Abstract
Wound healing is an articulated process that can be impaired in different steps in chronic wounds. Chronic leg ulcers are a special type of non‐healing wounds that represent an important cause of morbidity and public cost in western countries. Because of their common recurrence after conventional managements and increasing prevalence due to an ageing population, newer approaches are needed. Over the last decade, the research has been focused on innovative treatment strategies, including stem‐cell‐based therapies. After the initial interest in embryonic pluripotent cells, several different types of adult stem cells have been studied because of ethical issues. Specific types of adult stem cells have shown a high potentiality in tissue healing, in both in vitro and in vivo studies. Aim of this review is to clearly report the newest insights on tissue regeneration medicine, with particular regard for chronic leg ulcers.
Keywords: Adult tissue‐derived stem cells, Chronic leg ulcers, Stem cells, Wound healing
Introduction
Chronic leg ulcers (CLUs) affect 1% of the adult population and 3·6% of people older than 65 years representing one of the main cause of morbidity among older subjects, especially women in the western world; the prevalence of leg ulcers in Europe is about 0·18–1% 1, 2, 3.
CLUs occur more commonly in elderly people and their prevalence, in western countries, is rising due to an increase in both life expectancy and risk factors for atherosclerotic stenosis, that is smoking, obesity and diabetes 4. They are responsible for the high cost of caring for leg ulcers, including diagnosis, investigations, treatment, nursing care and rehabilitation: approximately 1% of the total health care costs in the western world are likely to be used for management of CLUs. Venous ulcers are the most common type of leg ulcers, accounting for approximately 70% of cases. Arterial disease accounts for another 5–10% of leg ulcers; most of the others are due to either neuropathy (usually diabetic) or a combination of those diseases 5, 6, 7, 8. They are characterised by significant morbidity, loss of productivity and reduced quality of life, especially among women 9. Furthermore, although the exact amount is not well established 10, the direct and the indirect social costs for the health care system associated with CLUs are very high, with the only diabetic ulcer costing $30 000 to $50 000 11, 12.
Various approaches have been developed for wound healing, but most of these have centred on one facet of wound healing, such as inflammation or growth factors 13, 14, 15, 16. Furthermore, evidences have shown that stem cell therapy can be an excellent option for patients with CLUs 17, 18, 19: these therapies can provide a comprehensive solution by addressing multiple factors during wound healing, including cell proliferation, extracellular matrix (ECM) synthesis, growth factor release and vascularisation 20.
The aim of this study is to perform a systematic analysis of the most recent scientific literature on the role of adult tissue‐derived stem cells in CLUs and the future prospects in regenerative medicine.
Materials and methods
PubMed and ScienceDirect databases were searched for articles using the terms: Chronic Leg Ulcers, Stem Cells Therapy, Angiogenesis, Wound Healing and Adult Tissue‐Derived Stem Cells.
Only publications in English were included. Titles and abstracts were screened by one author (F. F.) to identify potentially relevant studies. All potentially eligible studies were subsequently evaluated in detail by one reviewer (F. F.) through consideration of the full text. Reference lists of retrieved articles were also searched for relevant publications.
Inclusion required clinical trials, case reports, meta‐analysis and systematic reviews in which therapy with adult tissue‐derived stem cells were provided in CLU patients. Studies were excluded if performed in languages other than English, if the patient cohort, in human studies, was defined by the presence of CLU and an additional confounding disease process or if CLU‐specific results could not be distinguished from those of a larger population consisting of individuals without CLU. Studies were also excluded when the primary focus was other than chronic wounds.
Results
Study selection
Initial database searches yielded 34 studies from PubMed and 2302 from Science Direct in the last 5 years. We evaluated 115 eligible full text articles (Figure 1).
Figure 1.
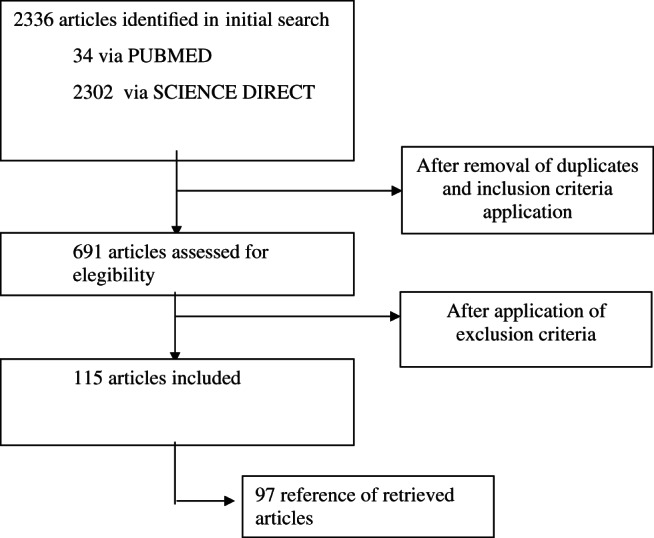
Flow of papers identified from search strategy.
The pathophysiology of CLUs and their correlation with delayed wound healing, the current therapeutic approaches for CLUs found in literature, and the description of the application of the adult tissue‐derived stem cell therapy in patients with CLUs are given below.
Pathophysiology of chronic wound and CLUs
Both local and systemic factors can be involved in chronic wound etiopathogenesis. Among local factors infection, ischaemia, arterial/venous insufficiency, local toxins, trauma and radiations are of great importance and inevitably characterise all the cases of CLUs in different amounts. Among systemic factors ageing, chronic diseases, alcoholism, smoking, drugs, nutritional deficiencies, chronic kidney disease and neuropathies appear to be the most important 21. Non‐healing wounds usually result from an impairment of one or more of the four phases of normal healing (haemostasis, inflammation, proliferation and remodelling). They are characterised by an incessant inflammation of which neutrophils represent a marker 22. This chronic inflammatory state is the base of the ECM degradation and is due to loss of important wound healing products such as platelet‐derived growth factor (PDGF) and hepatocyte growth factor (HGF), respectively broken down by reactive oxygen species or MMPs and elastases secreted by neutrophils 23. This picture is confirmed by the analysis of chronic wound fluid (CWF) that, when compared with acute wound fluid (AWF), presents enhanced pro‐inflammatory cytokines, MMPs, neutrophil elastases along with reduced amount of growth factors 22, 24, 25 and characterises, in particular, both chronic diabetic and venous ulcers 26. Moreover, in case of chronic venous insufficiency, fibroblasts appear to be qualitatively altered 27, 28.
Wound healing in diabetic ulcers appears to be affected in a more specific way. First of all, the cellular activity is altered, with keratinocytes, epidermal cells and fibroblasts showing increased level of apoptosis and impaired migration and functioning 29, 30, 31. In addition, epidermal stem cells present a lower capacity of differentiation 32, while adipose‐derived stem cells (ADSCs) were not impaired. Because of their ability to produce growth factors, cytokines and type I collagen, the latter cells can represent a potential role in diabetic ulcers treatment 33. ECM synthesis is reduced in diabetic wounds, mainly because of an impaired fibroblast activity 34. In the same time, its degradation is faster because of the higher levels of MMPs 31. Both angiogenesis and neovascularisation are impaired in diabetic wounds, the latter because of a senesce in endothelial progenitor cells (EPCs) 17. Macro and micro angiopathy further complicate this picture.
The dermal layer is the main source of keratinocytes 35. If this structure in the depth of the wound is destroyed (e.g. deep CLUs), the only source of new regenerating cells is the dermal region all around the injury and reepithelialisation is slow, uncompleted and complicated by scarring and the conventional treatment is more often failing 36.
Current treatments for CLUs
The treatment of chronic ulcers of the lower extremities presents a therapeutic challenge. First of all, it should be focused on the causal conditions. Sanitary measures together with both surgical and medical strategies represent the basic of a comprehensive management of CLUs. In particular (i) leg elevation, compression therapy and anticoagulant treatment and surgical reduction of reflux are employed in case of venous ulcers; (ii) revascularisation, antiplatelet medications and management of risk factors are the targets in case of arterial disease; (iii) neuropathic ulcers are managed with off‐loading of pressure and with topical growth factors; (iv) debridement is frequently performed in diabetic ulcers; and (v) pressure ulcers require an off‐loading of pressure and reduction of excessive moisture, sheer and friction along with adequate nutrition. However, ulcers frequently recur 9. Of note, although topically applied growth factors (e.g. PDGF, EFG and FGF) assist the chronic wound by speeding the formation of the granulation tissue or improving epidermal cell function and giving some benefits 37, 38, 39, these are frequently unsatisfactory probably because of the local degradation of such mediators due to the chronic inflammation 40.
CLUs surgery consists of: (i) skin transplantation, including skin autograft and allo/xenografts and (ii) tissue‐engineered skin substitutes. Autografting is usually performed with a split‐thickness skin graft (STSG), that is, a tangential excision of a skin graft that includes the epidermis and part of the dermis. The autologous origin of the graft guarantees a nil risk of rejection 41. However, although this procedure improves the early healing rate of the wound and the quality of life of the patient, the rate of success of such therapy is only partial 42 even if it can be improved by a topical application of PDGF 8. Allo‐ or xenografts are used as a temporary alternative to autografting and serve as barrier and potential source of tissue‐healing factor. However, they are inevitably rejected by the host after 1 week 41. Another approach for the management of tissue injuries consists of tissue‐engineered substitutes. An example is represented by the culture of allogeneic neonatal dermal fibroblasts on a polyglactin scaffold. These cells produce ECM proteins which, in turn, replace the previous mesh that is ultimately degraded. The final result is an allogenic dermal analogue that can be used to dress the wound. Being particularly used on diabetic foot ulcers, this allograft is punctually rejected, but appears to promote keratinocyte migration and restore of the dermis, with good outcomes. Another allogeneic skin graft consists of two layers, both dermis and epidermis, respectively obtained with fibroblast and keratinocytes taken from neonatal foreskin 43. As in the previous case, this skin substitute is also ultimately rejected. However, in recent years the treatment of CLUs has shown good clinical results 44. Despite their general good therapeutic outcomes, tissue‐engineered skin substitutes are characterised by important limitations for clinical purposes. The specific disadvantages such as slow vascularisation with poor integration, rejection an high cost, poor handling properties, a short life and an inability to reconstitute skin appendages 45, 46 make these strategies far from being the conclusive solution for wound healing. With such evidences, great interest has been directed towards potential application of stem cell biology in ulcer care.
Stem cells and CLUs
The most widely accepted stem cell definition is an undifferentiated cell with three unique capacities: (i) self‐renewal (i.e. the ability to produce unaltered daughter cells by symmetric cell division), (ii) long‐term viability and (iii) potency (i.e. the possibility to generate different specialised cell types) 47, 48. Those cells that are capable of giving rise to a whole, intact organism (including both somatic and germal cell types) are defined as totipotent; pluripotent and multipotent (organ‐specific) stem cells can instead give rise to cells belonging to all three germ layers or a single organ or tissue, respectively 49.
As they can be harvested from embryonic or adult tissues, two types of stem cells can be identified: (i) pluripotent embryonic stem cells (ESCs), derived from the inner mass of the blastocysts or primordial germ cells in the germinal ridges of later embryos and (ii) uni‐ or multipotent adult stem cells (ASCs), which reside in some differentiated, adult tissues, do not complete their differentiation programme and are able to give one or few cell lineages 50. These two categories can be recognised by different expression of cell surface receptors and transcription factor, along with morphological, cytological and histological characteristics.
After the initial enthusiasm due to the possibility to obtain epidermal and dermal components, ESCs had to face essential problems that have limited their clinical applicability. First, there are important ethical issues regarding the use of human embryos for cell harvesting. However, nowadays this aspect can be, at least in part, circumvented by using a single‐cell biopsy and a single blastomer without interfering with the embryo's developmental potential 51. Second, as for the ESCs derived from other species, those obtained from human embryo are highly incapable of differentiating in specific tissues, both in vivo 52 and in vitro 50. The former phenomenon demonstrates that adult tissues cannot provide a complete environment to direct the site‐specific differentiation of ESCs 50. Nevertheless, reports have recently showed a successful differentiation of ESC‐derived skin in vitro, giving hope and drive for future researches in this field 53. Third, teratocarcinomas have arisen from the ESCs 54. Fourth, and may be more importantly, ESC‐derived skin still represents an allogeneic substitute and cannot guarantee permanent wound coverage. As allogeneic and xenogeneic grafts are already available at more moderate cost, the clinical advantage of using ESC‐derived skin in not clear.
In view of these evidences, research has largely focused its attention on ASCs. ASCs can have an endodermal, mesodermal or ectodermal origin and reside in several tissues such as central nervous system, epidermis, intestine, liver, lung and retina 55. The primary function of these cells is to serve as self‐renewing stem cells and regenerate site‐specific tissues in case of both physiological and pathological stimuli.
The rationale of the speculated employment of such cells in the clinical practice of CLUs is that: (i) despite traditional comprehensive wound management, including vascular reconstruction, many patients present non‐responding wounds, which often resulting in amputation; (ii) ASCs could help replace lost tissues as well as create those skin appendages missing in the tissue‐engineered skin substitutes 45, 46.
We will now focus on ASC therapies, including mesenchymal stem cells (MSCs), EPCs, bone‐marrow‐derived mononuclear cells (BM‐MNCs) and fibrocytes (Table 1). The large majority of preclinical studies regarding MSCs and CLUs have been conducted on murine diabetic wounds because of the more feasible nature of such models.
Table 1.
Stem cells and their therapeutic effects
| Cell type | Cell markers | Role in wound healing |
|---|---|---|
| BM‐MSCS | CD105+, CD73+, CD90+, CD45−, CD34−, CD14−, CD11b−, CD79 alpha, CD19− and HLA‐DR− | Increase cell proliferation, collagen synthesis, growth factor release, wound contraction, neovascularisation and cellular recruitment to wounds |
| ADSCs | CD31−, CD34+/−, CD45−, CD90+, CD105− and CD146− | Promote cell proliferation, collagen synthesis, promote neovessel formation and tissue remodelling |
| EPCs | CD34+, VEGFR‐2+ and CD133+ | Promote vascularisation secrete proangiogenic growth factors and cytokines, and differentiate into endothelial cells |
| BM‐MNCs | haematopoietic progenitor cell markers: CD133+, CD117+ and CD34 | Secrete angiogenic growth factors decrease local inflammation, and promote vascularisation differentiate into endothelial cells |
| MSCs markers and endothelial progenitor population: CD34+/−, CD133+ and VEGFR2+ | ||
| Fibrocytes | CD 34+, CD11b+, CD13+, MHC II+, CD86+, CD45+, collagen‐1+, procollagen‐1+, CD3−, CD4−, CD8−, CD19− and CD25− | Increasing cell proliferation ECM deposition, wound contraction and vascularisation. |
| Secrete of growth factors and chemokines |
ADSCs, adipose‐derived stem cells; BM‐MNCs, bone‐marrow‐derived mononuclear cells; BM‐MSCs, bone‐marrow‐derived mesenchymal stem cells; EPCs, endothelial progenitor cells.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs), also called mesenchymal stromal cells, are a group of non‐haematopoietic ASCs that have a mesodermal origin. First described in 1966 by Friedenstein et al. 56, they are capable of differentiating in a far greater number of lineages than their normal mesoderm fate and can give arise to endodermic and ectodermic cells, skin included 57, 58, 59, 60. MSCs can be found in almost every tissue (periosteum, tendon, muscle, synovial membrane and normal skin among the others) 61. To date, neither surface nor stemness marker allowing an accurate classification of these cells have been found, and the exact identity of MSCs in vivo is not yet clear 62. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy defines MSCs as cells that (i) are plastic adherent in standard culture conditions; (ii) express CD105, CD73 and CD90 while lacking CD45, CD34, CD14 or CD11b, CD79 alpha or CD19 and HLA‐DR molecules; (iii) can differentiate into chondroblasts, osteoblasts and adipocytes in vitro. This definition is the most commonly used in research 63. Their wide distribution along with multipotency firmly suggests an important role for MSCs in wound healing and replacement of cells that are lost in both physiological and pathological conditions. They also contribute to the digestive system, liver, musculoskeletal system, periodontal tissue and neurological homeostasis 64.
An important characteristic of MSCs is their capacity to be home to the damaged tissue sites, even when administered from an exogenous source. Central in this phenomenon is the inflammation at the site of wound, with chemokines (e.g. CXCL12, CXCL4 and CCR2) 65, 66, adhesion molecules (such as P‐selectin and VCAM‐1) 67 and matrix metalloproteinases (MMPs, such as MMP‐2) being the most implicated. Furthermore, all these molecules are induced by inflammatory cytokines such as TNF and IL‐1 68, 69, which ultimately control stem cells' contribution at the site of injury.
Along with a multilineage differentiation potentiality, MSCs are involved in all four phases of wound repair. First, they can interact with cells of both the innate and adaptive immune systems and possess anti‐inflammatory responses 70, 71, 72. In one study, the application of MSCs to an active inflammatory site resulted in a decrease of pro‐inflammatory cytokines (such as TNF‐α and interferon‐γ) with a concomitant increase of anti‐inflammatory cytokines (namely IL‐10 and IL‐4) and T‐reg cells 73. Moreover, MSCs possess an anti‐microbial activity that is of great importance in wound and CLUs healing. This is mediated by both direct (i.e. the secretion of anti‐microbial factors) and indirect (i.e. the enhancement of the immune response of the host) mechanisms 74. The secretion of paracrine mediators at the site of inflammation is another way of the mesenchymal cell support in wound healing. In particular, growth factors (such as VEGF, PDGF, EGF, bFGF, FGF‐23 and TGF‐β) 75, 76 and cytokines (such as IL‐6 and CCL‐2) 77, 78, 79 are responsible for angiogenesis and both recruitment and functioning of macrophages, endothelial cells, keratinocytes and fibroblasts, which are the main actors of the physiological wound healing process. Of note, the secretion of VEGF and HGF, together with the maintenance of a good balance between tween TGF‐β1 and TGF‐β3 makes MSCs important in prevention of scarring 80, 81, 82. Although capable of transdifferentiating into vascular endothelial cells and skin components 83, it is currently believed that the paracrine activity of MSCs represents the primary mechanism by which these cells contribute to tissue healing mainly because of poor engraftment and survival of MSCs at the site of injury 75.
The unique anti‐inflammatory activity of MSCs is capable of limiting the host immunoreaction against themselves in case of allogeneic transplantation. In addition, although presenting MHC Class I alloantigens, MSCs are characterised by minimal levels of surface immunostimulatory antigens such as MHC Class II alloantigens and co‐stimulatory molecules including CD80 (B7‐1), CD86 (B7‐2) and CD40 84. These evidences support a low immunogenicity and a high anti‐rejection activity of the allogeneic MSCs, at least in the short term and in particular transplanting routes and microenvironments 84, 85, 86 and little or no rejection was observed after transplantation when allogeneic MSCs were administered systemically 87, 88.
In view of the above, MSCs have been employed in tissue regeneration medicine in two different ways: (i) replacing the lost tissue, via transplantation or construction of bio‐engineered tissues and (ii) attracting in vivo resident stem cells of the patient to the site of injury.
MSCs can easily be obtained from the bone marrow, adipose tissue, umbilical cord, human placenta, muscle, dermis, nerve tissue and lung, and can be further expanded in vitro and cryopreserved 87, 89, 90, 91, 92, 93, 94, 95, 96. Thus, at least in theory, all the above can be used in tissue regenerative medicine. However, because of practical and ethical issues, most of the preclinical and clinical studies were conducted on bone‐marrow‐derived mesenchymal stem cells (BM‐MSCs) and ADSCs and to date there is a huge amount of data exalting their important contribution to tissue healing, including limb ulcer models, in every route of administration 97, 98, 99, 100, 101, 102, 103, 104, 105, 106.
BM‐MSCs, also known as marrow stromal cells, are self‐renewing stem cells that are localised in the bone marrow. They represent a rare population of bone marrow cells (0·001–0·01% of the nucleated figures and 1/10 of HSCs), but are expandable in vitro 83. Although there is still a paucity of clinical data, their contribution to CLU healing is easily conceivable in light of the above. In their study, Kwon et al. demonstrated that systemic and local administration of BM‐MSCs improved wound healing in a diabetic rat; this was mainly because of an increased production of collagen types I and V at the site of injury 107.
ADSCs, also known as adipose‐derived stromal cells, adipose‐derived mesenchymal progenitor cells and processed lipoaspirate cells (PLAs), have such variegated nomenclature mainly because of a lack of consensus and a still changing knowledge of both phenotype and function of these cells 17. However, as reported in Table 1, the International Society for Cellular Therapy considers both CD34+ and CD34 as ADSCs 108. Recent evidences suggest that CD34+ ADSCs can be characterised as having a greater proliferative potentiality, while CD34− ADSCs have a greater differentiating capacity 108, 109.
As they can be extracted in large amounts with minor donor site morbidity and they have major proliferative capacities when compared to BM‐MSCs, ADSCs represent an intriguing tool for both chronic wound and CLU treatment. However, clinical trials on CLUs are still lacking 110, 111.
An important limitation of MSC employment in both chronic wound and ulcer management is represented by the long duration and complex procedures required for their expansion in vitro 17.
Endothelial progenitor cells
Human EPCs are a subset of circulating bone‐marrow‐derived figures that have been generally defined as cells (i) expressing a surface antigenic panel similar to that characterising the vascular endothelial cells; (ii) capable of adhering to the endothelium at the site of hypoxia/ischaemia; and (iii) capable of participating in neovascularisation 112. To date no specific marker has been known by which EPCs can be defined, although they express CD34, KDR (VEGFR‐2) and CD133 markers 17.
Since EPCs can be recruited from bone marrow and peripheral blood to the sites of hypoxia/ischaemia and are able to participate in neovascularisation processes, it is currently believed that these cells can be important actors in tissue healing and numerous preclinical studied have been published to this effect 113, 114. They indirectly participate in wound healing by secreting important mediators such as VEGF, hepatic growth factor (HGF), angiogenin 1, stroma derived factor (SDF)‐1α, insulin‐like growth factor (IGF)‐1, along with inducing endothelial nitric oxide synthase (eNOS)/inducible nitric oxide synthase (iNOS) 115.
Clinical data regarding EPCs and leg ulcers is still lacking. Several works performed in murine models of diabetic wounds have found that both augmented neovascularisation and re‐epithelialisation can be linked to the direct and indirect effects of ESC‐derived EPCs applied on wounds 116.
However, EPCs are characterised by similar problems as in MSCs when applied to clinical practice 117.
Bone‐marrow‐derived mononuclear cells
The term BM‐MNCs identifies a wide group of cells encompassing both staminal and differentiated figures in which haematopoietic stem cells, MSCs, EPCs and precursor cells along with their progeny are included 118. Because of their abundance in both peripheral blood and bone marrow, MNCs do not need in vitro expansion and are therefore a feasible source of staminal cells 118.
Because of their heterogeneity, several cell markers characterise BM‐MNCs. Two cell sets are mostly involved in the angiogenetic process: (i) haematopoietic progenitor cells, which are CD133+, CD117+ and CD34+, and MSCs, particularly the endothelial progenitor population composed mainly of CD34−/CD133+/VEGFR2+ and CD34+/CD133+/VEGFR2+ cells 119.
Several clinical trials firmly demonstrate that MNCs improve leg ulcers 120, 121. However, the specific therapeutic mechanisms still remain unknown. One hypothesis suggests that an augmented angiogenesis represents a central point in MNC‐mediated wound healing. Such speculation is supported by the elevated expression of angiogenic growth factors found after MNC transplantation 17. It appears that MNCs can even differentiate into endothelial cells, thus improving the neovascularisation at the wound site 119, 122, 123, 124. Finally, an anti‐inflammatory role of MNCs has been invoked 83. It can be concluded that although the complex makeup of MNCs makes it difficult to study them in a detailed manner, these cells represent a practical future tool for the clinical setting mainly because of their avoidance of an in vitro expansion.
Fibrocytes
In 1994, Bucala et al. 125 found that circulating, bone‐marrow‐derived ‘fibrocytes’ had the ability to adopt a mesenchymal phenotype and participated in scar formation. Fibrocytes represent a small subset (0·1–0·5%) of leukocytes and can be mostly found in the peripheral blood 126. They are characterised by a spindle shape when cultured in vitro and present a combination of markers (such as CD34, CD11b+, CD13+, MHC II+, CD86+ and CD45+) which is common to both fibroblasts and monocytes. Stromal cell markers (like collagen I, vimentin and fibronectin) further distinguish these cells 17.
Fibrocytes showed a great capacity to migrate to wound or chronic inflamed sites and localise to areas of ongoing ECM deposition 127 and an important role of these cells in wound healing is supported by several works. In some studies fibrocytes showed increase in ECM deposition, vascularisation and wound contraction 128. Moreover, they have been found capable of improving reepithelialisation, angiogenesis and local cell proliferation 127. A paracrine secretion is also speculated, with growth factors (VEGF, bFGF, TGF‐β, ODGF), chemokines and ECM augmented in wounds treated with fibrocytes 127, 128, 129. Although differentiation into mesenchymal cells and contractile myofibroblast has been reported 126, 127, their ability to do it in vivo is still controversial.
Fibrocytes are currently studied in several diseases, such as human hypertrophic scars, nephrogenic systemic fibrosis, human atherosclerotic pulmonary diseases characterised by repeated cycles of inflammation and repair (such as asthma), chronic pancreatitis, chronic cystitis and tumour metastasis 126, 127. To date there are still few studies exploring the therapeutic potential of circulating fibrocytes in CLUs. However, in their study, Behjati et al. 130 were not able to use the patient's fibrocytes on leg diabetic ulcers because of the rarity of such cells in the peripheral blood.
Stem cells and the future of regenerative medicine
The aim of the novel field of regenerative medicine is to restore structure and function of damaged tissues, and stem cells represent a promising approach to wound healing through the release of soluble mediators that modulate chronic inflammation thereby speeding up healing processes. However, significant drags remain on improving progenitor cell selection and tissue delivery. Innovative techniques such as microfluidic single‐cell characterisation seem to be promising for identifying and isolating the most appropriate cells for therapeutic use, as well as new and effective delivery vehicles in order to ameliorate the targeting of damaged tissues 131.
In the new era of regenerative medicine a new class of stem cells, has recently been discovered, the induced pluripotent stem cells (iPSCs). The use of iPSCs may allow the generation of autologous pluripotent stem cell population derived from differentiated adult tissues, being also non‐immunogenic. In this context, iPSCs have at the same time combined advantages of the pluripotency of ESCs and the availability of ASCs, but still some concerns remain with the utilisation of ASCs: difficulties with genetic manipulation, safety profile, efficiency and cost‐effectiveness 131, 132.
Conclusions
Chronic leg ulceration still represents an important problem, especially in the western countries, and new therapeutic strategies are needed. The stem‐cell‐based tissue regeneration medicine is proving its potentiality in tissue healing and regeneration. Although functional stem cell units have been described throughout all layers of human skin, other niches can be found throughout the body. Both bone marrow and adipose tissue derived stem cells appear to be important in tissue healing, but a necessity of a long‐lasting and complicated in vivo expansion still limits their clinical practice. BM‐MNCs are easily found in the peripheral blood, do not need a culture and are now extensively evaluated for leg ulcer treatment. Finally, more studies are needed to completely understand the physiological and pathological role of EPC fibrocytes and the new promising iPSCs. Considering the current available evidence regarding therapeutic potential of ASCs in tissue healing, we are strongly convinced that, in the next future, they will represent a reality in clinical practice of leg ulceration.
References
- 1. Serra R, Grande R, Butrico L, Montemurro R, De Caridi G, Fugetto F, Dominijanni A, Gallelli L, Greto Ciriaco A, Vitagliano T, Greco M, de Franciscis S. Skin grafting and topical application of platelet gel in the treatment of vascular lower extremity ulcers. Acta Phlebol 2014;15:129–36. [Google Scholar]
- 2. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Vitagliano T, Greco M, de Franciscis S. Skin grafting followed by low‐molecular‐weight heparin long‐term therapy in chronic venous leg ulcers. Ann Vasc Surg 2012;26:190–7. [DOI] [PubMed] [Google Scholar]
- 3. Serra R, Butrico L, Ruggiero M, Rossi A, Buffone G, Fugetto F, De Caridi G, Massara M, Falasconi C, Rizzuto A, Settimio UF, Perri P, Dardano G, Grande R, de Franciscis S. Epidemiology, diagnosis and treatment of chronic leg ulcers: a systematic review. Acta Phlebol 2014;15:1–2. [Google Scholar]
- 4. Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, Amato B, Gallelli L, de Franciscis S. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus . Expert Rev Anti Infect Ther 2015;13:605–13. [DOI] [PubMed] [Google Scholar]
- 5. Mekkes JR, Loots MA, Van Der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003;148:388–401. [DOI] [PubMed] [Google Scholar]
- 6. de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, Buffone G, Serra R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J 2015;12:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serra R, Grande R, Scarcello E, Buffone G, de Franciscis S. Angiosome‐targeted revascularisation in diabetic foot ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J 2013;10:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 2013;413604:9. [Google Scholar]
- 10. Nelzen O. Leg ulcers: economic aspects. Phlebology 2000;15:110–4. [Google Scholar]
- 11. Beckrich K, Aronovitch SA. Hospital‐acquired pressure ulcers: a comparison of costs in medical versus surgical patients. Nurs Econ 1999;17:263–71. [PubMed] [Google Scholar]
- 12. Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg 2002;183:445–56. [DOI] [PubMed] [Google Scholar]
- 13. Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Acta Phlebol 2013;14:99–107. [Google Scholar]
- 14. Serra R, Grande R, Butrico L, Buffone G, Caliò FG, Squillace A, Rizzo BA, Massara M, Spinelli F, Ferrarese AG, De Caridi G, Gallelli L, de Franciscis S. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J 2014. DOI: 10.1111/iwj.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2015;12:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serra R, Gallelli L, Conti A, De Caridi G, Massara M, Spinelli F, Buffone G, Caliò FG, Amato B, Ceglia S, Spaziano G, Scaramuzzino L, Ferrarese AG, Grande R, de Franciscis S. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and ulcers of lower limbs. Drug Des Devel Ther 2014;8:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang M, Sheng L, Zhang TR, Li Q. Stem cell therapy for lower extremity diabetic ulcers: where do we stand? Biomed Res Int 2013;2013:462179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Compagna R, Amato B, Massa S, Amato M, Grande R, Butrico L, de Franciscis S, Serra R. Cell Therapy in patients with Critical Limb Ischemia. Stem Cells Int 2015;2015:931420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorrell JM, Caplan AI. Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res Ther 2010;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amato B, Compagna R, Amato M, Grande R, Butrico L, Rossi A, Naso A, Ruggiero M, de Franciscis S, Serra R. Adult vascular wall‐resident multipotent vascular stem cells, matrix metalloproteinases and arterial aneurysms. Stem Cells Int 2015;2015:434962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson L, Lee AJ, Gallagher K, Carmichael SJ, Evans CJ, McKinstry BH, Fraser SC, Allan PL, Weller D, Ruckley CV, Fowkes FG. Risk factors for chronic ulceration in patients with varicose veins: a case control study. J Vasc Surg 2009;49:1490–8. [DOI] [PubMed] [Google Scholar]
- 22. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 23. Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, Grande R, Serra R, de Franciscis S. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999;81:189–95. [DOI] [PubMed] [Google Scholar]
- 25. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–8. [DOI] [PubMed] [Google Scholar]
- 26. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–7. [DOI] [PubMed] [Google Scholar]
- 27. Mendez MV, Stanley A, Phillips T, Murphy M, Menzoian JO, Park HY. Fibroblasts cultured from distal lower extremities in patients with venous reflux display cellular characteristics of senescence. J Vasc Surg 1998;28:1040–50. [DOI] [PubMed] [Google Scholar]
- 28. Raffetto JD, Mendez MV, Marien BJ, Byers HR, Phillips TJ, Park HY, Menzoian JO. Changes in cellular motility and cytoskeletal actin in fibroblasts from patients with chronic venous insufficiency and in neonatal fibroblasts in the presence of chronic wound fluid. J Vasc Surg 2001;33:1233–41. [DOI] [PubMed] [Google Scholar]
- 29. Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem 2008;56:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desta T, Li J, Chino T, Graves DT. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. J Dent Res 2010;89:609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 2003;162:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhong QL, Liu FR, Liu DW, Peng Y, Zhang XR. Expression of β‐catenin and cyclin D1 in epidermal stem cells of diabetic rats. Mol Med Rep 2011;4:377–81. [DOI] [PubMed] [Google Scholar]
- 33. Nambu M, Ishihara M, Kishimoto S, Yanagibayashi S, Yamamoto N, Azuma R, Kanatani Y, Kiyosawa T, Mizuno H. Stimulatory effect of autologous adipose tissue‐derived stromal cells in an atelocollagen matrix on wound healing in diabetic db/db mice. J Tissue Eng 2011;2011:158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lateef H, Stevens MJ, Varani J. All‐trans‐retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture. Am J Pathol 2004;165:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werner GR. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 36. Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng 2009;37:399–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 38. Falanga V, Eaglstein WH, Bucalo B, Katz MH, Harris B, Carson P. Topical use of human recombinant epidermal growth factor (h‐EGF) in venous ulcers. J Dermatol Surg Oncol 1992;18:604–6. [DOI] [PubMed] [Google Scholar]
- 39. Loot MA, Kenter SB, Au FL, van Galen WJ, Middelkoop E, Bos JD, Mekkes JR. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF‐I, bFGF and PDGF‐AB compared to controls. Eur J Cell Biol 2002;81:153–60. [DOI] [PubMed] [Google Scholar]
- 40. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg 2003;90:133–46. [DOI] [PubMed] [Google Scholar]
- 41. Tzaneva S, Heere‐Ress E, Kittler H, Böhler K. Surgical treatment of large vascular leg ulcers: a retrospective review evaluating risk factors for healing and recurrence. Dermatol Surg 2014;40:1240–8. [DOI] [PubMed] [Google Scholar]
- 42. Kirsner RS, Eaglstein WH, Kerdel FA. Split‐thickness skin grafting for lower extremity ulcerations. Dermatol Surg 1997;23:85–91. [DOI] [PubMed] [Google Scholar]
- 43. Saap LJ, Donohue K, Falanga V. Clinical classification of bioengineered skin use and its correlation with healing of diabetic and venous ulcers. Dermatol Surg 2004;30:1095–100. [DOI] [PubMed] [Google Scholar]
- 44. Jones JE, Nelson EA, Al‐Hity A. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev 2013;31:CD001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hassan WU, Greiser U, Wang W. Role of adipose‐derived stem cells in wound healing. Wound Repair Regen 2014;22:313–25. [DOI] [PubMed] [Google Scholar]
- 46. Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns 2007;33:282–91. [DOI] [PubMed] [Google Scholar]
- 47. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell 2000;100:157–68. [DOI] [PubMed] [Google Scholar]
- 48. Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol 2007;25:73–8. [DOI] [PubMed] [Google Scholar]
- 49. Sharma RK, John JR. Role of stem cells in the management of chronic wounds. Indian J Plast Surg 2012;45:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stocum DL. Stem cells in regenerative biology and medicine. Wound Repair Regen 2001;9:429–42. [DOI] [PubMed] [Google Scholar]
- 51. Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature 2006;444:481–5. [DOI] [PubMed] [Google Scholar]
- 52. Moodley Y, Thompson P, Warburton D. Stem cells: a recapitulation of development. Respirology 2013;18:1167–76. [DOI] [PubMed] [Google Scholar]
- 53. Coraux C, Hilmi C, Rouleau M, Spadafora A, Hinnrasky J, Ortonne JP, Dani C, Aberdam D. Reconstituted skin from murine embryonic stem cells. Curr Biol 2003;13:849–53. [DOI] [PubMed] [Google Scholar]
- 54. Evans MJ, Kaufman M. Pluripotential cells grown directly from normal mouse embryos. Cancer Surv 1983;2:185–208. [Google Scholar]
- 55. Can A. A concise review on the classification and nomenclature of stem cells. Turk J Hematol 2008;25:57–9. [PubMed] [Google Scholar]
- 56. Friedenstein AJ, Piatetzky‐Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966;16:381–90. [PubMed] [Google Scholar]
- 57. Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol 2011;26:941–51. [DOI] [PubMed] [Google Scholar]
- 58. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–4. [DOI] [PubMed] [Google Scholar]
- 59. Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin‐substitute wound healing. Br J Dermatol 2005;153:29–36. [DOI] [PubMed] [Google Scholar]
- 60. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell 2001;105:369–77. [DOI] [PubMed] [Google Scholar]
- 61. Sellheyer K, Krahl D. Cutaneous mesenchymal stem cells. Current status of research and potential clinical applications. Hautarzt 2010;61:429–34. [DOI] [PubMed] [Google Scholar]
- 62. Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 2014;32:1408–19. [DOI] [PubMed] [Google Scholar]
- 63. Tsai TL, Wang B, Squire MW, Guo LW, Li WJ. Endothelial cells direct human mesenchymal stem cells for osteo‐ and chondro‐lineage differentiation through endothelin‐1 and AKT signaling. Stem Cell Res Ther 2015. May 1;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 2013;34:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wynn RF, Hart CA, Corradi‐Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004;104:2643–5. [DOI] [PubMed] [Google Scholar]
- 66. Belema‐Bedada F, Uchida S, Martire A, Kostin S, Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT‐mediated clustering of CCR2. Cell Stem Cell 2008;2:566–75. [DOI] [PubMed] [Google Scholar]
- 67. Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006;108:3938–44. [DOI] [PubMed] [Google Scholar]
- 68. Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica 2007;92:897–904. [DOI] [PubMed] [Google Scholar]
- 69. Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine‐induced intercellular adhesion molecule‐1 and vascular cell adhesion molecule‐1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010;184:2321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Newman RE, Yoo D, LeRoux MA, Danilkovitch‐Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets 2009;8:110–23. [DOI] [PubMed] [Google Scholar]
- 71. Uccelli A, Moretta L, Vito PV. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–36. [DOI] [PubMed] [Google Scholar]
- 72. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011;6:457–78. [DOI] [PubMed] [Google Scholar]
- 73. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–22. [DOI] [PubMed] [Google Scholar]
- 74. Maxson S, Lopez EA, Yoo D, Danilkovitch‐Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–84. [DOI] [PubMed] [Google Scholar]
- 78. Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL‐6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun 2007;361:745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, Sun K, Jiang GC, Zhao X, Li R, Gao L, Zhao QD, Wu MC, Wei LX. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem 2011;286:25007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ono I, Yamashita T, Hida T, Jin HI, Ito Y, Hamada H, Akasaka Y, Ishii T, Jimbow K. Combined administration of basic fibroblast growth factor protein and the hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. Wound Repair Regen 2004;12:67–79. [DOI] [PubMed] [Google Scholar]
- 81. Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF‐beta 1 and TGF‐beta 2 or exogenous addition of TGF‐beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108:985–1002. [DOI] [PubMed] [Google Scholar]
- 82. Colwell AS, Beanes SR, Soo C, Dang C, Ting K, Longaker MT, Atkinson JB, Lorenz HP. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg 2005;115:204–12. [PubMed] [Google Scholar]
- 83. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–59. [DOI] [PubMed] [Google Scholar]
- 84. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 2005;12:47–57. [DOI] [PubMed] [Google Scholar]
- 85. Gu LH, Zhang TT, Li Y, Yan HJ, Qi H, Li FR. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell Mol Immunol 2015;12:444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti‐donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol 2013;91:40–51. [DOI] [PubMed] [Google Scholar]
- 87. Mansilla E, Marin GH, Sturla F, Drago HE, Gil MA, Salas E, Gardiner MC, Piccinelli G, Bossi S, Salas E, Petrelli L, Iorio G, Ramos CA, Soratti C. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc 2005;37:292–4. [DOI] [PubMed] [Google Scholar]
- 88. Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol 2006;176:2864–71. [DOI] [PubMed] [Google Scholar]
- 89. Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008;2:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 91. Anjos‐Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA‐1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood 2007;109:1298–306. [DOI] [PubMed] [Google Scholar]
- 92. In't Anker PS, Scherjon SA, Kleijburg‐van der Keur C, de Groot‐Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22:1338–45. [DOI] [PubMed] [Google Scholar]
- 93. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MK. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng 2001;7:211–28. [DOI] [PubMed] [Google Scholar]
- 94. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Zie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow‐derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double‐blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36. [DOI] [PubMed] [Google Scholar]
- 95. Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow‐derived cells. Arch Dermatol 2003;139:510–6. [DOI] [PubMed] [Google Scholar]
- 96. Vojtassák J, Danisovic L, Kubes M, Bakos D, Jarábek L, Ulicná M, Blasko M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett 2006;27(Suppl 2):134–7. [PubMed] [Google Scholar]
- 97. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow‐derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–312. [DOI] [PubMed] [Google Scholar]
- 98. Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, Takakura Y, Okuchi K, Nonomura A. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 2008;121:860–77. [DOI] [PubMed] [Google Scholar]
- 99. Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–66. [DOI] [PubMed] [Google Scholar]
- 100. Kesting MR, Loeffelbein DJ, Steinstraesser L, Muecke T, Demtroeder C, Sommerer F, Hoelzle F, Wolff KD. Cryopreserved human amniotic membrane for soft tissue repair in rats. Ann Plast Surg 2008;60:684–91. [DOI] [PubMed] [Google Scholar]
- 101. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24. [DOI] [PubMed] [Google Scholar]
- 102. Blanton MW, Hadad I, Johnstone BH, Mund JA, Rogers PI, Eppley BL, March KL. Adipose stromal cells and platelet‐rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg 2009;123(2 Suppl):56S–64. [DOI] [PubMed] [Google Scholar]
- 103. Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose‐derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 2009;53:96–102. [DOI] [PubMed] [Google Scholar]
- 104. Nambu M, Ishihara M, Nakamura S, Mizuno H, Yanagibayashi S, Kanatani Y, Hattori H, Takase B, Ishizuka T, Kishimoto S, Amano Y, Yamamoto N, Azuma R, Kiyosawa T. Enhanced healing of mitomycin C‐treated wounds in rats using inbred adipose tissue‐derived stromal cells within an atelocollagen matrix. Wound Repair Regen 2007;15:505–10. [DOI] [PubMed] [Google Scholar]
- 105. Uysal AC, Mizuno H, Tobita M, Ogawa R, Hyakusoku H. The effect of adipose‐derived stem cells on ischemia‐reperfusion injury: immunohistochemical and ultrastructural evaluation. Plast Reconstr Surg 2009;124:804–15. [DOI] [PubMed] [Google Scholar]
- 106. Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, Rodeheaver GT, Peirce SM, Katz AJ. Human adipose‐derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A 2010;16:1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, Chopp M, McIntosh K, Arbab AS, Dulchavsky SA, Gautam SC. Treatment with bone marrow‐derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 2008;5:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose‐derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 2012;24:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bailey AM, Kapur S, Katz AJ. Characterization of adipose‐derived stem cells: an update. Curr Stem Cell Res Ther 2010;5:95–102. [DOI] [PubMed] [Google Scholar]
- 110. Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS. Safety and effect of adipose tissue‐derived stem cell implantation in patients with critical limb ischemia. Circ J 2012;76:1750–60. [DOI] [PubMed] [Google Scholar]
- 111. Sarasua JG, Lopez SP, Viejo MA, Basterrechea MP, Rodriguez AF, Gutierrez AF, Gala JG, Menendez YM, Augusto DE, Arias AP, Hernandez JO. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med 2011;34:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2012;2:a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Deng X, Szabo S, Chen L, Paunovic B, Khomenko T, Tolstanova G, Tarnawski AS, Jones MK, Sandor Z. New cell therapy using bone marrow‐derived stem cells/endothelial progenitor cells to accelerate neovascularization in healing of experimental ulcerative colitis. Curr Pharm Des 2011;17:1643–51. [DOI] [PubMed] [Google Scholar]
- 114. Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 2005;23:1571–8. [DOI] [PubMed] [Google Scholar]
- 115. Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011;29:1650–5. [DOI] [PubMed] [Google Scholar]
- 116. Lee MJ, Kim J, Lee KI, Shin JM, Chae JI, Chung HM. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy 2011;13:165–78. [DOI] [PubMed] [Google Scholar]
- 117. Sheng L, Yang M, Du Z, Yang Y, Li Q. Transplantation of stromal vascular fraction as an alternative for accelerating tissue expansion. J Plast Reconstr Aesthet Surg 2013;66:551–7. [DOI] [PubMed] [Google Scholar]
- 118. Yang M, Sheng L, Li H, Weng R, Li QF. Improvement of the skin flap survival with the bone marrow‐derived mononuclear cells transplantation in a rat model. Microsurgery 2010;30:275–81. [DOI] [PubMed] [Google Scholar]
- 119. Murphy MP, Lawson JH, Rapp BM, Dalsing MC, Klein J, Wilson MG, Hutchins GD, March KL. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation‐free survival in patients with critical limb ischemia. J Vasc Surg 2011;53:1565–74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jain P, Perakath B, Jesudason MR, Nayak S. The effect of autologous bone marrow‐derived cells on healing chronic lower extremity wounds: results of a randomized controlled study. Ostomy Wound Manage 2011;57:38–44. [PubMed] [Google Scholar]
- 121. Yamaguchi Y, Yoshida S, Sumikawa Y, Kubo T, Hosokawa K, Ozawa K, Hearing VJ, Yoshikawa K, Itami S. Rapid healing of intractable diabetic foot ulcers with exposed bones following a novel therapy of exposing bone marrow cells and then grafting epidermal sheets. Br J Dermatol 2004;151:1019–28. [DOI] [PubMed] [Google Scholar]
- 122. Pedroso DC, Tellechea A, Moura L, Fidalgo‐Carvalho I, Duarte J, Carvalho E, Ferreira L. Improved survival, vascular differentiation and wound healing potential of stem cells co‐cultured with endothelial cells. PLoS One 2011;6:e16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Okuno Y, Nakamura‐Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow‐derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 2012;117:5264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, Tonn T, Seeger F, Dimmeler S, Lindhoff‐Last E, Zeiher AM, PROVASA Investigators . Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized‐start, placebo‐controlled pilot trial (PROVASA). Circ Cardiovasc Interv 2011. Feb 1;4:26–37. [DOI] [PubMed] [Google Scholar]
- 125. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 126. Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen‐secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004;36:598–606. [DOI] [PubMed] [Google Scholar]
- 127. Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow‐derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007;87:858–70. [Epub 2007 Jul 2]. [DOI] [PubMed] [Google Scholar]
- 128. Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re‐epithelialization, contraction, and angiogenesis. Ann Surg 2011;254:1066–74. [DOI] [PubMed] [Google Scholar]
- 129. Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 2001;15:2215–24. [DOI] [PubMed] [Google Scholar]
- 130. Behjati M, Hashemi M, Shoarayenejati A, Karbalaie K, Nasr‐Esfahani MH. Safety, efficacy and pitfalls of fibrocyte application in the treatment of diabetic foot ulcer. Int Wound J 2015;12:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC. Stem cells in wound healing: the future of regenerative medicine? a mini‐review. Gerontology 2015. DOI: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 132. Mizuno H, Tobita M, Uysal AC. Concise review: adipose‐derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012;30:804–10. [DOI] [PubMed] [Google Scholar]


