on day 5's assessment, better than any other treatment group. Although the assessment of angiogenesis was semi‐quantitative, the blinded assessment by a trained pathologist prompts furthermore quantitative assessment. The vWF quantification carried out in our previous study clearly indicated that FPEG hydrogels promote better vascularisation than no treatment 25, which substantiates our present finding.
It is well documented that bacterial load aggravates wound damage 39. From our histological results, we demonstrate that the SSD‐CSM‐FPEG hydrogel group showed significant improvement in epithelial thickness (150·9 ± 13·12 µm) and wound bed granulation on day 7. This result is consistent with early and complete clearance of bacteria throughout the wound. Inhibition of infection and increased healing is also shown through the appearance of reticular dermis on day 11 in the wounds treated with SSD‐CSM‐FPEG hydrogel. The results of the current investigation indicate that the controlled release of SSD inhibits infection. As the release of SSD from CSM maintains a steady‐state release of silver at therapeutic levels (data not shown) and not a burst release, there is no risk of Ag+ ions accumulation elsewhere in the body. Earlier dressings were developed mainly with synthetic polymers and did not provide controlled release from dressing. Therefore, this study provides a new dressing for controlled delivery of SSD using CSM in a FPEG hydrogel to reduce bacterial bioburden with one‐time application and improves wound healing by promoting angiogenesis and granulation tissue formation, followed by accelerated maturation of dermal and epidermal tissue.
Conclusion
The treatment of infected porcine wounds with SSD‐CSM‐FPEG hydrogel dressing effectively controls P. aeruginosa infection. The ability of the dressing to promote early angiogenesis supports our hypothesis of providing material mimicking a provisional matrix to enhance the healing process. In fact, the wounds treated with an FPEG‐based hydrogel formulation exhibited better reepithelialisation and a granulating wound bed, indicating the positive role of fibrin during the healing process. Therefore, there is a great potential for implementing SSD‐CSM and further incorporating them within a FPEG hydrogel not only to control infection but also to provide an environment to positively promote healing of combat‐associated skin injuries.
Disclaimer
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or Department of Army. The authors are employees of the US Government, and this work was prepared as part of their official duties. This study has been conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations and the principles of the Guide for the Care and Use of Laboratory Animals. The studies received Institutional Animal Care and Use Committee (IACUC) approval from the University of Miami.
Acknowledgement
This research was funded by the US Army Medical Research and Materiel Command.
References
- 1. Keen EF 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, Murray CK. Prevalence of multidrug‐resistant organisms recovered at a military burn center. Burns 2010;36:819–25. [DOI] [PubMed] [Google Scholar]
- 2. Keen EF 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, Murray CK. Incidence and bacteriology of burn infections at a military burn center. Burns 2010;36:461–8. [DOI] [PubMed] [Google Scholar]
- 3. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19:403–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Avignon LC, Saffle JR, Chung KK, Cancio LC. Prevention and management of infections associated with burns in the combat casualty. J Trauma 2008;64:S277–86. [DOI] [PubMed] [Google Scholar]
- 5. Kaspar RL, Griffith ME, Mann PB, Lehman DJ, Conger NG, Hospenthal DR, Murray CK. Association of bacterial colonization at the time of presentation to a combat support hospital in a combat zone with subsequent 30‐day colonization or infection. Mil Med 2009;174:899–903. [DOI] [PubMed] [Google Scholar]
- 6. Hospenthal DR, Murray CK, Andersen RC, Blice JP, Calhoun JH, Cancio LC, Chung KK, Conger NG, Crouch HK, D'Avignon LC, Dunne JR, Ficke JR, Hale RG, Hayes DK, Hirsch EF, Hsu JR, Jenkins DH, Keeling JJ, Martin RR, Moores LE, Petersen K, Saffle JR, Solomkin JS, Tasker SA, Valadka AB, Wiesen AR, Wortmann GW, Holcomb JB. Guidelines for the prevention of infection after combat‐related injuries. J Trauma 2008;64:S211–20. [DOI] [PubMed] [Google Scholar]
- 7. Murray CK. Epidemiology of infections associated with combat‐related injuries in Iraq and Afghanistan. J Trauma 2008;64:S232–8. [DOI] [PubMed] [Google Scholar]
- 8. Oncul O, Oksuz S, Acar A, Ulkur E, Turhan V, Uygur F, Ulcay A, Erdem H, Ozyurt M, Gorenek L. Nosocomial infection characteristics in a burn intensive care unit: analysis of an eleven‐year active surveillance. Burns 2014;40:835–41. [DOI] [PubMed] [Google Scholar]
- 9. Ilangovan A, Fletcher M, Rampioni G, Pustelny C, Rumbaugh K, Heeb S, Camara M, Truman A, Chhabra SR, Emsley J, Williams P. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR). PLoS Pathog 2013;9:e1003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jakobsen TH, Bjarnsholt T, Jensen PO, Givskov M, Hoiby N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol 2013;8:901–21. [DOI] [PubMed] [Google Scholar]
- 11. Black JS, Drake DB. A prospective randomized trial comparing silver sulfadiazine cream with a water‐soluble polyantimicrobial gel in partial‐thickness burn wounds. Plast Surg Nurs 2015;35:46–9. [DOI] [PubMed] [Google Scholar]
- 12. Tang H, Lv G, Fu J, Niu X, Li Y, Zhang M, Zhang G, Hu D, Chen X, Lei J, Qi H, Xia Z. An open, parallel, randomized, comparative, multicenter investigation evaluating the efficacy and tolerability of Mepilex Ag versus silver sulfadiazine in the treatment of deep partial‐thickness burn injuries. J Trauma Acute Care Surg 2015;78:1000–7. [DOI] [PubMed] [Google Scholar]
- 13. Modak SM, Fox CL Jr. Binding of silver sulfadiazine to the cellular components of Pseudomonas aeruginosa . Biochem Pharmacol 1973;22:2391–404. [DOI] [PubMed] [Google Scholar]
- 14. Rosenkranz HS, Carr HS. The determination of the susceptibility of bacterial isolates to silver sulfadiazine. Chemotherapy 1978;24:143–5. [DOI] [PubMed] [Google Scholar]
- 15. Wlodkowski TJ, Rosenkranz HS. Antifungal activity of silver sulphadiazine. Lancet 1973;2:739–40. [DOI] [PubMed] [Google Scholar]
- 16. Sano S, Fujimori R, Takashima M, Itokawa Y. Absorption, excretion and tissue distribution of silver sulphadiazine. Burns Incl Therm Inj 1982;8:278–85. [DOI] [PubMed] [Google Scholar]
- 17. Shanmugasundaram N, Uma TS, Ramyaa Lakshmi TS, Babu M. Efficiency of controlled topical delivery of silver sulfadiazine in infected burn wounds. J Biomed Mater Res A 2009;89:472–82. [DOI] [PubMed] [Google Scholar]
- 18. Duttagupta DS, Jadhav VM, Kadam VJ. Chitosan: a propitious biopolymer for drug delivery. Curr Drug Deliv 2015;12:369–81. [DOI] [PubMed] [Google Scholar]
- 19. Radulescu M, Ficai D, Oprea O, Ficai A, Andronescu E, Holban AM. Antimicrobial Chitosan based formulations with impact on different biomedical applications. Curr Pharm Biotechnol 2015;16:128–36. [DOI] [PubMed] [Google Scholar]
- 20. Wu QX, Lin DQ, Yao SJ. Design of chitosan and its water soluble derivatives‐based drug carriers with polyelectrolyte complexes. Mar Drugs 2014;12:6236–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu L, Sun Y, Wu Y. Advances in chitosan‐based drug delivery vehicles. Nanoscale 2013;5:3103–11. [DOI] [PubMed] [Google Scholar]
- 22. Moreno‐Arotzena O, Meier JG, Del Amo C, Garcia‐Aznar JM. Characterization of fibrin and collagen gels for engineering wound healing models. Materials (Basel) 2015;8:1636–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hojo M, Inokuchi S, Kidokoro M, Fukuyama N, Tanaka E, Tsuji C, Miyasaka M, Tanino R, Nakazawa H. Induction of vascular endothelial growth factor by fibrin as a dermal substrate for cultured skin substitute. Plast Reconstr Surg 2003;111:1638–45. [DOI] [PubMed] [Google Scholar]
- 24. Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A bilayer construct controls adipose‐derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A 2011;17:941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zamora DO, Natesan S, Becerra S, Wrice N, Chung E, Suggs LJ, Christy RJ. Enhanced wound vascularization using a dsASCs seeded FPEG scaffold. Angiogenesis 2013;16:745–57. [DOI] [PubMed] [Google Scholar]
- 26. Seetharaman S, Natesan S, Stowers RS, Mullens C, Baer DG, Suggs LJ, Christy RJ. A PEGylated fibrin‐based wound dressing with antimicrobial and angiogenic activity. Acta Biomater 2011;7:2787–96. [DOI] [PubMed] [Google Scholar]
- 27. Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm‐associated wound colonization in vivo. Wound Repair Regen 2008;16:23–9. [DOI] [PubMed] [Google Scholar]
- 28. Davis SC, Cazzaniga AL, Eaglstein WH, Mertz PM. Over‐the‐counter topical antimicrobials: effective treatments? Arch Dermatol Res 2005;297:190–5. [DOI] [PubMed] [Google Scholar]
- 29. Davis SC, Mertz PM. Determining the effect of an oak bark formulation on methicillin‐resistant staphylococcus aureus and wound healing in porcine wound models. Ostomy Wound Manage 2008;54:16–8, 20, 2–5. [PubMed] [Google Scholar]
- 30. Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng 2006;12:9–19. [DOI] [PubMed] [Google Scholar]
- 31. Rajan N, Habermehl J, Cote MF, Doillon CJ, Mantovani D. Preparation of ready‐to‐use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Protoc 2006;1:2753–8. [DOI] [PubMed] [Google Scholar]
- 32. Natesan S, Baer DG, Walters TJ, Babu M, Christy RJ. Adipose‐derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng Part A 2010;16:1369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prasitsilp M, Jenwithisuk R, Kongsuwan K, Damrongchai N, Watts P. Cellular responses to chitosan in vitro: the importance of deacetylation. J Mater Sci Mater Med 2000;11:773–8. [DOI] [PubMed] [Google Scholar]
- 34. Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 2008;26:1–21. [DOI] [PubMed] [Google Scholar]
- 35. Demidova‐Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care 2012;25:349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burd A. Evaluating the use of hydrogel sheet dressings in comprehensive burn wound care. Ostomy Wound Manage 2007;53:52–62. [PubMed] [Google Scholar]
- 37. Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 2009;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martineau L, Doillon CJ. Angiogenic response of endothelial cells seeded dispersed versus on beads in fibrin gels. Angiogenesis 2007;10:269–77. [DOI] [PubMed] [Google Scholar]
- 39. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]


