Abstract
Pyoderma gangrenosum (PG) is an uncommon inflammatory and ulcerative skin disorder, which is commonly associated with systemic conditions such as inflammatory bowel disease, arthritis and haematological malignancies. It is widely stated that control of the underlying diseases may lead to resolution of PG. However, standard of care dictates that patients suffering with monoclonal gammopathy of undetermined significance or smouldering multiple myeloma (MM) should not receive therapy unless they progress to symptomatic MM. Here, we report a woman in her 40s with a disseminated corticodependent PG, resistant to any treatment attempted, including anti‐tumoral necrosis factor (TNF) therapy in which bortezomib–dexamethasone regimen results in dramatic healing of all lesions in only a month. This case supports the belief that treatment of the underlying monoclonal gammopathy could be necessary when PG presents as an aggressive, non‐responding skin disease.
Keywords: Monoclonal gammopathies, Multiple myeloma, Neutrophilic dermatosis, Pyoderma gangrenosum
Introduction
Pyoderma gangrenosum (PG) is a rare, non‐infectious, neutrophilic dermatosis that is associated with systemic illnesses, such as inflammatory bowel disease, arthritis and haematological malignancies, in at least 50% of patients. The association between PG and monoclonal gammopathies has been widely reported, but incidence varies among different series. Recent studies describe a strong association between IgA isotype and neutrophilic dermatoses 1.
The mainstay of the treatment for PG is usually long‐term immunosuppression, but it is known that in patients with an underlying disease, effective therapy for the associated condition may be linked to an improvement or resolution of the cutaneous process as well. However, according to standard of care, treatment is not recommended for monoclonal gammopathies until they progress to symptomatic multiple myeloma (MM). The case presented here provides additional insight suggesting the usefulness of novel regimens of treatment for MM in monoclonal gammopathies‐related dermatological disorders. These regimens may permit improvement and resolution of the PG in case of ineffectiveness of conventional therapies.
Case report
A woman in her 40s without any other significant medical history presented in 2011 with a 15‐day history of a painful ulcer on the pretibial area of her right lower limb. Physical examination revealed a sharply demarcated ulcer with purple undermined borders, consistent with PG diagnosis. Histological analysis revealed a large neutrophilic infiltrate that caused necrosis. An extensive laboratory study was made, and corticosteroid therapy was started with initial response. Complementary tests revealed an IgA lambda paraprotein, only detected by immunofixation. This M‐protein was consistent with a monoclonal gammopathy of undetermined significance (MGUS). Nonetheless, in the following 5 years, the patient developed new flares of cutaneous lesions on other parts of her body. For this reason, she required almost continuous corticoid treatment. For this reason, she required almost continuous corticoid treatment, and due to its poor evolution, treatment with cyclosporine, colchicine, mycophenolate and dapsone was associated at different times of these years. However, the disease was not controlled with any of them. Thereafter, etanercept treatment was attempted at a dose of 50 mg subcutaneously twice weekly. Despite slight initial improvement, the disease could not be controlled after 4 months of treatment. In that moment, the patient showed an ill appearance with multiple painful cutaneous lesions (Figure 1). Coinciding with the skin worsening, a small increase in paraproteinaemia (0·7 g/dl) was detected, which motivated the reassessment of her MGUS. A bone marrow infiltration with 19% pathological plasmatic cells without any hyperCalcemia, Renal failure, Anemia, Bone lesions (CRAB) criteria or high‐risk factors was revealed. This led to the diagnosis of smouldering MM 2. Smouldering MM is a disorder that does not necessarily need a specific treatment, but because of PG evolution, symptomatic MM treatment was initiated. With a bortezomib–dexamethasone regimen (because of thalidomide intolerance) a complete remission of haematological disease was obtained after five cycles. This was accompanied with a dramatic resolution of patient's skin disease only 1 month after initiating therapy, with complete healing of all ulcers with atrophic scars (Figure 2). Six months later, the patient continues the MM treatment, with no recurrence of PG.
Figure 1.
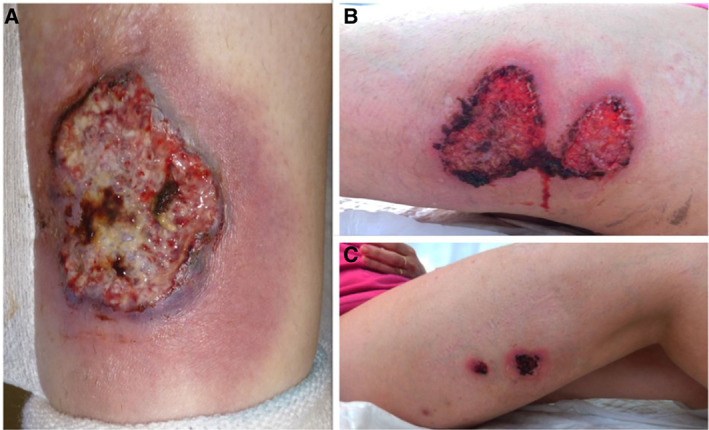
Multiple classic pyoderma gangrenosum lesions on the patient's limbs.
Figure 2.
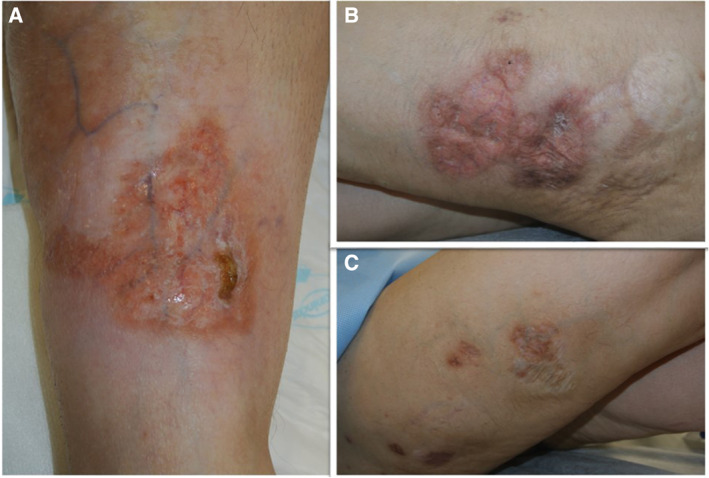
All the lesions were healed with this atrophic appearance after 1 month of bortezomib and dexamethasone therapy.
Discussion
PG is a challenging disease regarding therapeutics. Because of its low incidence, there are no specific schemes of treatment. Among immunosuppressive agents, corticosteroids and cyclosporine are the best characterised. More recently, tumour necrosis factor‐alpha blockers and other injectable biologicals have been used with some success 3. In patients with an underlying disease, therapy addressing the associated condition can be linked to a control of the cutaneous process. MGUS and smouldering MM are conditions that usually do not require any treatment unless they progress to symptomatic MM.
As pathogenesis is not well understood, standard treatment to paraprotein‐related dermatological disorders remains controversial 4. Conventional therapies focused at the skin may result in improvement and, occasionally, remission. Sometimes, a specific treatment of the underlying monoclonal gammopathy has been used in refractory cases 5, and a standard MM regimen in a PG‐associated IgA κ‐type MM has been recently documented 6.
Even though evidence about MGUS reveals that this disease does not usually need treatment, this case supports the fact that when PG‐associated‐monoclonal‐gammopathy presents as an aggressive, non‐responding skin disease, it is reasonable to treat this scenario as symptomatic MM. However, further knowledge about how monoclonal gammopathy is involved in PG aetiopathogenesis may improve the treatment of these patients.
References
- 1. Szalat R, Monsel G, Le Goff W, Battistella M, Bengouffa D, Schlageter MH, Bouaziz JD, Arnulf B, Vignon M, Lesnik P, Saussine A, Malphettes M, Lazareth A, Vignon‐Pennamen MD, Bagot M, Brouet JC, Fermand JP, Rybojad M, Asli B. The spectrum of neutrophilic dermatoses associated with monoclonal gammopathy: association with IgA isotype and inflammatory profile. J Am Acad Dermatol 2015;73:809–20. [DOI] [PubMed] [Google Scholar]
- 2. Rajkumar SV, Dimopoulos MA, Palumbo A, Bladé J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48. [DOI] [PubMed] [Google Scholar]
- 3. Miller J, Yentzer BA, Clark A, Jorizzo JL, Feldman SR. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol 2010;62:646–54. [DOI] [PubMed] [Google Scholar]
- 4. Daoud MS, Lust JA, Kyle RA, Pittelkow MR. Monoclonal gammopathies and associated skin disorders. J Am Acad Dermatol 1999;40:507–35; quiz 36–8. [DOI] [PubMed] [Google Scholar]
- 5. Chang CM, Hwang WL, Hsieh ZY, Wang RC, Teng CL. Monoclonal gammopathy of undetermined significance related pyoderma gangrenosum successfully treated with autologous peripheral blood stem cell transplantation. Ann Hematol 2010;89:823–4. [DOI] [PubMed] [Google Scholar]
- 6. Kikuchi Y, Yoshimi M, Takahashi T. Pyoderma gangrenosum in a patient with multiple myeloma. Intern Med 2016;55:711. [DOI] [PubMed] [Google Scholar]


