Abstract
Despite consensus on the assessment and management of wound infection, there exist deficiencies in its recognition and management. A survey study involving 85 physicians and 3 other clinicians from across Canada was completed to determine current knowledge and attitude towards learning about topics relating to wound infection and its management. The results of the survey describe knowledge gaps and interests to develop expertise in the management of wound infection, suggesting a need for education on this subject. Low levels of current knowledge were reported for all biofilm‐related topics.
Keywords: Knowledge and attitude, Learning needs, Wound infection
Background
Wound healing involves complex biochemical and cellular events. Chronic wounds do not follow a predictable or expected healing trajectory and they may persist for months or years due to underlying disease processes, excessive or prolonged inflammation, recurrent injury and comorbid conditions 1. With an ageing population and increased prevalence of chronic diseases, the majority of wounds are becoming recalcitrant to healing, placing a significant burden on the health system and individual patients. According to a regional survey of 1331 home care clients in Ontario, Canada, 37% received services for wound management 1. Of all the wound clients who were surveyed, over 60% received care for over 4 weeks indicating the refractory nature of chronic wounds. It is estimated that chronic wounds accounted for an annual cost of approximately US$3·2 billion to US$4·68 billion in direct costs alone 2. People living with chronic wounds experience poor quality of life, and they often suffer from social isolation, loss of independence, fear of amputation, depression, pain and recurrent infection 3.
Increased bacterial burden and formation of biofilm has been recognised as one of the key factors contributing to delayed wound healing. The consequence of wound infection can be devastating, especially among individuals who are frail and immune‐compromised 4, 5. Among patients with diabetic foot ulcers, wound infection precedes about two third of all lower extremity amputations 6. Surgical site infection has been linked to prolonged hospitalisation and high mortality 7. With bacteremia secondary to uncontrolled infection in pressure ulcers, the mortality rate has been reported to be as high as 50% 8. The average cost associated with the treatment of infection and related complications in pressure ulcers is exorbitantly high; estimated close to US $130 000 for a single hospitalisation in the USA 9. To achieve optimal clinical outcomes, a systematised approach is recommended to manage wound infection including assessment of wound infection (or the need for antimicrobials), management of active infection and prevention of recurring infection (Figure 1).
Figure 1.
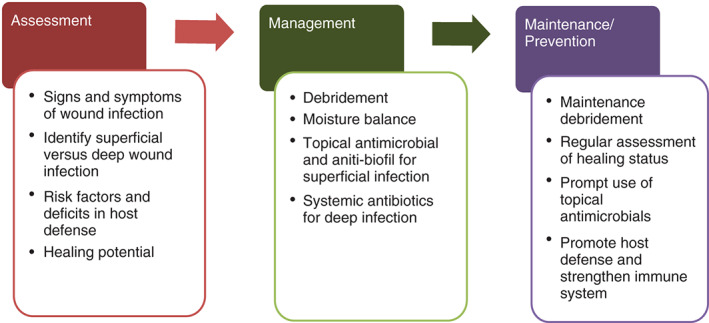
Systematic approach to wound infection.
Diagnosis of wound infection is a difficult task. Assessment should incorporate a comprehensive evaluation of factors that compromise host defence against pathogens, rendering individuals at high risk for infections such as malnutrition, immunosuppression, diabetes and poor vascular supply. Local changes in wound characteristics and symptoms (e.g. pain) are subtle but may provide valuable clues that are indicative of tissue damage from bacterial toxins and associated inflammatory response 10. Taking into account clinical presentations, clinicians must discern whether wound infection is localised or systemic for appropriate treatment 11. However, little is known whether physicians in the community are confident in their abilities to assess and diagnose wound infection.
Treatment of wound infection depends on the bacteria responsible for infection, wound types, host defence and whether the infection is localised versus deep and spreading. As bacterial pathogens proliferate, they may organise into communities, known as biofilms, which are encapsulated and protected by extracellular polymeric substance (EPS) against host defence and antimicrobial agents 12. As many as 60% of chronic wounds contain biofilm 5, and they are suspected to be the primary reason for excessive inflammatory damage in non‐healing wounds and recurrent infection 13. The mainstay of treatment for biofilms may include regular debridement and appropriate use of topical or systemic antimicrobial agents.
Despite existing guidelines, only half of patients with diabetic foot ulcers received appropriate antibiotic therapy for the treatment of infection 14. Prolonged and excessive use of systemic antibiotics often leads to undesirable adverse effects (e.g. Clostridium difficile colitis) and promotes the emergence of resistant bacteria 15, 16. The alternative approach involves early and judicious use of topical antimicrobial agents to limit the formation of biofilm and spread of infection into deeper tissue compartments. An ideal antimicrobial dressing should have the capacity to handle excessive wound exudate accompanied with high levels of oxidative enzymes, cytokines, leukocytes and proteases [e.g. metalloproteinases (MMPs)] due to inflammatory response. With the advent of a plethora of topical antimicrobial agents that have been developed in the last decades, clinicians are now challenged with decisions of when and what antimicrobial agents to use. In a retrospective review of 5541 chronic wounds, Fife et al. 17 reported that 71% of the wounds were treated with antimicrobial dressings by their physicians, but only two thirds of the prescriptions were supported by assessment findings. Further investigation is needed to determine whether physicians consider themselves knowledgeable in selecting topical antimicrobials. The purpose of this survey was to examine self‐rated knowledge and learning needs of physicians on the subject of wound infection, with specific reference to the emerging concept of wound biofilms and their role in chronic wound infections. Results of this survey will inform the development of an educational programme to address knowledge gaps among general practitioners in the community.
Methods
A literature review was conducted to identify core knowledge contents or practice issues that should be incorporated in a survey to determine the knowledge gaps and educational needs of physicians on the subject of chronic wound infection. To establish face validity, the draft survey was reviewed and modified by six interprofessional educators and clinicians from specialised wound care clinics. The final survey contains 6 questions focusing on respondents' practice patterns and 15 questions pertaining to their understanding of biofilm and wound infection (Table 1). Participants were asked to indicate, on a scale of 1 (low) to 4 (high), their levels of current knowledge and desired knowledge for each question representing a core knowledge area. The survey was formatted for the Internet and placed on the website of a Canadian provider of continuing medical education, (www.mdbriefcase.com). Registered users of the site were invited by email to participate in the educational needs assessment survey.
Table 1.
Items in the wound infection knowledge questionnaire
| Core knowledge item | Please rate your current level of knowledge (1 = low or poor to 4 = high or excellent) | |||
|---|---|---|---|---|
| 1. Barriers to wound healing | 1 | 2 | 3 | 4 |
| 2. Planktonic and biofilm microorganisms | 1 | 2 | 3 | 4 |
| 3. Differences between colonisation and wound infection | 1 | 2 | 3 | 4 |
| 4. Biofilm formation in wounds | 1 | 2 | 3 | 4 |
| 5. EPS and biofilm | 1 | 2 | 3 | 4 |
| 6. Relationship between biofilm and delayed wound healing | 1 | 2 | 3 | 4 |
| 7. Factors that can influence biofilm formation | 1 | 2 | 3 | 4 |
| 8. Assessment of wound infection | 1 | 2 | 3 | 4 |
| 9. Strategies to manage wound biofilm | 1 | 2 | 3 | 4 |
| 10. Appropriate use of topical antimicrobials (when to start and when to stop) | 1 | 2 | 3 | 4 |
| 11. Differences in the types of topical antimicrobials | 1 | 2 | 3 | 4 |
| 12. Wound debridement and cleansing | 1 | 2 | 3 | 4 |
| 13. Use of systemic antibiotics | 1 | 2 | 3 | 4 |
| 14. Agents and treatment modalities that are effective against wound biofilms | 1 | 2 | 3 | 4 |
| 15. Patient‐centred concerns | 1 | 2 | 3 | 4 |
Results
The survey was sent to 250 clinicians and a total of 88 individuals were participated in the survey, including family physicians (77%), specialist physicians (15%) and others (8%) (Figure 2). The respondents were from various Canadian provinces and territories, with the majority (58%) being from Ontario.
Figure 2.
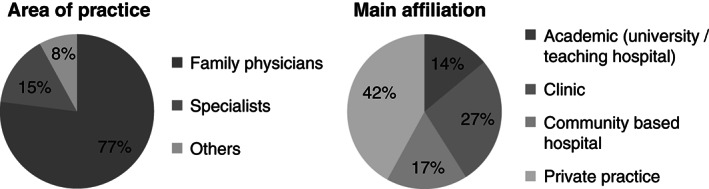
Characteristics of the participants in the survey (n = 88).
Participants listed a variety of acute and chronic wounds they encountered regularly in clinical practices. The most common wound types mentioned in the survey were diabetic foot ulcers, trauma wounds (laceration, abrasion, scratches and cuts), burns and post‐surgical wounds. All respondents of this survey had previous experiences assessing and managing wound infection. More than 80% (85·6%) of the respondents indicated that they encountered wounds with infection on a weekly basis and approximately one third (29·3%) were involved in the management of 2–5 infected wounds per week.
Rating of current and desired knowledge levels showed that for every item, the average level of current knowledge (range 1·73–2·74) of the participants was lower than that of desired knowledge (range 3·5–3·8) (Table 2).
Table 2.
Average current and desired knowledge levels of participants (n = 88)
| Average knowledge level | ||
|---|---|---|
| Topic | Current | Desired |
| Barriers to wound healing | 2·65 | 3·77 |
| Planktonic and biofilm microorganisms | 1·81 | 3·62 |
| Critical colonisation and wound infection | 1·91 | 3·68 |
| Biofilm formation in wounds | 1·89 | 3·67 |
| EPS | 1·77 | 3·59 |
| Biofilms and delayed wound healing | 1·81 | 3·70 |
| Factors influencing biofilm formation | 1·73 | 3·64 |
| Wound infection assessment and biofilm detection | 1·85 | 3·70 |
| Biofilm‐based wound care | 1·81 | 3·70 |
| When to start and when to stop treatment | 2·17 | 3·78 |
| Wound debridement and cleansing | 2·44 | 3·76 |
| Topical antimicrobials | 2·65 | 3·78 |
| When to use antibiotics | 2·74 | 3·78 |
| Anti‐biofilm agents and treatment modalities | 1·83 | 3·70 |
| Patient concerns and biofilms | 1·84 | 3·61 |
EPS, extracellular polymeric substance.
For all the topics listed in the survey, over 70% of participants expressed an interest to acquire the highest level (score 4·0) of knowledge. The content areas that were rated with the highest scores indicating most desirable for further learning include barriers to wound healing (79·55%), when to start and when to stop treatment (81·82%), wound debridement and cleansing (88·68%), topical antimicrobials (80·68%) and when to use antibiotics (82·95%). Low levels of current knowledge (range 1·77–1·91) were particularly notable for all biofilm‐related topics. Over 35% of participants gave themselves the lowest score (score of 1) for current knowledge on the following topics: planktonic and biofilm microorganisms (39·77%), basics of biofilms, critical colonisation and wound infection (35·23%), biofilm formation in wounds (36·35%), EPS (45·45%), biofilms and delayed wound healing (42·05%), factors influencing biofilm formation (45·45%), wound infection assessment and biofilm detection (38·64), biofilm‐based wound care (40·91%), anti‐biofilm agents and treatment modalities (42·05%) and patient concerns and biofilms (40·91%).
Discussion
With the raising prevalence, mortality, suffering and health care cost associated with chronic wounds, the need to deliver evidence‐based wound care is taking on a new urgency 18, 19. Treatment of chronic wounds requires a systematised approach under the tenets of ‘wound bed preparation’, which highlights the key individual components of wound care. Best practices that have been demonstrated to prepare the wound bed for healing include debriding of unhealthy and non‐viable tissue, controlling infection and bacterial bioburden, maintaining moisture balance and addressing the unhealthy wound edges.
Wound infection and biofilm is one of the most exciting yet controversial topic in chronic wound management. Recent advances in the understanding of wound infection pathogenesis have generated tremendous interest in biofilm and how it is related to wound healing. As evident from findings of this survey, the physicians who responded to this survey identified a significant knowledge gap in wound management, especially around wound infection and biofilm. The respondents also acknowledged their roles in wound care and expressed a keen interest to further their knowledge. The types of wounds that were managed by the respondents were diverse including diabetic foot ulcers, trauma wounds and surgical wounds. Of persons with diabetes, 2–3% will develop a foot ulcer annually, while the lifetime risk of developing a foot ulcer is as high as 25% due primarily to neuropathy and potential coexisting vascular disease. Individuals with diabetes are susceptible to wound infection as a result of immunodeficiency, neuropathy and vascular insufficiency. Surgical site infection is a growing concern in the community. There is a need to enhance the knowledge and build capacity for physicians to assess and treat wound infection and biofilms.
The diagnosis of wound infection is complex integrating a number of patient (host) factors, wound characteristics and clinical presentations. Differentiation of critical colonisation, deep wound infection and biofilm is a challenging but an important task. It is generally accepted that topical antimicrobial agents should be considered for localised wound infection, and systemic agents are introduced for wound infection that involves soft tissue. Almost 80% of the respondents rated their ability to assess for wound infection and biofilm to be poor to fair, demonstrating a need for further education in this area.
Evidence has demonstrated that the use of antibiotics and topical antimicrobials is inconsistent and even inappropriate 16, 20. In this survey, most participants rated their current knowledge at the higher levels (levels 3 or 4) for these topics, e.g. topical antimicrobials (65%), and when to use antibiotics (69%), although these topics were also among those for which they also sought the highest level of knowledge. Patient‐centred concerns such as pain and quality of life are important considerations in the management of chronic wound infections. However, pain management has been reported to be suboptimal in patients with chronic wounds 21. In this survey, 77% of respondents reported low levels (levels 1 or 2) of current knowledge for patient‐centred concerns.
Sharp debridement is integral to the management of wound infection by physically disrupting the resilient biofilm structure, removing devitalised tissue, eliminating foreign material (even gauze material) and reducing bacteria sequestrum 22, 23. Emerging evidence suggests that other mechanical modalities such as ultrasound, electrical therapy, hydrosurgery and vigorous cleansing may also be effective against biofilm 23. Following debridement and other means of mechanical disruption, topical antimicrobial dressing should be considered to prevent the re‐establishment of biofilm that could occur within 24–48 hours after initial debridement 24, 25, 26. Respondents of this survey acknowledged the importance of wound debridement and expressed an interest in learning more about various debridement methods.
Limitations
Physicians who participated in this study were registered users of an online continuing medical education platform. The respondents represent a group of motivated learners; their knowledge and attitudes towards the management of wound infection may not represent other general practitioners in the community. Results of the survey may not be generalisable to clinicians who did not respond to the survey. There remains a need to explore why some clinicians may not perceive a deficit in their current knowledge to manage wound infection. The survey consists of 15 self‐rated items. Self‐evaluation of knowledge levels may be subjected to bias. Considering adherence to best practices among general practitioners is poor, further investigation should examine strategies to overcome perceived barriers due to time restraints, insufficient evidence and accessibility of educational material. Despite potential limitations, this study describes the existing knowledge gaps and learning needs on a variety of wound‐infection‐related topics. It is apparent that physicians will benefit from educational programmes to enhance their care for chronic wounds.
Conclusion
Assessment and management of wound infection and related biofilm is complex. Physicians play a key role in the management of chronic wounds, especially in the community. Results of this survey highlight the knowledge gap and learning needs to enhance the capacity of general practitioners to independently manage wound infection. An effective learning programme should be self‐directed and tailored to meet their needs.
References
- 1. Woo KY, Houghton D, Sibbald RG. Prevalence of chronic wounds in a regional survey in central west Ontario. Poster presented at the Canadian Association of Wound Care Annual Conference; 2010, 11 November; London, Ontario.
- 2. Harding K, Queen D. Chronic wounds and their management and prevention is a significant public health issue. Int Wound J 2010;7:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards H, Finlayson K, Courtney M, Graves N, Gibb M, Parker C. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC Health Serv Res 2013;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woo KY, Keast D, Parsons N, Sibbald RG, Mittmann N. The cost of wound debridement: a Canadian perspective. Int Wound J 2013; doi: 10.1111/iwj.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 6. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. [DOI] [PubMed] [Google Scholar]
- 7. Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis 2006;43:322–30. [DOI] [PubMed] [Google Scholar]
- 8. Braga IA, Pirett CC, Ribas RM, Gontijo Filho PP, Diogo FA. Bacterial colonization of pressure ulcers: assessment of risk for bloodstream infection and impact on patient outcomes. J Hosp Infect 2013 Apr;83:314–20. [DOI] [PubMed] [Google Scholar]
- 9. Brem H, Maggi J, Nierman D, Rolnitzky L, Bell D, Rennert R, Golinko M, Yan A, Lyder C, Vladeck B. High cost of stage IV pressure ulcers. Am J Surg 2010;200:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enoch S, Harding K. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–29. [Google Scholar]
- 11. Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC, Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis 2005;41:1373–406. [DOI] [PubMed] [Google Scholar]
- 12. Fleming H‐C, Wingender J. The biofilm matrix. Nat Rev Microbiol 2010;8:623–33. [DOI] [PubMed] [Google Scholar]
- 13. Percival SL, Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004;16:234–40. [Google Scholar]
- 14. Lipsky BA. Medical treatment of diabetic foot infections. Clin Infect Dis 2004;39(Suppl 2):S104–14. [DOI] [PubMed] [Google Scholar]
- 15. Slimings C, Riley TV. Antibiotics and hospital‐acquired Clostridium difficile infection: update of systematic review and meta‐analysis. J Antimicrob Chemother 2014;69:881–91. [DOI] [PubMed] [Google Scholar]
- 16. Howell‐Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother 2005;55:143–9. [DOI] [PubMed] [Google Scholar]
- 17. Fife CE, Carter MJ, Walker D, Thomson B. A retrospective data analysis of antimicrobial dressing usage in 3,084 patients. Ostomy Wound Manage 2010;56:28–42. [PubMed] [Google Scholar]
- 18. Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence‐based management strategies for treatment of chronic wounds. Eplasty 2009;9:e19. [PMC free article] [PubMed] [Google Scholar]
- 19. Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. Wound Care 2004:S6–15. [DOI] [PubMed] [Google Scholar]
- 20. Stubbs N, Sandoe J, Mc Ginnis E, Edmunds H. The development, implementation and evaluation of a cross organisational clinical guideline for the management and prevention of wound infection. J Tissue Viability 2012;21:112–4. [DOI] [PubMed] [Google Scholar]
- 21. Taverner T, Closs SJ, Briggs M. Painful leg ulcers: community nurses' knowledge and beliefs, a feasibility study. Prim Health Care Res Dev 2011;12:379–92. [DOI] [PubMed] [Google Scholar]
- 22. Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, Dowd SE. Biofilm maturity studies indicate sharp debridement opens a time‐ dependent therapeutic window. J Wound Care 2010;19:320–8. [DOI] [PubMed] [Google Scholar]
- 23. Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012;9(Suppl 2):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woo KY. The use of antimicrobial dressings in chronic wounds: NERDS and STONEES principles. Surg Technol Int 2010;20:73–82. [PubMed] [Google Scholar]
- 25. Lipp C, Kirker K, Agostinho A, James G, Stewart P. Testing wound dressings using an in vitro wound model. J Wound Care 2010;19:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wound infection in clinical practice. An international consensus. Int Wound J 2008;5:iii–11. [DOI] [PMC free article] [PubMed]


