Abstract
Diabetic foot ulcerations may determine minor or major amputation, with a high impact on patients' life expectation and quality of life and on economic burden. Among minor amputations, transmetatarsal amputation (TMA) appears to be the most effective in terms of limb salvage rates and in maintaining foot and ankle biomechanics. In spite of this, TMA needs particular pre‐ and postoperative management in order to avoid the frequent failure rates.
A systematic review was undertaken of studies concerning TMA and its care in diabetic foot gangrene. Studies were identified by searching the MEDLINE, Scopus and Science Direct databases until 13 January 2016. All studies were assessed using the Downs and Black quality checklist.
Of the 348 records found, 86 matched our inclusion criteria.
After reading the full‐text articles, we decided to exclude 35 manuscripts because of the following reasons: (1) no innovative or important content, (2) no multivariable analysis, (3) insufficient data, (4) no clear potential biases or strategies to solve them, (5) no clear endpoints and (6) inconsistent or arbitrary conclusions. The final set included 51 articles.
In the current literature, there are less data about TMA, indication for the selection of patients, outcomes and complications.
Generally, the judgment of an experienced physician is one of the best indicators of subsequent healing. Ankle brachial indices, toe pressures, laser Doppler skin perfusion pressures, angiography and Doppler assessment of foot vasculature may help physicians in this decision.
In any case, despite the presumed lower healing rate, it is reasonable to pursue a TMA in a patient with a higher likelihood of continued ambulation.
Furthermore, tailored wound closure, adjuvant local treatments and the choice of the most appropriate antibiotic therapy, when infection occurs, are pivotal elements for the success of TMA procedures.
TMA is a valuable option for diabetic foot gangrene that can prevent major limb loss and minimise loss of function, thus improving the quality of life for diabetic patients.
Keywords: Diabetic foot, Gangrene, Transmetatarsal amputation, Ulcer
Introduction
Diabetic foot is considered a syndrome because of several aspects of diabetic disease, such as peripheral arterial disease, both microangiopathy and macroangiopathy and peripheral neuropathy leading to foot ulcerations that affect one in ten diabetics during their lifetime 1. Diabetic foot ulcerations may determine minor or major amputation, especially when wound infection or osteomyelitis is involved, with a high impact on patients' life expectation and quality of life and on economic burden. Furthermore, studies have shown that about 50% of patients with diabetic foot infections treated with distal amputations expire within 5 years 2.
These data are also alarming because the incidence and prevalence of diabetes is rising in last years. Therefore, it is important to prevent diabetic foot and its complications, such as ulcers and infection, acting primarily on the risk factors: close monitoring of hyperglycaemia, smoking and obesity 3, 4. Moreover, complete and careful foot care is also needed through daily foot checks, removing callosity because of diabetic neuropathy, daily foot hydration, regular toenail cutting and appropriate or custom‐made footwear 5, 6.
For many patients with infection or distal foot gangrene, a transmetatarsal amputation (TMA) is the last hope for partial foot salvage. The preservation of a sensitive heel is desirable for maintaining ambulatory function. However, wound healing of TMA is frequently a major challenge. Although transtibial amputations heal more reliably than TMAs, patients are often resistant to this procedure, and subsequent ambulation with a prosthesis is often more difficult than ambulation on the native heel and forefoot 7, 8.
The poor ambulatory performance of atherosclerotic patients with transtibial amputations is well documented. Therefore, despite the accepted lower healing rates, it is reasonable to pursue a TMA in patients with a higher likelihood of continued ambulation. It is often difficult to determine this preoperatively because most patients express a wish to walk again. So, for patients in whom ambulation is clearly a reasonable future goal, TMA is an optimal option despite its low healing rate and the need for additional operations and hospitalisations. For the superior functional outcomes of patients with successful amputation healing, TMA should be offered to patients with favourable prospects for postoperative ambulation. In patients with poor rehabilitation prospects, TMA may lead to additional procedures and hospitalisations, and a more proximal amputation with a higher likelihood of healing may be preferable 9.
Moreover, advanced wound care is required to improve diabetic ulcer healing and TMA outcome.
Methods
This systematic review was conducted and is reported in accordance with the PRISMA guidelines.
Inclusion and exclusion criteria
We decided to include all the studies conducted about TMA in diabetic foot. Randomised trials, cohort studies and reviews were contemplated in order to give a breadth of clinical data. Only publications in English were considered. We excluded all the studies with insufficient statistical analysis, possible biases and contradictions, no clear endpoints and inconsistent or arbitrary conclusions.
Search strategy
Two members of the research team (MA, LB) performed a comprehensive literature search using terms identified and agreed on by the authors. Medline, Scopus and Science Direct were searched from January 2006 to April 2016 using the keywords ‘Transmetatarsal amputation and diabetic foot’.
Data extraction and risk of bias assessment
Two review authors (SdF, RS) independently assessed both titles and abstracts of potentially eligible studies found in Medline, Scopus and Science Direct. In case of an ambiguous or unclear result, the study was retrieved in full and assessed further by all authors independently and included if pertinent. All studies were assessed using the Downs and Black quality checklist 10, 11.
Results
Study selection
A total of 348 records were found, and 86 matched our inclusion criteria (Figure 1).
Figure 1.
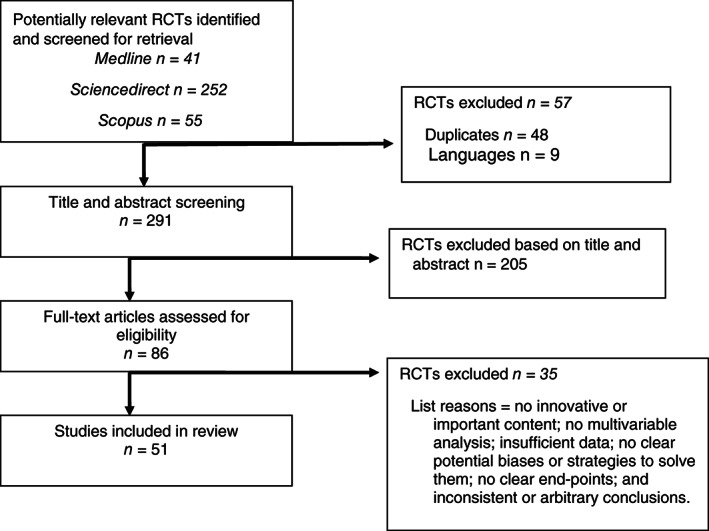
PRISMA diagram.
After reading the full‐text articles, we decided to exclude 35 manuscripts because of the following reasons: (i) no innovative or important content, (ii) no multivariable analysis, (iii) insufficient data, (iv) no clear potential biases or strategies to solve them, (v) no clear endpoints and (vi) inconsistent or arbitrary conclusions. The final set included 51 articles.
Diabetic foot epidemiology
The term ‘diabetic foot’ will be taken to encompass any foot lesion occurring as a result of diabetes and its complications. Globally, the diabetic foot remains a major medical, social and economic problem that is seen in every country. People at the greatest risk of ulceration can easily be identified by careful clinical examination of the feet; education and frequent follow‐up is indicated for these patients. When assessing the economic effects of diabetic foot disease, it is important to remember that rates of recurrence of foot ulcers are very high, being greater than 50% after 3 years. Costing should therefore include not only the immediate ulcer episode but also social services, home care and subsequent ulcer episodes. A broader view of total resource use should include some estimate of the quality of life and the final outcome. An integrated care approach with regular screening and education of patients at risk requires low expenditure and has the potential to reduce the cost of health care 12, 13.
However, the reported frequencies of amputation and ulceration do vary considerably as a consequence of the different diagnostic criteria used as well as regional differences 14, 15.
Up to 25% of patients with diabetes will develop a foot ulcer sometime during their lives, and up to 2% of patients may already have undergone amputation. Diabetes remains the major cause of non‐traumatic amputation in most Western countries; rates are as much as 15 times higher than in the non‐diabetic population 16, 17.
It is difficult to make a direct comparison between studies/countries for methodological issues. First, the definition as to what constitutes a foot ulcer varies, and secondly, surveys invariably include only patients with previously diagnosed diabetes, whereas in type 2 diabetes, foot problems may be the presenting feature. In one study from the United Kingdom, for example, 15% of patients undergoing amputation were first diagnosed with diabetes on that hospital admission 18, 19.
Such observations clearly indicate the need for all diabetes services to have a regular screening programme to identify such high‐risk individuals. It is well recognised that a number of contributory factors working together ultimately result in the final pathway to foot ulceration in diabetic patients. The most common component in this pathway include peripheral neuropathy, foot deformity, external trauma, peripheral vascular disease and peripheral oedema 20, 21.
With the exception of trauma, none of the abovementioned risk factors will cause ulceration in isolation. Ethnicity and gender also have associations with neuropathy. In Western countries, foot ulcers are more common in male patients, and in mixed populations, foot ulceration is more common among those of European origin when compared to Asians and African–Caribbeans 22, 23.
An important prospective study evaluated in detail the predictive factors for limb salvage in patients with diabetic foot problems in Singapore: comorbidities, infections, complications, sensory neuropathy, gangrene and pathogens 24.
However, the greatest single risk factor for foot ulceration is a past history of either ulceration or amputation 20, 21.
Much progress has been made in the last 20 years in our understanding of the pathogenesis and management of diabetic foot problems, and the volume of research activity in this area is rapidly increasing. However, the current ‘epidemic’ of type 2 diabetes that is being witnessed throughout the world is resulting in an ever‐increasing population of diabetic patients with lower limb complications. The challenge in the years before the next international meeting is to continue increasing the awareness of diabetic foot problems, their causes and management.
Functional impact of TMA
Risk factors associated with TMA were male gender, physical impairment, cardiovascular complications, smoking, obesity, nephropathy, peripheral arterial disease characterised by intermittent claudication and impaired peripheral pulses, neuropathy, previous diabetic ulcers, gangrene and contralateral amputation 25.
Several authors consider TMA better than transtibial amputation because of its ability to preserve the ankle and a part of the foot, permitting independent gait and avoiding a prosthetic device 26, 27.
After TMA, there are several patho‐mechanical changes in the amputated limb that cause abnormalities of gait; among these, a shorter and deformed foot, with loss of toes, plantar aponeurosis, intrinsic muscles and toe proprioceptors, causes an inadequate plantar flexor lever arm and forces patients to walk with reduced gait velocity and decreased ankle moments. Furthermore, higher pressures over the mid‐foot of the amputation limb because of reduced surface area are detected 28.
As a result of these factors, the patients had remarkable difficulty in any activity that required shifting weight to the forefoot, like climbing stairs and picking up something from the floor.
Indeed, the contralateral limb shows greater ankle moments and heel high pressure, causing an asymmetrical ankle during the gain, which can undergo a plantar tissue injury 29, 30.
Patients should wear regular shoes and toe fillers to improve function and to prevent skin breakdown. Mueller et al. recommend the full‐length shoes, total‐contact insert and a rigid rocker‐bottom sole for almost all patients 31.
Outcomes and complications
In the current literature, there are less data about TMA outcomes and complications. Unfortunately, no preoperative measures were able to accurately predict which patients would go on to achieve healing of their amputations, so clinical judgment still remains an important factor in deciding who should be offered this operation.
TMA was first described as a method of partial foot preservation by McKittrick et al. in 1949 32. Preservation of a sensitive heel is desirable for maintaining ambulatory function. However, wound healing of TMA is frequently a major challenge. Wound‐healing rates from multiple series range from approximately 40% to 70% 33, 34, 35, 36, 37, 38, 39 .
Clearly, patient choice becomes an important factor because patients in general wish to preserve as much of their limb as possible. Below‐knee amputation is still a stigmatised procedure that patients will most likely defer as long as possible and at all reasonable cost. However, several similar studies demonstrate the importance of tempering expectations. Patients need to be aware of the relatively low likelihood of the success of this operation. Although this awareness is not likely to dissuade patients from pursuing it, at least patients will be informed of the tenuous nature of limb salvage in their particular situation. It is human nature to believe that bad outcomes will happen to someone else and not them, but armed with the knowledge that their operation has a relatively low likelihood of success, patients may be more likely to be compliant with the prescribed postoperative care recommendations, not the least of which is maintaining a non‐weight‐bearing status until the amputation site has healed.
Although transtibial amputations heal more reliably than TMAs, patients are often resistant to this procedure, and subsequent ambulation with a prosthesis is often more difficult than ambulation on the native heel and forefoot. Given the difficulty in the healing of TMA, however, it would be desirable to predict which patients are less likely to heal and to possibly avoid the prolonged periods of medical care for these patients.
Typically, the judgment of an experienced physician is one of the best indicators of subsequent healing. Other adjunctive measures, such as ankle brachial indices, toe pressures, laser Doppler skin perfusion pressures, angiography and Doppler assessment of foot vasculature, are occasionally used to assist in this decision 35, 40, 41, 42.
Detailed data by Landry et al. showed that TMA should be pursued in patients with good rehabilitation potential. In this study, 62 TMAs were performed in 57 patients. On univariate analysis, significant predictors of mortality included renal failure (74% mortality in patients with renal failure versus 40% in patients without renal failure), non‐ambulation (62% versus 36%), non‐independent living (79% versus 21%) and pre‐TMA revascularisation (64% versus 31%). Multivariate predictors of mortality included renal failure [odds ratio (OR), 4·85; 95% confidence interval (CI), 1·01–23·30] non‐independent living [OR, 17·80; 95% CI, 3·03–104·80] and need for preoperative revascularisation (OR, 4·80; 95% CI, 1·24–18·50). The mean survival of the entire patient cohort was 16·5 months (range, 0–94 months) 43.
Other researchers, such as Pollard et al., suggest that TMA is associated with high complication rates in a diabetic and vasculopathic population. In this study, 90 patients underwent 101 TMAs. A healed stump was achieved in 58 cases (57·4%). Post‐surgical complications developed in 88 cases (87·1%). Patients were examined for any postoperative complications associated with TMA. Complications were defined as hospital mortality occurring less than 30 days postoperatively, stump infarction with or without more proximal amputation, postoperative infection, chronic stump ulceration, stump deformity in any of the three cardinal planes, wound dehiscence and equinus and calcaneus gait. The χ 2 tests of association were used to determine whether diabetes, a palpable pedal pulse, coronary artery disease, end‐stage renal disease, cerebral vascular accident or hypertension were predictive of or associated with healing. A documented palpable pedal pulse was a predictor of healing and of not requiring more proximal amputation 38.
Postoperative management of TMA
The aim of TMA is to stem forefoot infection by dislodging all necrotic and infected tissues in order to assist healing and to rescue the mid‐foot and rearfoot, keeping limb function to walk and bear the load. Therefore, adequate and optimal postoperative management is essential 44, 45, 46. If the amputation is subsequent to an infection, antibiotic therapy should be continued 47; evidence has shown that Gram‐positive (e.g. Staphylococcus aureus) and Gram‐negative (e.g. Pseudomonas aeruginosa) bacteria react to adverse environments, producing a biofilm that protects them against the host's immune response, 48 and it accounts for peripheral arterial disease and ischaemic gangrene 49. In this way, a good choice of antibiotics, ‘the most active’ and not ‘the most easy’ 48, is necessary to obtain a rapid improvement of symptoms in patients with infected chronic ulcers. However, antibiotic treatment is often blocked by antibiotic resistance, making stump management difficult in the post‐amputation period 48, 50.
Primary closure by the plantar flap is indicated when a plentiful arterial network, arising from the plantar artery, is present, and closure without skin tension and a satisfactory capillary fill time is needed to prevent wound dehiscence and ischaemia 51, 52. Surgical wound closure generally consists of non‐absorbable, simple, interrupted sutures, and deep sutures are not required because of the possible of dysvascularity 53, 54. If a plantar flap is not available, other options for closure should be explored 55, 56, such as the use of skin flaps and grafts on the basis of the available skin tissues. Local flaps, characterised by skin without the presence of local necrosis and infection, and the transposition of neighbouring vascularised soft tissue allow coverage of a large defect 34, 57. The split‐thickness skin graft may be used with meshing that allows coverage of a larger area and decreases complications, such as a seroma or haematoma 58, 59, 60. Sometimes, delayed primary closure is a valid option 61; diabetic post‐amputation wounds, in fact, tend to heal often by second intention, and the main disadvantage is the high complication rates in wound healing that range from approximately 40% to 70% 38, 43, 62, 63. Failure to heal occurs because of decreased vascularity, increased pressure, hyperglycaemia and concomitant infection 28, 64, 65.
Several approaches have been proposed to improve wound healing in diabetic patients with TMA. Negative pressure wound therapy (NPWT), the delivery of intermittent or continuous subatmospheric pressure through a specialised pump connected to a resilient, open‐celled, foam‐surface dressing covered with an adhesive drape to maintain a closed environment, heals a higher proportion of post‐amputation wounds, ensuring a faster time to wound closure, a more rapid and robust granulation tissue response and a potential trend towards reduced risk for a second amputation 66, 67, 68. Studies on the restoration of tissue integrity have shown the involvement of platelets in the wound‐healing process. Platelet activation can modulate wound healing by interacting with molecular signals, primarily cytokines and growth factors (GFs) 69, 70. Serra et al. documented that in 96·15% of patients, PG promoted the functional recovery of physiological tissue reparation after a TMA procedure. Therefore, PG application may be an effective adjuvant treatment to improve wound healing in diabetic dysvascular patients 70.
Discussion
The primary goal of a TMA is the removal of nonviable tissue and the subsequent maintaining of limb function by preserving the ankle joint and limb length, 44 and undoubtedly, a well‐healed TMA provides excellent function over time. Therefore, careful consideration of performing a TMA should take into account several factors such as the assessment of skin perfusion and foot vasculature as well as the likely ability of the patient to undergo successful rehabilitation 71. In fact, in this way, we can identify which patients are less likely to heal and to maintain the functionality of a TMA 34, 39, 40, 41 as it was also observed that limited mobility at the ankle joint with increased plantar pressure puts the post‐TMA diabetic patient at a high risk of tissue breakdown and major amputation 72.
After performing a TMA, wound closure is a pivotal element that must be tailored according to the wound's and the patient's conditions, and when appropriate, adjuvant local treatments must be considered (skin grafting, NPWT, application of PG) in order to speed up and maintain wound healing 58, 59, 60, 66, 67, 68, 71.
Furthermore, the choice of the most appropriate antibiotic therapy, in the case of infection, improves stump management in the post‐amputation period and avoids TMA failures 47, 48, 50.
TMA is a valuable option for diabetic foot gangrene that may prevent major limb loss and minimise loss of function, thus improving the quality of life for diabetic patients. Furthermore, interdisciplinary cooperation among the wound specialists 73 is needed in order to obtain the best results in terms of global health.
Acknowledgement
This work received no funding. The authors declare that they have no competing interests.
Reference
- 1. Amin N, Doupis J. Diabetic foot disease: from the evaluation of the "foot at risk" to the novel diabetic ulcer treatment modalities. World J Diabetes 2016;7:153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pemayun TG, Naibaho RM, Novitasari D, Amin N, Minuljo TT. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital‐based case–control study. Diabet Foot Ankle 2015;6:29629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Health Quality Ontario . Behavioural interventions for type 2 diabetes: an evidence‐based analysis. Ont Health Technol Assess Ser 2009;9:1–45. [PMC free article] [PubMed] [Google Scholar]
- 4. Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, Khunti K. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta‐analysis. Diabetes Care 2014;37:922–33. [DOI] [PubMed] [Google Scholar]
- 5. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ, International Working Group on the Diabetic Foot . The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence‐based global consensus. Diabetes Metab Res Rev 2016;1:2–6. [DOI] [PubMed] [Google Scholar]
- 6. Morey‐Vargas OL, Smith SA. BE SMART: strategies for foot care and prevention of foot complications in patients with diabetes. Prosthet Orthot Int 2015;39:48–60. [DOI] [PubMed] [Google Scholar]
- 7. Early JS. Transmetatarsal and midfoot amputations. Clin Orthop Relat Res 1999;361:85–90. [DOI] [PubMed] [Google Scholar]
- 8. Younger ASE, Awwad MA, Kalla TP, de Vries G. Risk factors for failure of transmetatarsal amputation in diabetic patients: a cohort study. Foot Ankle Int 2009;30:1177–82. [DOI] [PubMed] [Google Scholar]
- 9. Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, Strecker PK, Anderson MW, Jones DN, Whitehill TA, Moskowitz S, Krupski WC. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg 2003;38:7–14. [DOI] [PubMed] [Google Scholar]
- 10. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Collaborating Centre for Methods and Tools . Quality Checklist for Health Care Intervention Studies. Hamilton: McMaster University, 2008. URL http://www.nccmt.ca/resources/search/9 [accessed on 15 January 2016]. [Google Scholar]
- 12. Boulton AJM, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 13. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 2010;52:17S–22. [DOI] [PubMed] [Google Scholar]
- 14. van Houtum WH. Amputations and ulceration; pitfalls in assessing incidence. Diabetes Metab Res 2008;24 Suppl 1:S14–8. [DOI] [PubMed] [Google Scholar]
- 15. Lavery LA, Peters EJ, Williams JR, Murdoch DP, Hudson A, Lavery DC, International Working Group on the Diabetic Foot . Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2008;31:154–6. [DOI] [PubMed] [Google Scholar]
- 16. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 17. Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev 2010;5:CD001488. [DOI] [PubMed] [Google Scholar]
- 18. Deerochanawong C, Home PD, Alberti KG. A survey of lower limb amputations in diabetic patients. Diabet Med 1992;9:942–6. [DOI] [PubMed] [Google Scholar]
- 19. Yusof MI, Sulaiman AR, Muslim DA. Diabetic foot complications: a two‐year review of limb amputation in a Kelantanese population. Singapore Med J 2007;48:729–32. [PubMed] [Google Scholar]
- 20. Boulton AJM. The diabetic foot: from art to science. Diabetologia 2004;47:1343–53. [DOI] [PubMed] [Google Scholar]
- 21. Dalla Paola L, Faglia E. Treatment of diabetic foot ulcer: an overview strategies for clinical approach. Curr Diabetes Rev 2006;2:431–47. [DOI] [PubMed] [Google Scholar]
- 22. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ, North‐West Diabetes Foot Care Study . The North‐West Diabetes Foot Care Study: incidence of, and risk factors for new diabetic foot ulceration in a community‐based patient cohort. Diabet Med 2002;19:377–84. [DOI] [PubMed] [Google Scholar]
- 23. Wu L, Norman G, Dumville JC, O'Meara S, Bell‐Syer SE. Dressings for treating foot ulcers in people with diabetes: an overview of systematic reviews. Cochrane Database Syst Rev 2015;7:CD010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Internal Clinical Guidelines Team . Diabetic Foot Problems: Prevention and Management. National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK), 2015. [PubMed] [Google Scholar]
- 25. Nather A, Bee CS, Huak CY, Chew JL, Lin CB, Neo S, Sim EY. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications 2008;22:77–82. [DOI] [PubMed] [Google Scholar]
- 26. Salsich GB, Mueller MJ. Relationships between measures of function, strength and walking speed in patients with diabetes and transmetatarsal amputation. Clin Rehabil 1997;11:60–7. [DOI] [PubMed] [Google Scholar]
- 27. Muellera MJ, Salsicha GB, Bastiana AJ. Differences in the gait characteristics of people with diabetes and transmetatarsal amputation compared with age‐matched controls. Gait Posture 1998;7:200–206. [DOI] [PubMed] [Google Scholar]
- 28. Dudkiewicz I, Schwarz O, Heim M, Herman A, Siev‐Ner I. Trans‐metatarsal amputation in patients with a diabetic foot: reviewing 10 years experience. Foot (Edinb) 2009;19:201–4. [DOI] [PubMed] [Google Scholar]
- 29. Mueller MJ, Salsich GB, Strube MJ. Functional limitations in patients with diabetes and transmetatarsal amputations. Phys Ther 1997;77:937–43. [DOI] [PubMed] [Google Scholar]
- 30. Rajani VK, Van Deursen RWM, Patricia E, Price KGH. What happens to the contra‐lateral foot following diabetic foot amputations? Clin Biomech 2008;23:677–678. [Google Scholar]
- 31. Mueller MJ, Strube MJ. Therapeutic footwear: enhanced function in people with diabetes and transmetatarsal amputation. Arch Phys Med Rehabil 1997;78:952–6. [DOI] [PubMed] [Google Scholar]
- 32. McKittrick LS, McKittrick JB, Risley TS. Transmetatarsal amputation for infection or gangrene in patients with diabetes mellitus. Ann Surg 1949;130:826–42. [PubMed] [Google Scholar]
- 33. Nguyen TH, Gordon IL, Whalen D, Wilson SE. Transmetatarsal amputation: predictors of healing. Am Surg 2006;72:973–7. [PubMed] [Google Scholar]
- 34. Stone PA, Back MR, Armstrong PA, et al. Midfoot amputations expand limb salvage rates for diabetic foot infections. Ann Vasc Surg 2005;19:805–11. [DOI] [PubMed] [Google Scholar]
- 35. Toursarkissian B, Hagino RT, Khan K, Schoolfield J, Shireman PK, Harkless L. Healing of transmetatarsal amputation in the diabetic patient: is angiography predictive? Ann Vasc Surg 2005;19:769–73. [DOI] [PubMed] [Google Scholar]
- 36. Sheahan MG, Hamdan AD, Veraldi JR, McArthur CS, Skillman JJ, Campbell DR, Scovell SD, Logerfo FW, Pomposelli FB Jr. Lower extremity minor amputations: the roles of diabetes mellitus and timing of revascularization. J Vasc Surg 2005;42:476–80. [DOI] [PubMed] [Google Scholar]
- 37. Mwipatayi BP, Naidoo NG, Jeffery PC, Maraspini CD, Adams MZ, Cloete N. Transmetatarsal amputation: three‐year experience at Groote Schuur Hospital. World J Surg 2005;29:245–8. [DOI] [PubMed] [Google Scholar]
- 38. Pollard J, Hamilton GA, Rush SM, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. J Foot Ankle Surg 2006;45:91–7. [DOI] [PubMed] [Google Scholar]
- 39. Thomas SRYW, Perkins JMT, Magee TR, Galland RB. Transmetatarsal amputation: an 8‐year experience. Ann R Coll Surg Engl 2001;83:164–6. [PMC free article] [PubMed] [Google Scholar]
- 40. Vitti MJ, Robinson DV, Hauer‐Jensen M, Thompson BW, Ranval TJ, Barone G, Barnes RW, Eidt JF. Wound healing in forefoot amputations: the predictive value of toe pressure. Ann Vasc Surg 1994;8:99–106. [DOI] [PubMed] [Google Scholar]
- 41. Adera HM, James K, Castronuovo JJ Jr, Byrne M, Deshmukh R, Lohr J. Prediction of amputation wound healing with skin perfusion pressure. J Vasc Surg 1995;21:823–9. [DOI] [PubMed] [Google Scholar]
- 42. Attinger CE, Meyr AJ, Fitzgerald S, Steinberg JS. Preoperative doppler assessment for transmetatarsal amputation. J Foot Ankle Surg 2010;49:101–5. [DOI] [PubMed] [Google Scholar]
- 43. Landry GJ, Silverman DA, Liem TK, Mitchell EL, Moneta GL. Predictors of healing and functional outcome following transmetatarsal amputations. Arch Surg 2011;146:1005–9. [DOI] [PubMed] [Google Scholar]
- 44. Anthony T, Roberts J, Modrall JG, Huerta S, Asolati M, Neufeld J, Parker B, Yang W, Sarosi G. Transmetatarsal amputation: assessment of current selection criteria. Am J Surg 2006;192:e8–11. [DOI] [PubMed] [Google Scholar]
- 45. Yusof NM, Rahman JA, Zulkifly AH, Che‐Ahmad A, Khalid KA, Sulong AF. Vijayasingham 3. Predictors of major lower limb amputation among type II diabetic patients admitted for diabetic foot problems. Singapore Med J 2015;56:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woodbury MG. Diabetic foot risk assessment. Diabetes Metab Res Rev 2016;32:376–8. [DOI] [PubMed] [Google Scholar]
- 47. Krause FG, deVries G, Meakin C, Kalla TP, Younger AS. Outcome of transmetatarsal amputations in diabetics using antibiotic beads. Foot Ankle Int 2009;30:486–93. [DOI] [PubMed] [Google Scholar]
- 48. Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, Amato B, Gallelli L, de Franciscis S. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther 2015;13:605–13. [DOI] [PubMed] [Google Scholar]
- 49. Salahuddin O, Azhar M, Imtiaz A, Latif M. A developing world experience with distal foot amputations for diabetic limb salvage. Diabet Foot Ankle 2013; doi: 10.3402/dfa.v4i0.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nageen A. The most prevalent organism in diabetic foot ulcers and its drug sensitivity and resistance to different standard antibiotics. J Coll Physicians Surg Pak 2016;26:293–6. [PubMed] [Google Scholar]
- 51. Boffeli TJ, Reinking R. Plantar rotational flap technique for panmetatarsal head resection and transmetatarsal amputation: a revision approach for second metatarsal head transfer ulcers in patients with previous partial first ray amputation. J Foot Ankle Surg 2014;53:96–100. [DOI] [PubMed] [Google Scholar]
- 52. Altindas M, Cinar C. Promoting primary healing after ray amputations in the diabetic foot: the plantar dermo‐fat pad flap. Plast Reconstr Surg 2005;116:1029–34. [DOI] [PubMed] [Google Scholar]
- 53. Brown ML, Tang W, Patel A, Baumhauer JF. Partial foot amputation in patients with diabetic foot ulcers. Foot Ankle Int 2012;33:707–16. [DOI] [PubMed] [Google Scholar]
- 54. Baumgartner R. Forefoot and midfoot amputations. Oper Orthop Traumatol 2011;23:254–64. [DOI] [PubMed] [Google Scholar]
- 55. Matamoros R, Riepe G, Drees P. Minor amputations: a maxi task :part 2: from transmetatarsal amputation to hindfoot amputation. Chirurg 2012;83:999–1012. [DOI] [PubMed] [Google Scholar]
- 56. DeCotiis MA. Lisfranc and Chopart amputations. Clin Podiatr Med Surg 2005;22:385–93. [DOI] [PubMed] [Google Scholar]
- 57. Elsharawy MA. Outcome of midfoot amputations in diabetic gangrene. Ann Vasc Surg 2011;25:778–82. [DOI] [PubMed] [Google Scholar]
- 58. Boffeli TJ, Thompson JC. Partial foot amputations for salvage of the diabetic lower extremity. Clin Podiatr Med Surg 2014;31:103–26. [DOI] [PubMed] [Google Scholar]
- 59. Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2016;2:CD011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rose JF, Giovinco N, Mills JL, Najafi B, Pappalardo J, Armstrong DG. Split‐thickness skin grafting the high‐risk diabetic foot. J Vasc Surg 2014;59:1657–63. [DOI] [PubMed] [Google Scholar]
- 61. Serra R, Grande R, Butrico L, Montemurro R, De Caridi G, Fugetto F, Dominijanni A, Gallelli L, Greto Ciriaco A, Vitagliano T, Greco M, de Franciscis S. Skin grafting and topical application of platelet gel in the treatment of vascular lower extremity ulcers. Acta Phlebol 2014;15:129–36. [Google Scholar]
- 62. Snyder RJ, Shimozaki K, Tallis A, Kerzner M, Reyzelman A, Lintzeris D, Bell D, Rutan RL, Rosenblum B. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds 2016;28:70–7. [PubMed] [Google Scholar]
- 63. Nehler MR, Whitehill TA, Bowers SP, Jones DN, Hiatt WR, Rutherford RB, Krupski WC. Intermediate‐term outcome of primary digit amputations in patients with diabetes mellitus who have forefoot sepsis requiring hospitalization and presumed adequate circulatory status. J Vasc Surg 1999;30:509–17. [DOI] [PubMed] [Google Scholar]
- 64. Chu YJ, Li XW, Wang PH, Xu J, Sun HJ, Ding M, Jiao J, Ji XY, Feng SH. Clinical outcomes of toe amputation in patients with type 2 diabetes in Tianjin, China. Int Wound J 2016;13:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yarwood‐Ross L, Dignon AM. NPWT and moist wound dressings in the treatment of the diabetic foot. Br J Nurs 2012;21:S26, S28, S30–2. [DOI] [PubMed] [Google Scholar]
- 66. Armstrong DG, Lavery LA, Boulton AJ. Negative pressure wound therapy via vacuum‐assisted closure following partial foot amputation: what is the role of wound chronicity? Int Wound J 2007;4:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Armstrong DG, Lavery LA. Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 68. Ma H, Huang Q, Wang M, Xu K. Intra‐wound injection of platelet‐rich plasma in addition to vacuum‐assisted closure for non‐healing wounds in patients with diabetes mellitus. Surg Infect (Larchmt) 2016;17:378–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martinez‐Zapata MJ, Martí‐Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, Garcia J, Zaror C. Autologous platelet‐rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;5:CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J 2013;10:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marshall C, Brakat T, Stansby G. Amputation and rehabilitation. Surgery (Oxford) 2016;34:188–91. [Google Scholar]
- 72. Armstrong DG, Lavery LA. Plantar pressures are higher in diabetic patients following partial foot amputation. Ostomy Wound Manage 1998;44:30–2. [PubMed] [Google Scholar]
- 73. Matamoros R, Riepe G, Drees P. Minor amputations ‐ a maxi task. Part 1: from the principles to transmetatarsal amputation. Chirurg 2012;83:923–33. [DOI] [PubMed] [Google Scholar]


