Abstract
The aims of this study were to identify, assess, and summarise available evidence about the effectiveness of static air mattress overlays to prevent pressure ulcers. The primary outcome was the incidence of pressure ulcers. Secondary outcomes included costs and patient comfort. This study was a systematic review. Six electronic databases were consulted: Cochrane Library, EMBASE, PubMed (Medline), CINAHL (EBSCOhost interface), Science direct, and Web of Science. In addition, a hand search through reviews, conference proceedings, and the reference lists of the included studies was performed to identify additional studies. Potential studies were reviewed and assessed by 2 independent authors based on the title and abstract. Decisions regarding inclusion or exclusion of the studies were based on a consensus between the authors. Studies were included if the following criteria were met: reporting an original study; the outcome was the incidence of pressure ulcer categories I to IV when using a static air mattress overlay and/or in comparison with other pressure‐redistribution device(s); and studies published in English, French, and Dutch. No limitation was set on study setting, design, and date of publication. The methodological quality assessment was evaluated using the Critical Appraisal Skills Program Tool. Results were reported in a descriptive way to reflect the exploratory nature of the review. The searches included 13 studies: randomised controlled trials (n = 11) and cohort studies (n = 2). The mean pressure ulcer incidence figures found in the different settings were, respectively, 7.8% pressure ulcers of categories II to IV in nursing homes, 9.06% pressure ulcers of categories I to IV in intensive care settings, and 12% pressure ulcers of categories I to IV in orthopaedic wards. Seven comparative studies reported a lower incidence in the groups of patients on a static air mattress overlay. Three studies reported a statistical (P < .1) lower incidence compared with a standard hospital mattress (10 cm thick, density 35 kg/m3), a foam mattress (15 cm thick), and a viscoelastic foam mattress (15 cm thick). No significant difference in incidence, purchase costs, and patient comfort was found compared with dynamic air mattresses. This review focused on the effectiveness of static air mattress overlays to prevent pressure ulcers. There are indications that these mattress overlays are more effective in preventing pressure ulcers compared with the use of a standard mattress or a pressure‐reducing foam mattress in nursing homes and intensive care settings. However, interpretation of the evidence should be performed with caution due to the wide variety of methodological and/or reporting quality levels of the included studies.
Keywords: incidence, low‐technology support surfaces, pressure ulcers, prevention, static air support surfaces
1. INTRODUCTION
“A pressure ulcer (PU) is defined as a localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear. A number of contributing factors are also associated with pressure ulcer; the significance of these factors is yet to be elucidated”.1
The presence of PUs is internationally accepted as an important indicator of the quality of care. Prevention should include the identification of at‐risk patients and the introduction of preventive interventions tailored to the patient. Primarily, the interventions should focus more on prevention rather than treatment of PUs.2 The decision to use pressure‐redistributing resources is determined by risk assessment, patient comfort, general health, training, and the availability of materials and resources.2, 3 International and national guidelines recommend regular repositioning to prevent PUs.1, 2 Repositioning must be performed in patients with an increased risk of PUs. The presence of non‐blanchable redness indicates that the frequency of repositioning and/or pressure‐redistributing resources is not efficacious.4 Further research is needed regarding the use of repositioning on different pressure‐redistribution support surfaces.
In general, the use of a static air mattress overlay is lower compared with the alternating pressure mattress.5 In a study by Serraes and Beeckman6, 31% of the participants received no specified PU prevention interventions, and 43% was lying on a viscoelastic foam mattress. A significantly lower number of participants were lying on an alternating pressure mattress or a static air mattress overlay—24% and 2% of the participants, respectively.
A Cochrane systematic review defined 3 groups of pressure‐redistribution support surfaces (PRSS): high‐technology support surfaces (electrically powered, alternating pressure devices), low‐technology support surfaces (not electrically powered, constant low‐pressure devices), and other support surfaces (operating table mattress pad, rotating beds, cushions, and limb protectors).7 The high‐technology supported surfaces include alternating‐pressure mattresses/overlays, air‐fluidised beds, and low‐air‐loss beds. The high‐technology supported surfaces provide pressure redistribution by cyclic inflated and deflated air characterised by frequency, duration, amplitude, and rate of change, with or without the body weight of the patient resting on the surface.8 The low‐technology support surfaces include sheepskins and mattresses or overlays filled with standard foam, alternative foam, gel, fibre, air, water, and bead.7
Support surfaces conform to the contours of the body to spread the load of the patient over a maximum area when a person lies on the mattress.6, 9, 10 Pressure redistribution is based on the principles of envelopment and immersion to increase contact surface.4 “Envelopment” is the ability of a support surface to conform (fit or mould) around irregularities of the body.8 “Immersion” refers to the depth of penetration or “sinking” into a support surface.8 Envelopment and immersion are possible with a static air mattress overlay.4, 11, 12 The static air mattress overlay is compact and low in weight. It consists of several compartments; the air moves over a large area when a person lies on the mattress.6, 9, 13, 14
Currently, there is no systematic review that specifically assesses the effectiveness of a static air mattress overlay in the prevention of PUs.
2. AIM AND OBJECTIVES
The aim of this systematic review was to identify, assess, and summarise evidence to prevent the development of PUs on a static air mattress overlay. One primary outcome (PU incidence) and 2 secondary outcomes (cost and patient comfort) were carefully selected to guide the reporting of the data from the original studies. All data were reported in a descriptive way. The aim was not to develop broader statements about effectiveness of the devices because of the exploratory nature of the review.
3. METHOD
3.1. Eligibility criteria, information sources, and search
A 2‐step search strategy was applied to identify relevant studies. Firstly, 6 electronic databases were systematically searched: the Cochrane Library, EMBASE, PubMed (Medline), CINAHL (EBSCOhost interface), Science Direct, and Web of Science. The terms and synonymies for PUs, static air mattress overlay, and prevention were combined (see Table 1).
Table 1.
Search terms used in: PubMed (Medline), Cinahl (EBSCOHOST interface), Science Direct, and Web of Science
| Search terms | Mesh terms |
|---|---|
| 1. Pressure ulcer | (pressure ulcer) OR (ulcers pressure) OR (decubitus) OR (pressure damage) OR (bed sore) OR (bedsores) OR (pressure sore) OR (pressure sores) OR (scores, pressure) |
| 2. Static air‐filled mattress overlay | (static mattress) OR (static cushion) OR (static overlay) OR (air‐filled overlay) OR (air‐filled cushion) OR (air cushion) OR (air mattress) OR (air overlay) OR (low tech overlay) OR (low tech mattress) OR (non‐powered mattress) OR (non‐powered overlay) OR (constant low‐pressure support mattress) OR (constant low‐pressure support surfaces) OR (constant low mattress) |
| 3. Prevention | (prevention) OR (primary prevention) OR (preventive therapy) OR (prophylaxis) OR (preventive measures) OR (accident prevention) |
Search strategy 1 AND 2 AND 3.
Search term used in Cochrane Library were (“pressure ulcer” AND ”static”).
Search term used in Embase were (“pressure ulcer” AND “static” AND “Prevention”).
Secondly, a hand search through reviews, conference proceedings (European Wound Management Association, European Pressure Ulcer Advisory Panel), and the reference lists of the included studies was performed to identify additional studies.
Studies were included if the following criteria were met: (1) reporting an original study; (2) the outcome was the incidence of PUs Categories I to IV when using a static air mattress overlay and/or in comparison with other pressure‐redistribution device(s); and (3) studies published in English, French, and Dutch. No limitation was set on study design, date of publication, and patient population. If no full text could be found, 1 or more authors were contacted by mail. Studies were excluded if: (1) the support surfaces considered static air‐filled seat cushions and/or heel wedges and (2) there were insufficient data on primary and secondary outcomes.
The results of the systematic search were initially screened on titles and abstracts by researchers. The full texts of the potential studies were obtained and assessed for inclusion by 2 independent researchers.
3.2. Data abstraction and quality appraisal
The included studies were analysed in more detail. A template for data extraction was developed, including the following elements: (1) year of publication, (2) design, (3) sample, (4) setting, (5) primary outcome, (6) secondary outcome, (7) PU classification system used to assess the outcomes, and (8) follow‐up period of participants (see Table 3: summary included studies, and Appendix S1 [Supporting information]).
Two researchers independently extracted the data and performed the quality appraisals of the included studies. The methodological quality assessment evaluated the risk of bias using the Critical Appraisal Skills Program (CASP). The Randomised Control Trail (RCT) CASP tool consists of 11 questions for a systematic assessment of the quality of an RCT. The COHORT CASP tool consists of 12 questions for a systematic assessment of the quality of a Cohort Study.15 Disagreements regarding the quality assessment were discussed until consensus was reached. The inter‐rater reliability of the quality assessment between the 2 researchers was calculated using the percentage of agreement and the inter‐rater reliability analysis (Cohen's kappa, κ).
4. RESULTS
The systematic search resulted in 925 studies. The hand search resulted in 10 studies from reference lists of reviews and conference proceedings. The authors of 2 studies were contacted; 1 of the authors responded to the request for the full‐text publication. Table 2 reflects the results of the study selection.
Table 2.
Summary of included/excluded studies
| Results | Cochrane library | Embase | Pubmed (Medline) | Cinahl | ScienceDirect | Web of Science | Hand search |
|---|---|---|---|---|---|---|---|
| Total | 15 | 173 | 237 | 115 | 297 | 88 | — |
| Excluded | 10 | 160 | 219 | 107 | 295 | 79 | — |
| Included | 5 | 13 | 18 | 8 | 2 | 9 | 10 |
In total, 65 potential studies were included based on the screening of title and abstract. Based on the inclusion criteria and after excluding duplicates, 52 studies were excluded (see Figure 1 Flowchart). The reasons for exclusion were irrelevant outcome and insufficient data. The remaining 13 studies were included for further data extraction and are shown in Table 3.
Figure 1.
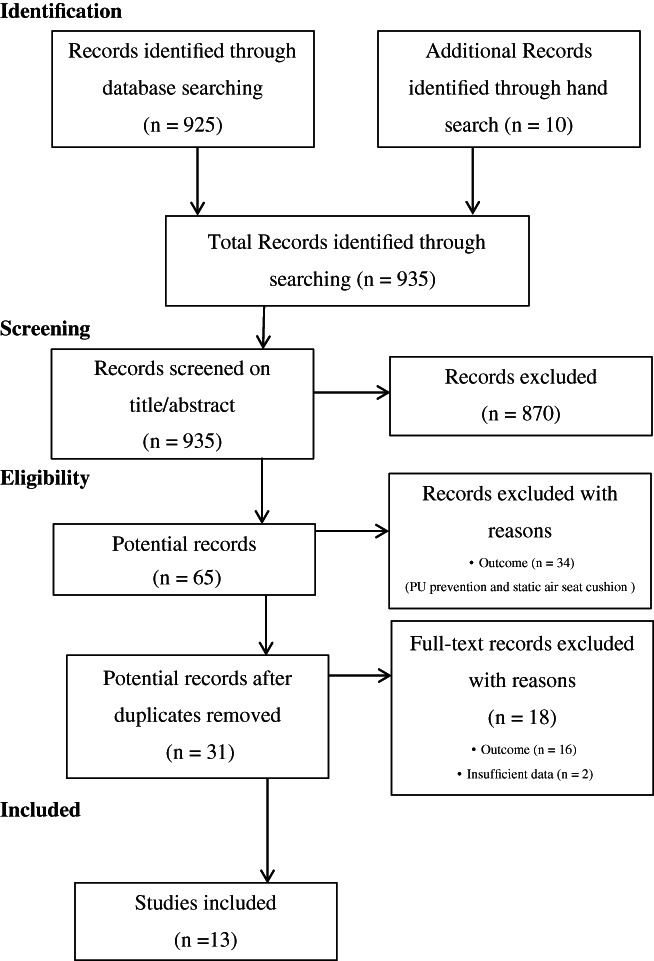
Flowchart
Table 3.
Summary of the included studies
| Author | Design, sample and setting | Primary outcome | Secondary outcome | Classification PU | Follow up | |
|---|---|---|---|---|---|---|
| Lazzara and Buschmann, 199117 |
RCT n = 74 Nursing homes |
Exp.: Air ‐filled overlay (SOF CARE) PU Categories I‐II n = 32.2% (n = 10/31) PU Categories I n = 16.1% (n = 5/31) PU Categories II n = 16.1% (n = 5/31) Contr. Gel mattress (No specification) PU Categories I‐II n = 31.7% (n = 8/26) PU Categories I n = 15.8% (n = 4/26) PU Categories II n = 15.8% (n = 4/26) P = not reported |
PU Healing: Air‐filled overlay 58% (n = 7/12) PU improved or decreased. 41.7% (n = 5/12) increased or unchanged Gel mattress 60% (n = 9/15) improved, 40% (n = 6/15) worsened or unchanged P = not reported |
Not reported |
6 months Weekly skin assessments |
|
| Sideranko et al, 199220 |
RCT n = 57 Hospital Surgical intensive care units |
Exp.: Static air mattress overlay (4‐in. thick, Gay Mar Sof Care bed, Unikion Gay Mare industries Inc. Orchard park, New York) PU n = 5% (n = 1/20) Contr.1: Alternating air mattress overlay (1 ½ inch thick, alternating air mattress Lapidus Airfloat system, American Hospital supply Corp., Valencia, California) PU n = 25% (n = 5/20) Contr.2: Water mattress overlay (4 in. thick water mattress Lotus RXM 3666, Connecticut Artcraft Corp. Naugatuck, Connecticut) PU n = 12% (n = 2/17) NS |
Mean pressure position: Alt 3800 mmHg Static air 2500 mmHg Water 2330 mmHg |
Not reported |
Total days not reported Mean follow up 9.4 days |
|
| Takala et al, 199621 |
RCT n = 40 Hospital Acute respiratory failure, intensive care, no traumatised patients |
Exp.: Carital air‐float system (series of 21 double air cells). (Carital Optima, Carital Ltd.) PU Categories I‐II n = 0% Contr.: Standard hospital mattress 10 cm thick foam mattress, density 35 kg/m3 (Espe Inc. Kouvola, Finland) PU Categories I‐II n = 36.8% (n = 7/19) PU Categories I n = 12 PU Categories II n = 1 P <.005 |
SkinT° at area pressure expose was lower at air mattress (P < .001) Pressure interface between skin—mattress was lower in air mattress, most prominent at sacrum (different days ranging from P < .001 to NS) |
Grading of Shea |
1‐year study 14 days follow up |
|
| Cobb et al, 199716 |
RCT n = 123 Military hospital, critical care |
Exp.: EHOB Waffle static air mattress (EHOB, Indianapolis, Indiana) PU Categories >I n = 19.7% (n = 12/61)Categories I: n = 2% (1/61)Categories II: n = 18% (11/61)Eschar: n = 0% Contr.: Low‐air‐loss specialty (KinAir) PU Categories >I 12·9% (n = 8/62)Categories I: n = 5% (3/62)Categories II: n = 5% (3/62)Eschar: n = 3% (2/62) NS |
Cost EHOB Waffle $59 000 bed rental fees were saved. Statistical difference cost for PU prevention and treatment (P = .017) |
Not reported |
14 month Frequency of skin assessment unclear |
|
| Cooper et al, 199825 |
RCT n = 100 Hospital emergency trauma, orthopaedic wards |
Exp.: ROHO mattress (Bellville, Illinois) PU Categories I n = 11.6% (n = 5/43) Contr. Sofflex mattress PU Categories I‐II n = 7.3% (n = 3/41) PU Categories I n = 4.9% (n = 2/41)PU Categories II n = 2.4% (n = 1/41) P = not reported |
Comfort: (5‐point scale) ROHO comfortable or high comfortable 79% (n = 34/43) SOFFLEX comfortable or high comfortable 90% (n = 37/41)Uncomfortable ROHO n = 5/43 (12%) vs SOFFLEX n = 0 P = not reported |
Stirling pressure ulcers severity scale |
10 months Weekly skin assessments |
|
| Price et al 199913 |
RCT n = 80 Hospital Orthopaedic ward, neck of femur fracture |
Exp.: Low‐pressure inflatable mattress and cushion (Repose, Frontier Therapeutic Ltd.) PU Categories I‐III n = 20.8% (n = 5/24) (Day 14) PU Categories I n = 8.3% (n = 2/24) PU Categories II n = 0 PU Categories III n = 12.5% (n = 3/24) Contr.: Dynamic support mattress (Nimbus II, ArjoHuntleigh, Luton Bedfordshire, UK) and alternating pressure cushion (Alpha Transcell) PU Categories I‐III n = 15.4% (n = 4/26) (Day14) PU Categories I n = 7.7% (n = 2/26) PU Categories II n = 3.8% (n = 1/26) PU Categories III n = 3.8% (n = 1/26) NS |
Comfort Low tech (n = 24) mean 67 (SD = 18)High tech (n = 26) mean 60 (SD = 25 NS Cost Low tech single patient less £ 5000 (50% of the cost high tech. |
EPUAP |
14 days Weekly skin assessments |
|
| Malbrain et al, 23 |
RCT n = 16 Hospital Intensive care unit |
Exp.: Static air mattress (ROHO) PU Categories I n = 1/8 (12.5%) PU Categories II‐IV n = 1/8 (12.5%) Contr.: Active alternating pressure mattress (Nimbus III) PU Categories I n = 2/8 (25%) PU Categories II‐IV n = 0/8 (0%) NS |
Treatment Pu healing: Improve: Nimbus III; 82% and static 0% (P = .002) Remained stable: Nimbus III 18% and static 33% (NS) Deterioration: Nimbus III 0% and static 67% (P = .006) PU Categories III Nimbus III 0% and static 66.7% (n = 4) (P = .008) |
EPUAP |
1‐19 days Mean study duration was 12.2 days |
|
| Rich et al, 201118 |
Secondary analysis Prospective cohort n = 651 Hospitals hip fracture |
Global non‐powered PRSS (high‐density foam, static air of gel‐filled mattress or overlay) PU Categories I‐IV n = 3.6% (n = 54/1496) No PRSS PU Categories I‐IV n = 4.2% (n = 195/4632) Powered mattress (Alternating mattress, low‐air‐loss mattress, alternating pressure overlay) PU Categories I‐IV n = 4.5% (n = 28/623) NS |
4‐point scale + unstageable, slightly different from EPUAP | 0‐21 days | ||
| Van Leen et al, 201119 |
RCT n = 83 Nursing home |
Exp.: Static air overlay (Repose, Frontier Therapeutic Ltd.) on top of cold foam mattress (15 cm thick) PU Categories II‐III n = 4.8% (n = 2/42) PU Categories II n = 1 PU Categories III n = 1 Contr.: Cold foam mattress (15 cm thick, Silhouette, Comfortex, Winoma) PU Categories II‐III n = 17.1% (n = 7/41) PU Categories II n = 2 PU Categories III n = 5 NS P = .088 |
Healing PU: PU on cold foam (n = 5/7) mattress showed no healing with the Institutional PU protocol vs (n = 2/2) all PU on static air mattress showed healing with Pu protocol Risk factor: No difference of PU incidence Norton score 5‐8 (high risk) vs Norton score 9‐12 (median risk) |
EPUAP |
6 months Weekly skin assessment |
|
| Vermette et al, 201222 |
RCT n = 110 Hospital Acute care facility (medical, surgical, active geriatric, intensive care unit wards |
Exp.: Inflated static overlay (ISO) (EHOB Waffle, EHOB, Indianapolis, Indiana) PU n = 3.6% (n = 2/55) Contr.:Micro fluid static overlay (RIK) OR Low‐air‐loss dynamic mattress (TheraKair, KCI, medical, San Antonio, Texas) Pu n = 11% (n = 5/55) NS |
Comfort: (5‐point scale) MSO/LALM were more comfortable (n = 90%, 27/30) than ISO (n = 85%, n = 29/34) NS Cost: MSO/LALM $13 606 CAD ISO $3.364CAD P < .001 |
NPUAP |
Max 14 days, follow up Weekly skin assessment (Monday, Wednesday, Friday) |
|
| Van Leen et al, 201324 |
RCT cross over n = 41 Nursing home |
Exp.: Static air overlay (Repose, Frontier Therapeutic Ltd.) on top of viscoelastic foam mattress PU Categories II‐IV n = 5.2% (n = 2/39) Contr.: Viscoelastic foam mattress, 15 cm, Duosmart, Kabelwerk, Eupen, Belgium) PU Categories II‐IV n = 22.2% (n = 8/40) NS P = .087 |
Healing PU: Exp.: 100% (n = 2/2) healed with Pu protocol. Contr.: 25% (n = 2/8) no signs of healing with Pu protocol. Risk factor: Difference regarding PU incidence between pp. with a very low Braden score between 6 and 12, and pp with a mean score between 13 and 19 |
EPUAP |
1 Year (2×6 month) Weekly skin assessment |
|
| Jiang et al, 201426 |
RCT n = 1074 Hospitals Intensive care unit, surgical wards |
Exp.: EHOB Waffle static air mattress, EHOB PU Categories I‐II n = 1.07% (n = 6/562) Contr.: Power pressure air mattress (PPAM) (Sanma mattress manufacturing factory, Shanghai, China) PU Categories I‐II n = 0.98% (n = 5/512) NS |
Comfort NS | NPUAP | 0‐5 days | |
| Serraes and Beeckman, 20166 |
Prospective Cohort n = 174 Nursing homes |
Static air mattress, cushion, wedge (Repose, Frontier Therapeutic Ltd.) PU Categories II‐III n = 5.1% (n = 9/174) PU Categories II n = 3.4% (n = 6/9) PU Categories III n = 1.7% (n = 3/9) |
Time to develop PU: 16 Days (IQR 2‐26) PU location: Sacral region 89% (n = 8/9) Risk factors: Time of sitting in chair (PU Categories I‐III) |
EPUAP |
30 days Daily skin assessment |
|
Abbreviations: NS, not significant; PU, pressure ulcer; RCT, randomised controlled trail; S, significant.
The overall agreement for study selection based on title/abstract between the reviewers was high, and the inter‐rater reliability analysis (Cohen's kappa, κ) showed an almost perfect degree of agreement—respectively, 83.9% (n = 26) and a Cohen's kappa of 0.832 (P < .001), 95% CI (0.634, 1.000).
The quality of the 13 studies was assessed. No study was excluded based on the quality assessments (internal and external validity). The most important methodological limitations were a lack of information or clarification about (1) mattress specification (n = 4),16, 17, 18, 19 (2) randomisation method (n = 3),20, 21, 22 (3) withdrawals and dropouts,16, 17, 18, 19, 20, 22, 23 (4) blinding method (n = 9),13, 16, 17, 19, 20, 22, 23, 24, 25, 26 (5) similar from baseline (n = 6), and groups treated equally (n = 5) (see Table 4: judgement about the methodological quality of the studies).
Table 4.
Judgement about the methodological quality of the studies
| Lazzara and Buschmann, 199117 | Sideranko et al, 199220 | Takala et al, 199621 | Cobb et al, 199716 | Cooper et al, 199825 | Price et al, 199913 | Malbrain et al, 201023 | Van Leen et al, 201119 | Vermette et al, 201222 | Van Leen et al, 201324 | Jiang et al, 201426 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CASP tool for RCT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Did the trial address a clearly focused issue? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the assignment of patients to treatments randomised? | Y | ? | ? | Y | Y | Y | Y | Y | ? | Y | Y |
| Were patients, health workers, and study personnel blinded? | ? | ? | Y | ? | ? | N | ? | N | N | N | N |
| Were the groups similar at the start of the trial? | Y | ? | Y | N | Y | Y | Y | ? | N | ? | ? |
| Aside from the experimental intervention, were the groups treated equally? | Y | ? | ? | N | Y | Y | N | Y | Y | Y | ? |
| Were all of the patients who entered the trial accounted for at its conclusion? | N | Y | N | Y | N | Y | N | Y | Y | Y | Y |
|
How large was the treatment effect? |
N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| How precise was the estimate of the treatment effect? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Can the results be applied in your context? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were all clinically important outcomes considered? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are the benefits worth the harms and costs? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Rich et al, 201118 | Serraes and Beeckman, 20166 | |
|---|---|---|
| CASP Tool for cohort studies | Y | Y |
| Did the trial address a clearly focused issue? | Y | Y |
| Was the cohort recruited in an acceptable way? | Y | Y |
| Was the exposure accurately measured to minimise bias? | ? | Y |
| Was the outcome accurately measured to minimise bias? | ? | Y |
| Have the authors identified all important confounding factors? | ? | ? |
| Have they taken in account of the confounding factors in the design and/or analysis? | ? | ? |
| Was the follow up of subjects complete enough? | Y | Y |
| Was the follow up of subjects long enough? | Y | Y |
| What are the results of this study? | N/A | N/A |
| How precise are the results? | N/A | N/A |
| Do you believe the results? | Y | Y |
| Can the results be applied to the local population? | Y | Y |
| Do the results of this study fit with other available evidence? | N | Y |
| What are the implications of this study for practice? | N/A | N/A |
Abbreviations: ?, unclear; N, no; N/A, not applicable; Y, yes.
The overall agreement rate and inter‐rater reliability analysis (Cohen's kappa, κ) for the quality assessments between researchers was high to almost perfect—respectively, an overall agreement of 96.7% (n = 117) and a Cohen's kappa of 0.905 (P < .001), 95% CI (0.791, 0.980).
4.1. Characteristics of the included studies
The main outcome of this review was the incidence of PUs of categories I to IV when using a static air mattress overlay and/or in comparison with other pressure‐redistribution device(s) for the prevention of PUs (see Table 5). The data of the included studies were heterogeneous (see Table 3 and Appendix S1).
Table 5.
Specification static air mattress overlay
| Specification mattress overlay | Studies (n) | References |
|---|---|---|
| EHOB Waffle static air mattress overlay | 4 | 16, 19, 22, 26 |
| Repose low‐pressure inflatable mattress | 3 | 6, 13, 24 |
| ROHO reactive mattress overlay | 2 | 23, 25 |
| SOFFLEX mattress: similar to the ROHO mattress but consists of lager cells and therefore requires only 3 sections to make a full mattress | 1 | 25 |
| Sof Care air‐filled overlay | 1 | 17 |
| Gay Mar Sof Care static air | 1 | 20 |
| Carital air‐float system | 1 | 21 |
The included studies were RCTs (n = 11) and cohort studies (n = 2) conducted in nursing homes, intensive care units, surgical wards, and orthopaedic wards. Sample size ranged from 16 to 1074 participants. The most used baseline characteristics were patient age, evaluation of PU at admission, and risk assessment scales. The age of the participants ranged from >18 years (n = 3) to >60 years (n = 1) and >65 years (n = 5). In 4 studies, age was not reported.
Various risk assessment scales and cut‐off points were used in the included studies: Norton score 5 to 12 (n = 1), Norton score < 8 (n = 2), Norton score > 15 (n = 1); Braden score 9 to 16 (n = 1), Braden score ≤ 12 (n = 1), Braden score ≤ 14 (n = 1), Braden score ≤ 16 (n = 1), Braden score < 18 (n = 1); Waterlow score > 15 (n = 1); and Medley score > 25 (n = 1). Two studies reported no risk assessment scales. Four studies included participants with PUs at admission.
There was a variety of classification systems for the assessment of the skin: European Pressure Ulcers Scale (EPUAP), National Pressure Ulcers Scale (NPUAP) (n = 2), Stirling Pressure Score Severity Scale (n = 1), Grading or Shea (n = 1), formulated own classification system different from EPUAP/NPUAP (n = 3), and no reported classification (n = 2).
In some studies, the static air mattress overlay was compared with a high‐technology supported surface or compared with another low‐technology support surface, a gel mattress, a water mattress overlay, a (cold) foam mattress, a viscoelastic foam mattress, and a Micro Static fluid Overlay (MSO) based on foam (see Table 3 and Appendix S1).
4.2. Primary outcome: PU incidence
In 6 studies, a static air mattress overlay was compared with a high‐technology supported surface.13, 16, 18, 20, 23, 26 Two studies, each in a different setting (hospital hip fracture and surgical intensive care), reported a lower overall incidence of PU in the group where the static air mattress overlay was used.18, 20 Another study in a similar orthopaedic ward (n = 1)13 and studies in intensive care wards (n = 3) reported a lower incidence of PUs in the group where the high‐technology supported surfaces was used.16, 23, 26 None of the included studies found significant differences in the incidence of PUs between a static air mattress overlay and a high‐technology supported surface in a variety of settings: intensive care and orthopaedic wards.
In 7 studies, a static air mattress overlay was compared with another low‐technology support surface (not electrically powered, constant low‐pressure devices). A study conducted in a nursing home reported a higher incidence of PUs of categories I to II in the group where a static air mattress overlay was used, compared with a gel mattress, respectively, 32.2% and 31.7%.17 The other 6 studies conducted in nursing homes, intensive care units, and emergency orthopaedic trauma wards reported a lower PU incidence rate in the groups where the static air mattress overlay was used.19, 20, 21, 22, 24, 25 Of these, 3 studies showed a difference at a significance level of <.1. PU incidence compared with a standard hospital mattress (10 cm thick, density 35 kg/m3) (P = P < 0.005), a foam mattress (15 cm thick) (P = 0.088), and a viscoelastic foam mattress (15 cm thick) (P = 0.087).19, 21, 24 The studies were conducted in an intensive care unit and nursing homes (see Table 3 and Appendix S1). A multicentre cohort study in nursing homes reported an incidence of 5.1% (n = 9/176) PUs of categories II to IV.6 This incidence figure is similar to other studies.18, 19, 20, 21, 22, 24 The mean PU incidence figures found in the different settings was 7.8% PUs of categories II to IV in nursing homes,6, 17, 19, 24 9.06% PUs of categories I to IV in intensive care settings,16, 20, 21, 22, 23, 26 and 12% PUs of categories I to IV in orthopaedic wards.13, 18, 25
4.3. Secondary outcomes
In this review, secondary outcome measures of cost (n = 3) and patient comfort (n = 4) were included (see Table 3).
4.3.1. Cost
The costs of static air mattress overlays was less expensive compared with high‐technology supported surfaces in the prevention and treatment of PUs.13, 16, 22 Price et al (1999) reported that the costs of a static air mattress overlay for single‐patient use would be less than £5000, which was a difference of 50% when using a dynamic support mattress in the prevention of PUs. Vermette et al22 found that the static air mattress overlays were less expensive in total costs compared with the MSO and low‐air‐loss dynamic mattress, respectively, $3364 CAD vs $13 606 CAD (P = .001). Cobb et al16 found a significant difference of $ 59 000 in rental fees (P = .017) compared with the costs of the low‐air‐loss mattress. Overall, there was a wide variety in the approach of the cost.
4.3.2. Patient comfort
Comfort of the patient was evaluated using a 5‐point rating scale.22, 25, 26 No significant difference in comfort rating was reported. One study reported a higher comfort level when using a static air mattress overlay compared with a dynamic support mattress.13
5. DISCUSSION
The aim of this review was to identify, assess, and summarise available evidence about the effectiveness of static air mattress overlays to prevent PUs. Generating broader statements about the effectiveness of these devices was not possible because of large heterogeneity in study designs, outcome reporting, surfaces being tested, and methodological limitations. The primary outcome of this review was the incidence of PUs of categories I to IV, and the data were reported in a descriptive way to reflect the exploratory nature of the review. Based on the included studies, a static air support surfaces appeared to be more effective compared with a standard mattress and pressure‐reducing foam mattress. However, the studies comparing the effectiveness of static air support surfaces with high‐technology support surfaces reported inconsistent evidence. It is not possible to conclude which support surface is superior. Overall, we found varying figures of mean PU incidence in a variety of settings: 7.8% PUs of categories II to IV in nursing homes, 9.06% PUs of categories I to IV in intensive care settings, and 12% PUs of categories I to IV in orthopaedic wards. We could not conclude that a static air support surface is more or less effective than other support surfaces in a variety of settings.
Secondary outcomes focused on costs and patient comfort. The costs of static air mattress overlays were lower compared with high‐technology supported surfaces.13, 16, 22 However, cost data were not always or were inconsequently reported in the primary studies. There was a wide variety in the methods used to collect cost data. One study compared rental fees with purchasing costs of the support surfaces.16 No formal economic model was designed because of the lack of information and the heterogeneity about economic data in the included studies. Further research should focus on performing and reporting economic data based on international guidelines. Patient comfort was evaluated in 4 studies.13, 22, 25, 26 Only 1 study reported a higher comfort level when using a static air mattress overlay compared with a dynamic support mattress. However, the method to evaluate the comfort was not clearly described.13 The incidence of PUs should be a primary outcome, but cost and especially patient comfort are also relevant and must be taken into account. All 3 measures provide important information for policymakers for decision making about purchasing support surfaces for their organisation. Therefore, further research is recommended on the cost‐effectiveness and patient comfort of the static air mattress overlay in the prevention and/or treatment of PUs in patients in different health care settings.
5.1. Limitations
The methodological quality of the studies was assessed using the CASP. Because of the exploratory character of the review, the authors decided not to exclude studies based on methodological quality. The CASP evaluation revealed that major issues remained at the level of blinding, reporting of comparability of study groups, reporting about confounding factors, follow up, and analyses. No differences were seen when we evaluated the quality of the reporting over time. Many resources are invested in designing clinical studies, with only limited contribution to the scientific community and clinical practice. The results of this review should be interpreted with caution and should take into account the wide variety of methodological and/or reporting quality of the included studies.
Core outcome sets (COS) for guiding the design of clinical studies and reporting of the results are currently widely being developed. The field of PUs will clearly benefit from the existence of such a COS. The use of valid outcomes and instruments to measure them in a reliable way will benefit the reporting and will reduce the heterogeneity of outcome selection. This will allow authors of systematic reviews to pool non‐heterogeneous data into meta‐analyses and to generate broader statements on the relative effectiveness of the different surfaces. This was not possible in this systematic review.
5.2. Implications for clinical practice and future research
The evidence about static air support surfaces is limited. Results suggest that these support surfaces are more effective compared with a standard mattress or a pressure‐reducing foam mattress in nursing homes and intensive care settings. However, evidence should be treated with caution. In a variety of settings, no support surface was found to be superior in effectiveness and in comfort for patients. No significant difference in purchase costs was found compared with dynamic air mattresses. The results of the studies indicated that the static air support surface was less expensive compared with the high‐technology mattresses.
The selection of the best support surface for each individual patient involves various factors and is, therefore, quite complex. The decision to use pressure‐redistributing resources is determined by the risk assessment, patient comfort, general health, training, and the availability of materials and resources.2, 3
Independent, well‐designed, multicentre RCTs are recommended to assess the efficacy and cost‐effectiveness of static air support surfaces in the prevention and treatment of PUs in a variety of settings. Studies with a clear and transparent methodology (clinical setting, clearly described baseline characteristics, inclusion and exclusion criteria, adequate follow‐up period, use of a risk assessment scale, an international PU classification system, a repositioning protocol, clear definition of the study mattresses, clearly described randomisation, dropouts, missing data, and data analysis) are essential. Researchers should take into account the limitations of previous studies when designing new research. Unfortunately, several limitations were similar between the included studies.
6. CONCLUSION
This review focused on the effectiveness of static air mattress overlays to prevent PUs. There are indications that these mattress overlays are more effective in preventing PUs compared with a standard mattress or a pressure‐reducing foam mattress in nursing homes and intensive care setting. No studies reported significant differences in effectiveness, patient comfort, and purchase costs between a static air mattress overlay compared with a high‐technology mattress. However, the available evidence should be treated with caution due the wide variety of methodological and/or reporting quality levels of the included studies. Study quality is a major issue as many resources are invested in designing clinical studies, with limited contribution to the scientific community and clinical practice.
Supporting information
Appendix S1 Extended evidence table.
ACKNOWLEDGEMENTS
The authors thank Mrs. Jolien Heirman RN, MSc, for proofreading the manuscript.
Conflict of interest
The authors declare no potential conflict of interests.
Serraes B, van Leen M, Schols J, Van Hecke A, Verhaeghe S, Beeckman D. Prevention of pressure ulcers with a static air support surface: A systematic review. Int Wound J. 2018;15:333–343. 10.1111/iwj.12870
Contributor Information
Brecht Serraes, Email: brechtserraes@hotmail.be.
Dimitri Beeckman, Email: dimitri.beeckman@ugent.be.
REFERENCE
- 1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance . Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Emily Haesler (Ed.). Cambridge Media: Perth, Australia; 2014. [Google Scholar]
- 2. Beeckman D, Matheï C, Van Lancker A, et al. Een nationale richtlijn voor Decubituspreventie. Good Clinical Practice (GCP). Brussel: Federaal Kenniscentrum voor de Gezondheidszorg (KCE). 2012. KCE Reports 193A. D/2012/10.273/95. [Google Scholar]
- 3. Halfens RJG, Meijers RJG, Meesterberends E, et al. Landelijke Prevalentiemeting Zorgproblemen rapportage resultaten 2014. Maastricht: Universiteit Maastricht; CAPHRI School for Public Health and Primary Care, Department of Health Services Research Focusing on Chronic Care and Ageing; 2014. [Google Scholar]
- 4. Wounds International . International review: Pressure ulcer prevention: pressure, shear, friction and microclimate in context. A consensus document. London: Wounds International; 2010. [Google Scholar]
- 5. Defloor T, Bouzegta N, Beeckman D, Vanderwee K, Gobert M, Van Durme T. Studie van de decubitusprevalentie in de Belgische ziekenhuizen; 2008.
- 6. Serraes B, Beeckman D. Static air support surfaces to prevent pressure injuries: a multicenter Cohart study in Belgian nursing homes. J Wound Ostomy Cont Nurs. 2016;43(4):375‐378. [DOI] [PubMed] [Google Scholar]
- 7. McInnes E, Jammali‐Blasi A, Bell‐Syer SEM, Dumville JC, Middleton V, Cullum N. Support Surfaces for Pressure Ulcer Prevention. 2015. Issue 9. CD001735. 10.1002/14651858.CD001735.pub5. Accessed December 1, 2016. [DOI] [PMC free article] [PubMed]
- 8. National Pressure Ulcer Advisory Panel . Terms and definitionsrelated to support surfaces. http://www.npuap.org/NPUAP_S3I_TD.pdf. last accessed date 1/12/2016.
- 9. Hampton S. Repose: the cost‐effective solution for prompt discharge of patients. Br J Nurs. 2000;9(21):2249‐2253. [DOI] [PubMed] [Google Scholar]
- 10. Collins F. Vicair academy mattress in the prevention of pressure damage. Br J Nurs. 2002;11(10):715‐718. [DOI] [PubMed] [Google Scholar]
- 11. Collins F. A guide to the selection of specialist beds and mattresses Patients at risk of pressure ulcers should be cared for on pressure‐reducing or relieving equipment. J Wound Care. 2004;13.5 SUPP:14–18. [Google Scholar]
- 12. Moysidis T, Niebel W, Bartsch K, et al. Prevention of pressure ulcers: interaction of body characteristics and different mattresses. Int Wound J. 2011;8:578‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price P, Bale S, Newcombe R, Harding K. Challenging thepressure sore paradigm. J Wound Care. 1999;8(4):187‐190. [DOI] [PubMed] [Google Scholar]
- 14. Clegg A, Smith S. Bedding aids. EUGMS. 2010;1(6):385‐390. 10.1016/j.eurger.2010.09.001 [DOI] [Google Scholar]
- 15. Critical Appraisal Skills Programme . Critical Appraisal Skills Programme (CASP) Making sense of evidence. CASP Checklists. 2004. Oxford. http://www.casp-uk.net/. Accessed December 2, 2015.
- 16. Cobb G. Pressure Ulcers: Patient Outcomes on a KinAir Bed or EHOB Waffle Mattress. Bethesda, MD: Nursing Research Service, Department of Nursing, Brooke Army Medical Center; 1995. [Google Scholar]
- 17. Lazzara DJD, Buschmann M. Prevention of pressure ulcers in elderly nursing home residents: are special support surfaces the answer? Decubitus. 1991;4(4):42‐44. 46, 48. [PubMed] [Google Scholar]
- 18. Rich S, Shardell M, Hawkes W, et al. Pressure‐redistributing support surface use and pressure ulcer incidence in elderly hip fracture patients. J Am Geriatr Soc. 2011;6:1052‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Leen M, Hovius S, Neyens J, Halfens R, Schols J. Pressure relief, cold foam or static air? A single center, prospective, controlled randomized clinical trial in a Dutch nursing home. J Tissue Viability. 2011;20(1):30‐34. [DOI] [PubMed] [Google Scholar]
- 20. Sideranko S, Quinn A, Burns K, Froman RD. Effects of position and mattress overlay on sacral and heel pressures in a clinical population. Res Nurs Health. 1992;15:245‐251. [DOI] [PubMed] [Google Scholar]
- 21. Takala J, Varmavuo S, Soppi E. Prevention of pressure sores in acute respiratory failure: a randomised controlled trial. Clin Intensive Care. 1996;7:228‐235. [Google Scholar]
- 22. Vermette S, Reeves I, Lemaire J. Cost effectiveness of an air‐inflated static overlay for pressure ulcer prevention: a randomized, controlled trial. Wounds. 2012;24(8):207‐214. [PubMed] [Google Scholar]
- 23. Malbrain M, Hendriks B, Wijnands P, et al. A pilot randomised controlled trial comparing reactive air and active alternating pressure mattresses in the prevention and treatment of pressure ulcers among medical ICU patients. J Tissue Viability. 2010;19(1):7‐15. [DOI] [PubMed] [Google Scholar]
- 24. Van Leen M, Hovius S, Halfens R, Neyens J, Schols J. Pressure relief with visco‐elastic foam or with combined static air overlay? A prospective, crossover randomized clinical trial in a Dutch nursing home. Wounds. 2013;25(10):287‐292. [PubMed] [Google Scholar]
- 25. Cooper PJ, Gray DG, Mollison J. A randomised controlled trial of two pressure reducing surfaces. J Wound Care. 1998;7:374‐376. [DOI] [PubMed] [Google Scholar]
- 26. Jiang Q, Li X, Zhang A, et al. Multicenter comparison of the efficacy on prevention of pressure ulcer in postoperative patients between two types of pressure‐relieving mattresses in China. Int J Clin Exp Med. 2014;7(9):2820‐2827. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Extended evidence table.


