Abstract
Patients undergoing surgery in the park‐bench position are at high risk of developing intraoperatively acquired pressure ulcers (IAPUs). The purpose was to examine retrospectively risk factors associated with IAPUs in the park‐bench position. This study was conducted at a general hospital during the period of September 2010 to September 2012. Twenty‐one potential risk factors were evaluated using data obtained from the hospital database. IAPUs developed in 30 of 277 patients (11%). Perspiration was statistically found to be independently associated with IAPUs [OR 3·09, 95% confidence interval (Cl) 1.07–8·58, P = 0·037]. A length of surgery of more than 6 hours was identified to be likely associated with IAPUs (OR 2·64, 95% Cl 0·84–9·08, P = 0·095) compared with less than 6 hours. Furthermore, there was an interaction between the length of surgery and the core temperature; that is, when the length of surgery was more than 6 hours, a core temperature of more than 38·1°C at the end of surgery had a higher odds ratio (8·45, 95% Cl 3·04–27·46, P < 0·001) than that at a lower core temperature (3·20, 95% Cl 1·23–8·78, P = 0·017). These results suggest that perspiration and core temperature are preventable causative factors of pressure ulcers, even under conditions of prolonged surgery in the park‐bench position.
Keywords: Core temperature, Perioperative nursing, Perspiration, Pressure ulcer prevention, Surgery
Introduction
Pressure ulcers are defined as ‘localized (sites of) injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure, or pressure in combination with shear’ 1. Patients undergoing surgery constitute a high‐risk group for pressure ulcers. The incidence of intraoperatively acquired pressure ulcers (IAPUs) is reported to range from 14% to 66% 2, 3, 4, as the patient must maintain the same position on the operating table for a prolonged time and is unable to move when under general anaesthesia. Although operating table mattresses and positioning devices are often used, repositioning, which is generally recommended to prevent pressure ulcers 1, is difficult during surgery because the safety and exposure of the surgical site are a priority.
Among the various surgical positions, including supine, prone and so on, the incidence of pressure ulcers is especially high in subjects placed in the park‐bench position. The park‐bench position is a specific lateral position used during neurosurgery to treat the cerebellopontine angle and vascular lesions (Figure 1) 5. IAPUs easily develop in the park‐bench position because the area of contact in this position is only half that obtained in the supine position as the patient's head and part of the trunk are fixed along the outside of the operating table. In our operating theatre, the incidence of IAPUs in the park‐bench position is as high as 30%, indicating the importance of preventing this complication 6.
Figure 1.
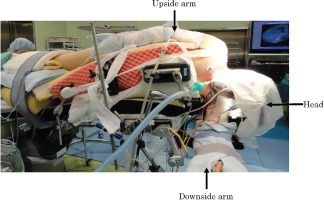
Park‐bench position, The patient's head is clamped in a Mayfield frame, and the lateral trunk is fixed on the main operating table with the upside arm positioned along the lateral trunk and the downside arm fixed on an external arm board.
Many previous studies have reported intrinsic factors for IAPUs, including age 7, gender 8, a low Braden scale score 9, low BMI 10, diabetes 11, 12, hypertension 12 and congestive heart failure 7, and extrinsic factors, including the length of surgery 11, 12, 13, 14, 15, 16, 17, surgical positioning 18, 19, 20, 21, surgical procedure 21, 22, 23, 24, hypotension 25, hypothermia 9, 26, amount of bleeding 27, American Society of Anesthesiologists Physical Status (ASA PS) classification 8, 9 and use of an operating table mattress 28 in the supine, prone, lithotomy and lateral positions.
Risk factors in the park‐bench position remain unclear, as some of these factors are not applicable for this position. For example, hypothermia rarely occurs in the park‐bench position because the patient's core temperature is usually maintained at high level under a full drape using a warming device to prevent adverse effects. Additionally, skin moisture due to perspiration may occur in the park‐bench position, whereas this factor is mostly caused by incontinence in the general unit and is not a factor in the operating theatre. However, the effects of perspiration on pressure ulcer formation are not well examined in surgical research because perspiration is rarely noted in other general surgical positions based on the surgical procedure, amount of bleeding and insensible water loss. Therefore, IAPU risk factors specific to the park‐bench position must be investigated. The purpose of this retrospective study was to clarify the risk factors for IAPUs in the park‐bench position.
Methods
Study design
We conducted a retrospective, observational study of all patients consecutively hospitalised for surgery in a 500‐bed acute care hospital in Japan between September 2010 and September 2012.
The institutional review board of Tokyo Metropolitan Police Hospital (#35) approved this study. Written informed consent for the use of data from the medical records was obtained from the patients on admission.
Setting and participants
Tokyo Metropolitan Police Hospital is an urban, acute care hospital. Approximately 5000 surgeries are performed at this hospital each year. We made the prediction that the incidence of pressure ulcers was 30%, α = 0·05, power of 80%, and the ratio of with and without perspiration was 1:6. Finally, we estimated that a sample size of 270 patients was needed for this study. The inclusion criteria, as determined according to the electronic records, were met in 309 patients. The exclusion criteria included patients who underwent emergency surgery or repeated surgery or demonstrated missing data for the risk assessment.
Procedures
In our hospital, surgery in the park‐bench position is conducted in accordance with the following standard procedures.
Preoperative phase
Autologous blood collection (400 ml) is performed 2–3 weeks before the scheduled date of surgery. The patient is admitted to the hospital 2 days before the day of surgery, and a blood test is carried out on admission. There were no limitations of mobility after hospitalisation.
During hospitalisation, preventive care for pressure ulcers is provided based on the Japanese guidelines 29. Risk factors associated with pressure ulcer development are evaluated according to the Japanese Ohura‐Hotta (OH) scale 30 on admission by unit nurses, which includes items of self‐sustainable ability, morbid bony prominences, joint contractures and oedema. Self‐sustainable ability to move unassisted is classified as assisted (3), intermediate (1·5) or unassisted (0). Morbid bony prominence is evaluated by the vertical interval between the centre of sacrum and the region 8 cm away from the sacrum and is classified as severe (3), intermediate (1·5) or none (0). Joint contracture is classified as presence (1) or none (0). Oedema is evaluated with the depth after releasing the gentle pressure of a finger at the dorsal foot, and it is classified as presence (1) or none (0). In addition, dryness of the skin is assessed macroscopically throughout the patient's body.
Intraoperative phase
The patient's head is fixed in the Mayfield clamp under total intravenous anaesthesia, and the needle electrode is fixed for cranial nerve monitoring on the face. The patient is subsequently placed in the park‐bench position on the operating table, which was a product of MAQUET; Gmph & Co. KG, Tokyo, Japan, made of urethane. And laid a urethane foam mattress and gel pads are used to achieve pressure distribution and a ‘vacuum bean bag’ and side plates are used for fixation, with the outside of the vacuum bean bag fixed at four locations to the side plate. The urethane foam mattress is composed of double layers measuring 6·5 cm in thickness (Soft Nurse Yellow Pink®, LAC Healthcare Ltd., Tokyo, Japan). The underside of the upper limbs in contact with the operating table is placed on armrests and the opposite side of the upper limbs is fixed along the trunk with cotton thread (8 cm in width) without being placed on the armrests. The lower limbs are fixed using few sponges (EM®, Toyo Soflantec Co., Tokyo, Japan) to prevent the knees from overlapping. During surgery, the patient's rectal temperature is monitored using a temperature probe (NOVATEMP® General Purpose Temperature Probes 10‐Fr 5–15610,NIHON KOHDEN Co., Tokyo, Japan), and a warming device (Bair Hugger ™ Temperature Management Units model 750, 3M Japan Ltd., Tokyo, Japan) with a whole‐body cover with preset temperature of 43°C is used to manage the core temperature prior to surgery by the anaesthesiologist for hypothermia prevention. Intraoperative warming device; Bair Hugger™, has three temperature settings, high 43°C, medium 38°C and low 32°C. The initial temperature was set at 43°C to reduce the magnitude of redistribution hypothermia. If the temperature rose up above the normal range, active warming was discontinued. The patient enters the intensive care unit (ICU) following extubation of the intratracheal tube after surgery.
Hyperthermia due to the effects of surgery and heat accumulation induced by the cover draping the patient often occur during neurosurgery involving prolonged procedures in the park‐bench position 31. In the present study, when the patient's core temperature reached 37·5°C to 38·0°C, the temperature management method was changed from active warming to thermal insulation. In patients undergoing neurosurgery, active surface cooling, which constitutes general care for hyperthermia during surgery, was not performed because postoperative shivering was induced by cooling, subsequently resulting in an increased oxygen uptake 32. Alternatively, treatment with medications (NSAIDs) and infusion was performed.
Determination of IAPUs
The patient's skin is assessed three times in the perioperative period: immediately after the induction of general anaesthesia, at the end of surgery and 24 hours after the surgery. The skin at regions of contact with the operating table is checked by two operating theatre nurses, and the presence of non‐blanchable erythema is evaluated using finger pressure. The operating theatre nurses document the results of the patient's skin assessment, and the presence of pressure ulcers is diagnosed based on the appearance of localised signs that continue for 24 hours. All pressure ulcers are classified according to the National Pressure Ulcer Advisory Panel (NPUAP) staging.
Data collection
Data regarding the incidence of IAPUs were obtained from the patients' electronic records as the primary outcome variable. Pressure ulcers were diagnosed as IAPUs if the following conditions were documented in the nursing records: no sign of ulcers at the time of admission and the appearance of redness immediately after surgery that remained 24 hours later. The data extracted from the database records were independently checked by operating theatre nurses, and the medical records were subsequently referred to once a week by operating theatre nurses who were members of the Records Commission at our hospital.
The operating theatre nurses collected the demographic data at the time of admission and during the surgical procedure and assessed the risk factors associated with IAPUs based on the patients' medical records. A total of 21 potential risk factors were examined according to a review of the literature: a portion of the OH scale for joint contractures, oedema and skin moisture (perspiration), age 7, gender 8, diabetes 11, 12, congestive heart failure 7, hypertension 12, rheumatism 33 , paralysis 33, history of smoking 12, skin dryness 34, body mass index (BMI) 10, haemoglobin level 11, length of surgery 13, 14, 15, 16, 17, core temperature at the end of surgery 6, amount of bleeding 27, ASA PS classification 8, 9, bony prominences on the lateral thorax and iliac crest 6, 35, use of rotation 36 and application of skin polyurethane film 37. The self‐sustainable ability and morbid bony prominences based on the OH scale were not assessed because these factors were not applicable to our patients, who were placed in the park‐bench position under general anaesthesia and received an indwelling bladder catheter during surgery. Although the core temperature was measured continuously during the surgical procedure, we collected data for the core temperature at the end of surgery as an independent variable, as other surgery‐related factors were evaluated only at the end of surgery. The core temperature data of this study were extracted every 30 minutes from the anaesthesia records. The threshold for classifying the length of surgery was more or less than 6 hours based on the guidelines of the Japanese Society of Pressure Ulcers 38 and a previous study 6.
Data analysis
Univariate analyses for each independent variable were performed using the χ 2 test or Fisher's exact test for categorical variables and the unpaired t‐test or Mann–Whitney U test for continuous variables. Variables with a P‐value of less than 0·05 were included in the subsequent multivariate analysis.
A multivariate logistic regression analysis was conducted according to the stepwise selection method. Before the analyses, correlations between potential independent variables were examined in order to avoid multi‐collinearity. If the correlation coefficients exceeded 0·4, either variable was selected. The optimal thresholds for the extracted continuous variables were then examined using a receiver operating characteristic (ROC) curve at the point showing the highest sum of the sensitivity and specificity. Interactions between the remaining independent variables were also assessed. If a significant interaction was observed, the combination of the interacting variables was alternatively included as an independent variable.
The statistical analyses were performed using the JMP for Windows ver. 10.0.2 software program (SAS Institute, Tokyo, Japan). The level of statistical significance was set at a P‐value of less than 0·05.
Results
Overall patient characteristics
Of the 309 patients enrolled in this study, 277 were finally selected for the analysis (Figure 2). Ten patients were excluded because they underwent emergency surgery (n = 6) or repeated surgery (n = 4). One patient was excluded because the amount of bleeding in that case was an outlier (2835 ml) (n = 1). An additional 21 patients were excluded because blood data were not available in their records. In total, 118 males and 159 females were included in the analyses. The mean ± SD age was 45·5 ± 13·8 years (range: 10–82). Comorbidities included hypertension (n = 19), diabetes (n = 9) and congestive heart failure (n = 1). A total of 151 patients were classified as having an ASA PS of 1, 124 were classified as having a PS of 2 and two were classified as having a PS of 3. The surgical procedures included cerebellopontine angle tumour removal in 266 cases and microvascular decompression in 11 cases. The mean ± SD length of surgery was 376 ± 89 minutes, and the mean ± SD core temperature at the end of surgery was 37·7 ± 0·7°C.
Figure 2.
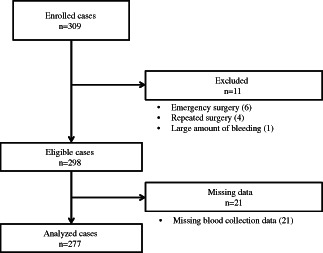
Flow of the participants throughout the study, 309 patients were enrolled. Excluding 31 patients, 277 patients were finally included in the analysis.
Pressure ulcers developed in 30 of the 277 patients 24 hours after surgery (11%). Twenty‐nine patients had stage I ulcers, and one patient had a stage II ulcer with a blister. Neither stage I nor stage II pressure ulcers were exacerbated to become deeper ulcers. The locations of pressure ulcer in the park‐bench position were the lateral thorax, greater trochanter, iliac, lateral knee and lateral malleolus. The sites of the pressure ulcers included the lateral thorax (n = 12), iliac crest (n = 6), greater trochanter (n = 5), lateral thorax and/or greater trochanter (n = 4), lateral thorax and/or iliac crest (n = 1) and other sites (n = 2).
Five variables were found to be significantly associated with IAPUs in the univariate analyses, including perspiration, a prolonged length of surgery, a high core temperature at the end of surgery and a large amount of bleeding (Table 1). We excluded the amount of bleeding from the following analysis because of its moderate correlation with the length of surgery (Pearson's r = 0·43).
Table 1.
Univariate analysis of risk factors associated with intraoperatively acquired pressure ulcers*
| With pressure ulcers (n = 30) | Without pressure ulcers (n = 247) | P value | |
|---|---|---|---|
| Joint contractures, n (%) | |||
| Yes | 0 | 0 | |
| No | 30 (12·1) | 247 (87·9) | ‐ |
| Oedema, n (%) | |||
| Yes | 0 | 0 | |
| No | 30 (12·1) | 247 (87·9) | ‐ |
| Perspiration, n (%) | |||
| Yes | 9 (34·6) | 17 (65·4) | |
| No | 21 (8·4) | 230 (91·6) | <0·001 |
| Age (years, mean ± SD) | 45·5 ± 11·5 | 45·5 ± 14·0 | 0·456 |
| Gender, n (%) | |||
| Male | 12 (10·7) | 100 (89·3) | |
| Female | 18 (10·9) | 147 (89·1) | 0·884 |
| Diabetes, n (%) | |||
| Yes | 1 (11·1) | 8 (88·9) | |
| No | 29 (10·8) | 239 (89·2) | 0·978 |
| Congestive heart failure, n (%) | |||
| Yes | 0 | 1 (100·0) | |
| No | 30 (10·9) | 246 (89·1) | 0·727 |
| Hypertension, n (%) | |||
| Yes | 2 (11·1) | 16 (88·9) | |
| No | 28 (10·8) | 231 (89·2) | 0·968 |
| Rheumatism, n (%) | |||
| Yes | 0 | 0 | |
| No | 30 (10·8) | 247 (89·2) | ‐ |
| Paralysis, n (%) | |||
| Yes | 1 (50·0) | 1 (50·0) | |
| No | 29 (10·5) | 246 (89·5) | 0·074 |
| History of smoking, n (%) | |||
| Yes | 4 (6·0) | 63 (94·0) | |
| No | 26 (12·4) | 184 (87·6) | 0·142 |
| Skin dryness, n (%) | |||
| Yes | 2 (16·7) | 10 (83·3) | |
| No | 28 (10·6) | 237 (89·4) | 0·506 |
| Body mass index (mean ± SD) | 23·5 ± 4·1 | 22·0 ± 3·1 | 0·988 |
| Haemoglobin level (g/dL; mean ± SD) | 13·6 ± 1·62 | 13·8 ± 8·22 | 0·357 |
| Length of surgery (minute, mean ± SD) | 443 ± 69·0 | 367·9 ± 87·9 | <0·001 |
| Core temperature at the end of surgery (°C, mean ± SD) | 38·2 ± 0·5 | 37·7 ± 0·66 | <0·001 |
| Amount of bleeding (ml, mean ± SD) | 306·9 ± 241·7 | 218·5 ± 199·2 | 0·008 |
| ASA‐Physical status classification, n (%) | |||
| 1 | 16 (10·6) | 135 (89·4) | |
| 2 | 14 (11·2) | 110 (88·8) | |
| 3 | 0 | 2 (100·0) | 0·928 |
| Bony prominence, n (%) | |||
| Yes | 7 (6·6) | 99 (93·4) | |
| No | 23 (13·5) | 148 (86·5) | 0·075 |
| Use of rotation, n (%) | |||
| Yes | 2 (9·5) | 19 (90·5) | |
| No | 28 (10·9) | 228 (89·1) | 0·841 |
| Use of skin polyurethane film, n (%)† | |||
| Yes | 30 (10·8) | 244 (89·2) | |
| No | 0 | 3 (100·0) | 0·544 |
ASA‐Physical status classification, American Society of Anesthesiologists Physical status Classification.
The data are reported as the mean ± SD for continuous variables or n (%) for categorical variables. Differences were assessed using the unpaired t‐test or Mann–Whitney U test for continuous variables and the chi‐square test or Fisher's exact probability test for categorical variables.
Use of skin polyurethane film, n (%): use for pressure ulcer prevention as a result of shear and friction.
We then examined the threshold for the core temperature at the end of surgery using ROC curves. Consequently, the development of pressure ulcers was more likely to occur at a core temperature of more than 38·1°C (Figure 3). In addition, the core temperatures were higher in the patients with pressure ulcers (38·2 ± 0·5°C) than in those without pressure ulcers (37·7 ± 0·7°C). Hence, the optimal cut‐off point for the core temperature was determined to be 38·1°C, with a sensitivity of 74·9% and a specificity of 73·4%. As the interaction between the length of surgery and the core temperature at the end of surgery was significant, we classified the patients into three groups for the logistic regression analysis: subjects with a length of surgery less than 6 hours, those with a length of surgery of 6 hours or longer with a core temperature of <38·1°C and those with a length of surgery of 6 hours or longer with a core temperature of ≥38·1°C. As the number of patients with a length of surgery less than 6 hours and a core temperature of ≥38·1°C was small (n = 5), this category was integrated into the first group.
Figure 3.
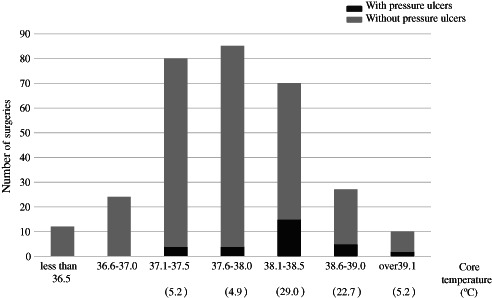
Relationship between the core temperature at the end of surgery and the incidence of pressure ulcers, The core temperature at the end of surgery was measured according to the rectal temperature at the end of surgery. The values below each range of core temperature show pressure ulcer incidence (%). The number of surgeries associated with pressure ulcers increased for a core temperature of more than 38·1°C at the end of surgery. No pressure ulcers developed at a core temperature of less than 37·1°C at the end of surgery.
According to the multivariate logistic regression analysis (Table 2), the risk factor independently associated with IAPUs included perspiration [P = 0·037, OR 3·09, 95% confidence interval (CI) 1·07–8·58], and the combination of the length of surgery and the core temperature showed a significant association with the development of pressure ulcers. In particular, when the core temperature was below 38·1°C, the likelihood of developing IAPUs increased for a length of surgery of more than 6 hours (P = 0·095, OR 2·64, 95%CI 0·84–9·08). Additionally, when the core temperature was more than 38·1°C, the odds ratio increased to 8·45 [P < 0·001, 95% confidence interval (CI) 3·04–27·46] compared with a length of surgery of <6 hours, and a core temperature of more than 38·1°C demonstrated an increased odds ratio for developing IAPUs compared with a core temperature below 38·1°C when the length of surgery was more than 6 hours (P = 0·017, OR 3·20, 95%CI 1·23–8·78).
Table 2.
Multivariate analysis of risk factors associated with intraoperatively acquired pressure ulcers (n = 277)
| Variables | Odds ratio | (95% CI) | P value |
|---|---|---|---|
| Perspiration | |||
| No | 1·00 | ||
| Yes | 3·09 | (1·07‐8·58) | 0·037 |
| Length of surgery and core temperature | |||
| Less than 6 hours | 1·00 | ||
| More than 6 hours and less than 38·1°C | 2·64 | (0·84‐9·08) | 0·095 |
| More than 6 hours and more than 38·1°C | 8·45 | (3·04‐27·46) | <0·001 |
Discussion
This study is the first to show that hyperthermia of more than 38·1°C was a risk factor for IAPUs in the park‐bench position, and that there was a synergetic effect between length of surgery and core temperature. These relationships could not be fully showed in our previous study because of the small sample size.
The incidence rate of IAPUs in the present report is 11%, although it was 30% in the author's previous report 6. We had changed the pressure distribution and fixation methods in the park‐bench position between this study and previous study. Although we have introduced additional intervention for interface pressure management by using a support surface as pressure ulcer prevention in the park‐bench position, the incidence of pressure ulcer is still higher than that in other positions. Therefore, it is necessary to consider the risk factor of pressure ulcer development other than interface pressure. It should be noted that our participants were generally younger than patients who develop pressure ulcers in the general unit or in nursing homes 39. In addition, the young participants displayed a good general condition on admission, with the exception of neurological disorders, indicating that general risk factors may not contribute to pressure ulcer development in this population. In this study, perspiration, length of surgery and core temperature were identified to be the risk factors for IAPUs, and there was a synergistic relationship between the core temperature and the length of surgery, in that a core temperature above 38·1°C exacerbated the effects of prolonged surgery for more than 6 hours on the development of pressure ulcers by approximately three‐fold. Although the length of surgery is not necessarily modifiable, regulating perspiration and the core temperature is a preventable strategy from a nursing perspective and entails collaboration with the anaesthesiologist for controlling the patient's thermal condition.
An increased core temperature was found to be a particular risk factor for IAPUs in the park‐bench position. In the park‐bench position, the patient's core temperature is increased because their body is covered with a full drape, except for the head. An increased core temperature further increases the skin temperature at areas of contact with the operating table. In a study of healthy individuals, an increase in skin temperature by 1·0°C was shown to activate tissue metabolism by 10% requiring the supply of more oxygen and nutrients 40. In addition, high interface pressure during surgery reduces or interferes with the blood flow, resulting in poor supply relative to the increased demand, thus causing tissue damage 41. This result is surprising because hyperthermia has been demonstrated to be a protective factor in the general unit based on the effects of vasodilation associated with an increased blood flow and oxygenation 39. In contrast, vasodilation does not occur in the park‐bench position as a result of persistent pressure on contact areas, which is higher than the pressure observed in the capillary. Therefore, the adverse effects of hyperthermia on tissue become apparent. Because the anaesthesiologists prioritised the prevention of adverse events caused by hypothermia, approximately one‐third of the patients showed a core temperature >38·1°C, even though we usually provided several interventions when the core temperature of the patient reached 37·5–38·0°C as advised by our anaesthesiologists. We, along with our anaesthesiologists, may be able to provide alternative interventions against hyperthermia considering the risk of PU by early cessation of active warming of the device or increasing heat exchange in local skin temperature on the operating table using sheets.
An interaction was observed between the core temperature and length of surgery in this study. The length of surgery is a well‐known risk factor for pressure ulcers in surgical units 13, 14, 15, 16, 17. However, the length of surgery is mostly dependent on the difficulty of the procedure, including factors such as the size or location of neoplasms and the amount of bleeding. It is difficult to shorten the length of surgery directly, considering the safety of the surgery. On the other hand, the core temperature can be adjusted by the operating theatre nurses by controlling the warming device and consulting the anaesthesiologist. In an experimental study, tissue damage within the deep and superficial layers was shown to be enhanced by increasing skin temperature, even under consistent pressure–time loads 42. These observations suggest that IAPUs may be prevented by maintaining the patient's core temperature at the appropriate level, even during prolonged surgeries.
Perspiration was also found to be independently associated with IAPUs in the park‐bench position in the present study. Perspiration may be associated with the presence of skin moisture, which subsequently weakens the crosslinks among collagen fibres in the dermis and thus promotes skin fragility 43. Moreover, the park‐bench position often produces a shearing force as a result of elevation of the patient's head on the operating table and the incline of the operating table towards the patient's abdomen so as to secure the surgeon's field of vision. Therefore, skin moisture resulting from perspiration is thought to increase the degree of friction 41, ultimately resulting in pressure ulcer development. Although perspiration is a reaction to hyperthermia in the general setting, perspiration may also occur independently from the core temperature in the park‐bench position. In the current study, perspiration was observed in only 10 of the 58 patients with a core temperature of more than 38·1°C. This inconsistency may be attributed to the effects of cytokines induced by surgical invasiveness and the use of anaesthesia, which increase the threshold of the core temperature for perspiration by altering the central temperature regulatory centre 44.
Strengths of the present study include the fact that the surgery‐related procedures were standardised and a relatively large sample size was used. Furthermore, all staff received adequate training for the position fixation method because scheduled surgery was conducted almost 3 days per week. In addition, anaesthesia was performed using total intravenous anaesthesia. Finally, the surgeries were conducted by the same neurosurgical procedure operator and the IAPUs were observed by the same individuals. Therefore, the effects of bias due to facility‐related factors were small.
Limitations
The first limitation of this study is the unclear timing of perspiration, hyperthermia and pressure ulcer development during the course of surgery. If the precise onset of these complications is identified in a future study using monitoring devices, the most appropriate timing to initiate proper interventions could be determined. The second limitation is the lack of measurements of the interface pressure in the retrospective data. However, the influence of interface pressure was not considered to be large because BMI and bony prominences were not extracted as risk factors in our results.
Conclusion
The present study identified specific risk factors, including perspiration, the length of surgery and the core temperature, for pressure ulcer development in the park‐bench position. In particular, a core temperature of more than 38·1°C demonstrated an interactive effect with a length of surgery of more than 6 hours, and perspiration was found to be independently related to IAPUs based on the core temperature. These findings suggest that clinicians may prevent IAPUs by managing the level of perspiration and controlling the patient's core temperature to less than 38·1°C in collaboration with the anaesthesiologist under conditions of prolonged surgery.
Acknowledgements
The authors greatly appreciate the Senior Nursing Officer S. Ohashi, WOCN. M. Toyoda, and the operating theatre nurses Ms. E. Hasegawa, Ms. N. Ishizono, Ms. T. Yamada and Ms. T. Kawashima at Tokyo Metropolitan Police Hospital for their support in the data collection. The authors declare no conflicts of interest.
References
- 1. National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel . Prevention and treatment of pressure ulcers: a clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel, 2009. [Google Scholar]
- 2. Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN J 1999;70:434–7. [DOI] [PubMed] [Google Scholar]
- 3. Jesurum J, Joseph K, Davis JM, Suki R. Balloons, beds, and breakdown. Effects of low‐air loss therapy on the development of pressure ulcers in cardiovascular surgical patients with intra‐aortic balloon pump support. Crit Care Nurs Clin North Am 1996;8:423–40. [PubMed] [Google Scholar]
- 4. Scott SM, Mayhew PA, Harris EA. Pressure ulcer development in the operating room. Nursing implications. AORN J 1992;56:242–50. [DOI] [PubMed] [Google Scholar]
- 5. St‐Arnaud D, Paquin MJ. Safe positioning for neurosurgical patients. AORN J 2008;87:1156–68. [DOI] [PubMed] [Google Scholar]
- 6. Yoshimura M, Nagata O, Kohno M, Yamasaki T, Mae T, Ohashi S. Perioperative risk factors associated with pressure ulcer in the park bench position (in Japanese). JJSCA 2013;33:75–83. [Google Scholar]
- 7. O'Brien DD, Shanks AM, Talsma A, Brenner PS, Ramachandran SK. Intraoperative risk factors associated with postoperative pressure ulcers in critically ill patients: a retrospective observational study. Crit Care Med 2014;42:40–7. [DOI] [PubMed] [Google Scholar]
- 8. Lindgren M, Unosson M, Krantz AM, Ek AC. Pressure ulcer risk factors in patients undergoing surgery. J Adv Nurs 2005;50:605–12. [DOI] [PubMed] [Google Scholar]
- 9. Fred C, Ford S, Wagner D, Vanbrackle L. Intraoperatively acquired pressure ulcers and perioperative normothermia: a look at relationships. AORN J 2012;96:251–60. [DOI] [PubMed] [Google Scholar]
- 10. Wu T, Wang ST, Lin PC, Liu CL, Chao YF. Effects of using a high‐density foam pad versus a viscoelastic polymer pad on the incidence of pressure ulcer development during spinal surgery. Biol Res Nurs 2011;13:419–24. [DOI] [PubMed] [Google Scholar]
- 11. Lewicki LJ, Mion L, Splane KG, Samstag D, Secic M. Patient risk factors for pressure ulcers during cardiac surgery. AORN J 1997;65:933–42. [DOI] [PubMed] [Google Scholar]
- 12. Papantonio CT, Wallop JM, Kolodner KB. Sacral ulcers following cardiac surgery: incidence and risks. Adv Wound Care 1994;7:24–36. [PubMed] [Google Scholar]
- 13. Grous CA, Reilly NJ, Gift AG. Skin integrity in patients undergoing prolonged operations. J Wound Ostomy Continence Nurs 1997;24:86–91. [DOI] [PubMed] [Google Scholar]
- 14. Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: an analysis of bedding materials. Res Nurs Health 1994;17:333–9. [DOI] [PubMed] [Google Scholar]
- 15. Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. J Wound Ostomy Continence Nurs 1999;26:130–6. [DOI] [PubMed] [Google Scholar]
- 16. Schoonhoven L, Defloor T, van der Tweel I, Buskens E, Grypdonck MH. Risk indicators for pressure ulcers during surgery. Appl Nurs Res 2002;15:163–73. [DOI] [PubMed] [Google Scholar]
- 17. Stevens J, Nichelson E, Linehan WM, Thompson N, Liewehr D, Venzon D, Walther MM. Risk factors for skin breakdown after renal and adrenal surgery. Urology 2004;64:246–9. [DOI] [PubMed] [Google Scholar]
- 18. Walton‐Geer PS. Prevention of pressure ulcers in the surgical patient. AORN J 2009;89:538–52. [DOI] [PubMed] [Google Scholar]
- 19. Beckett AE. Are we doing enough to prevent patient injury caused by positioning for surgery? J Perioper Pract 2010;20:26–9. [DOI] [PubMed] [Google Scholar]
- 20. Defloor T, De Schuijmer JD. Preventing pressure ulcers: an evaluation of four operating‐table mattresses. Appl Nurs Res 2000;13:134–41. [DOI] [PubMed] [Google Scholar]
- 21. Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. Adv Wound Care 1998;11(3 Suppl):8–9. [PubMed] [Google Scholar]
- 22. Bliss M, Simini B. When are the seeds of postoperative pressure sores sown? Often during surgery. BMJ 1999;319:863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Price MC, Whitney JD, King CA, Doughty D. Development of a risk assessment tool for intraoperative pressure ulcers. J Wound Ostomy Continence Nurs 2005;32:19–32. [DOI] [PubMed] [Google Scholar]
- 24. Scott EM, Buckland R. Pressure ulcer risk in the peri‐operative environment. Nurs Stand 2005;20:74, 76, 78. [DOI] [PubMed] [Google Scholar]
- 25. Connor T, Sledge JA, Bryant‐Wiersema L, Stamm L, Potter P. Identification of pre‐operative and intra‐operative variables predictive of pressure ulcer development in patients undergoing urologic surgical procedures. Urol Nurs 2010;30:289–95, 305. [PubMed] [Google Scholar]
- 26. Nixon J, Brown J, McElvenny D, Mason S, Bond S. Prognostic factors associated with pressure sore development in the immediate post‐operative period. Int J Nurs Stud 2000;37:279–89. [DOI] [PubMed] [Google Scholar]
- 27. Stotts NA. Predicting pressure ulcer development in surgical patients. Heart Lung 1988;17(6 pt 1):641–7. [PubMed] [Google Scholar]
- 28. Primiano M, Friend M, McClure C, Nardi S, Fix L, Schafer M, Savochka K, McNett M. Pressure ulcer prevalence and risk factors during prolonged surgical procedures. AORN J 2011;94:555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Japanese Society of Pressure Ulcers . Guideline for pressure ulcers prevention and management. Tokyo: Shorinsha Inc., 2005. [Google Scholar]
- 30. Hotta Y. Pressure ulcers of truth and OH scale, the pressure ulcer prevention by “OH scale” pressure ulcers risk factors of Japanese (in Japanese). Tokyo: Nissouken Inc., 2005. [Google Scholar]
- 31. Maruyama K. Perioperative nursing (in Japanese). Tokyo: Gakken Inc., 2007. [Google Scholar]
- 32. Ciofolo MJ, Clergue F, Devilliers C, Ben Ammar M, Viars P. Changes in ventilation, oxygen uptake, and carbon dioxide output during recovery from isoflurane anesthesia. Anesthesiology 1989;70:737–41. [DOI] [PubMed] [Google Scholar]
- 33. Flattau A, Blank AE. Risk factors for 90‐day and 180‐day mortality in hospitalized patients with pressure ulcers. Int Wound J 2014;11:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allman RM, Goode PS, Patrick MM, Burst N, Bartolucci AA. Pressure ulcer risk factors among hospitalized patients with activity limitation. JAMA 1995;273:865–70. [PubMed] [Google Scholar]
- 35. Furuno Y, Sasajima H, Goto Y, Taniyama I, Aita K, Owada K, Tatsuzawa K, Mineura K. Strategies to prevent positioning‐related complications associated with the lateral suboccipital approach. J Neurol Surg B Skull Base 2014;75:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kobara K, Fujita D, Osaka H, Ito T, Watanabe S. Influence of distance between the rotation axis of back support and the hip joint on shear force applied to buttocks in a reclining wheelchair's back support. Prosthet Orthot Int 2013;37:459–64. [DOI] [PubMed] [Google Scholar]
- 37. Nakagami G, Sanada H, Konya C, Kitagawa A, Tadaka E, Matsuyama Y. Evaluation of a new pressure ulcer preventive dressing containing ceramide 2 with low frictional outer layer. J Adv Nurs 2007;59:520–9. [DOI] [PubMed] [Google Scholar]
- 38. Japanese Society of Pressure Ulcers . Policy for revised reimbursement of medical services related to pressure ulcer items. Tokyo: Shorinsha Inc., 2006. [Google Scholar]
- 39. Nijs N, Toppets A, Defloor T, Bernaerts K, Milisen K, Van Den Berghe G. Incidence and risk factors for pressure ulcers in the intensive care unit. J Clin Nurs 2009;18:1258–66. [DOI] [PubMed] [Google Scholar]
- 40. Fisher SV, Szymke TE, Apte SY, Kosiak M. Wheelchair cushion effect on skin temperature. Arch Phys Med Rehabil 1978;59:68–72. [PubMed] [Google Scholar]
- 41. Wounds International . International review. Pressure ulcer prevention: pressure, shear, friction and microclimate in context. A consensus document. London: Wounds International, 2010. [Google Scholar]
- 42. Lachenbruch C, Tzen YT, Brienza DM, Karg PE, Lachenbruch PA. The relative contributions of interface pressure, shear stress, and temperature on tissue ischemia: a cross‐sectional pilot study. Ostomy Wound Manage 2013;59:25–34. [PubMed] [Google Scholar]
- 43. Mayrovitz HN, Sims N. Biophysical effects of water and synthetic urine on skin. Adv Skin Wound Care 2001;14:302–8. [DOI] [PubMed] [Google Scholar]
- 44. Frank SM, Kluger MJ, Kunkel SL. Elevated thermostatic setpoint in postoperative patients. Anesthesiology 2000;93:1426–31. [DOI] [PubMed] [Google Scholar]


