Abstract
Chronic venous disease (CVD) and its most frightening complication, chronic venous ulceration (CVU), represent an important socioeconomic burden in the western world. Metalloproteinases have been identified in the pathogenesis of several vascular diseases such as venous problems. The aim of this study was to evaluate a broad range of metalloproteinases, such as matrix metalloproteinases (MMPs), ADAMs (a disintegrin and metalloproteinases) and ADAMTSs (a disintegrin and metalloproteinases with thrombospondin motifs) and their inhibitors, tissue inhibitor of metalloproteinases (TIMPs) and a related protein, neutrophil gelatinase‐associated lipocalin (NGAL), in patients with CVD in order to correlate their serum levels with each stage of the disease. We performed a multicenter open‐label study that comprised the enrolment of 541 patients with CVD of clinical stages C1–C6, (178 males, 363 females; mean age 57·29, median age 53·72, age range 29–81); 29 subjects without CVD were included in this study (9 males and 20 females; mean age 54·44, median age 50, age range 28–84) as the control group. Enzyme‐linked immunosorbent assay (ELISA) was performed for measuring serum levels of proteases and related proteins. The study found that the serum elevation of MMP‐2, ADAMTS‐1 and ADAMTS‐7 appeared to be correlated with the initial stages of CVD, whereas the serum elevation of MMP‐1, MMP‐8, MMP‐9, NGAL, ADAM‐10, ADAM‐17 and ADAMTS‐4 was particularly involved in skin change complications. This study showed that each stage of CVD may be described by particular patterns of metalloproteinases, and this may have therapeutic implications in discovering new targets and new drugs for the treatment of CVD.
Keywords: Chronic venous disease, Metalloproteinases, Varicose veins, Skin changes, Venous ulcer
Introduction
Chronic venous disease (CVD) is a very common problem affecting the Western adult population, with a prevalence of <10% among individuals younger than 30 years for both genders and with a prevalence up to 77% in individuals aged ≥70 years 1, 2.
According to clinical classes (C) of the Clinical‐Aetiology‐Anatomy‐Pathophysiology (CEAP) classification 3, the spectrum of CVD ranges from various types of varices (telangiectasia, reticular veins and varicose veins) (classes C1–C2) to leg oedema (class C3) and serious skin changes, including hyperpigmentation, eczema, lipodermatosclerosis (class C4) and venous skin ulceration (classes C5–C6). Classes C3–C6 indicate a more advanced form of CVD called chronic venous insufficiency (CVI) 1.
A further sign of CVD, corona phlebectatica (fan‐shaped intradermal telangiectases on the medial or lateral aspects of the foot), has been poorly investigated as it is not yet included in CEAP classification, although it appears to correlate with the clinical severity in the progression of CVD 4, 5.
The pathogenesis of CVD encloses several theories focusing on endothelial dysfunction and extracellular matrix (ECM) unbalance. It has been particularly suggested that the balance between matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) plays a crucial role in all the clinical manifestations of CVD. Furthermore, MMP‐1, MMP‐2, MMP‐8, MMP‐9, TIMP‐1, TIMP‐2 and neutrophil gelatinase‐associated lipocalin (NGAL), which is directly involved in the regulation of MMPs activity, appear to be directly associated with venous disease 6, 7, 8, 9, 10, 11, 12, 13, 14.
There are also further members of different metalloproteinases families, such as ADAMs (a disintegrin and metalloproteinases) and ADAMTSs (a disintegrin and metalloproteinases with Thrombospondin motifs), that were investigated for the cardiac and the arterial side of cardiovascular disease (ADAM‐10, ADAM 12, ADAM‐17, ADAMTS‐1, ADAMTS‐4, ADAMTS‐5, ADAMTS‐7) 15, 16, but they have not yet been evaluated in venous disease.
The aim of this study is to describe the entire pattern of clinical manifestations of CVD related to serum levels of some members of the MMP, TIMP, ADAM and ADAMTS families and NGAL.
Materials and methods
Study design
We performed a multicenter open‐label study between 1 January 2013 and 30 June 2015. This study was approved by the Investigational Review Board (IRB) of the Interuniversity Center of Phlebolymphology (CIFL) International Research and Educational Program in Clinical and Experimental Biotechnology in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice. Before the beginning of the study, all participants provided written informed consent.
Population
Inclusion criteria
Patients with CVD, of both genders, older than 18 years, belonging to classes C1–C6 of the CEAP classification 3 were included.
Exclusion criteria
Patients with cancer, hepatic failure, infectious or autoimmune diseases, arthritis, arterial aneurysms, hernias, previous or active venous thromboembolism, nephritis, peripheral artery disease, fibrosis and other diseases associated with increased levels of MMPs were excluded from the study. Patients were treated with corticosteroids or cytostatic drugs.
On inclusion in the study, blood samples were collected from venipuncture in order to evaluate serum levels of MMP‐1, MMP‐2, MMP‐8, MMP‐9, TIMP‐1, TIMP‐2, NGAL, ADAM‐10, ADAM 12, ADAM‐17, ADAMTS‐1, ADAMTS‐4, ADAMTS‐5 and ADAMTS‐7.
Enzyme‐linked immunosorbent assay (ELISA)
Serum samples were used in this study. The blood samples were collected and allowed to clot before centrifugation. After centrifugation, serum was removed and stored at −20°C. An enzyme‐linked immunosorbent assay (ELISA) was performed to measure serum levels using commercially available kits (USCNK, USCN Life Science, Wuhan, China) following the manufacturer's instructions. Thereafter, 100 µl of the standards or the samples were added to each well and incubated for 2 hours at 37°C. The plates were then washed and incubated for 1 hour at 37°C with 100 µl/well of prepared detection reagent A. The plates were then washed and incubated with 100 µl/well of detection reagent B. After half an hour, at 37°C, the plates were washed 5 times, and 90 µl/well of substrate solution was added. After 15–25 minutes at 37°C, the reaction was stopped with 50 ml/well 2 M H2SO4, and the absorbance was read at 450 nm with a Multiskan™ GO Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Statistical analysis was performed using GraphPad Prism 2·01 (GraphPad Software, San Diego, CA, USA).
Results
During the study period, 541 patients with CVD, clinical stages C1–C6, were enrolled (178 males, 363 females; mean age 57·29, median age 53·72, age range 29–81), and they represented Group A.
Finally, 29 subjects without CVD were included in this study (9 males and 20 females; mean age 54·44, median age 50, age range 28–84) as the control group (Group B).
Complete demographic characteristics of both groups, and for each clinical stage, are shown in Table 1.
Table 1.
Demographics
| Group A | Group B | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | C1 | C2 | C3 | C4 | C5 | C6 | Total | |
| Males | 178 (32·90%) | 23 (13·86%) | 47 (27·01%) | 63 (54·78%) | 23 (52·27%) | 10 (52·63%) | 12 (52·17%) | 9 (31.03%) |
| Females | 363 (67·10%) | 143 (86·14%) | 127 (72·99%) | 52 (45·22%) | 21 (47·73%) | 9 (47·37%) | 11 (47·83%) | 20 (68·97%) |
| Mean age | 57·29 | 46·50 | 55 | 56·18 | 57·56 | 65 | 63·5 | 54·44 |
| Median age | 53·72 | 44 | 54·33 | 50 | 54·5 | 62 | 57·5 | 50 |
| Age range | 29–81 | 29–58 | 32–72 | 40–68 | 42–73 | 49–76 | 44–81 | 28–84 |
| BMI (mean) | 27·86 | 26·58 | 27·09 | 27·65 | 28·17 | 28·23 | 29·5 | 26·92 |
| Smoke | 182 (33·64%) | 53 (31·92%) | 58 (33·33%) | 37 (32·17%) | 17 (38·63%) | 8 (42·10%) | 9 (39·13%) | 9 (31·03%) |
| Diabetes mellitus | 62 (11·46%) | 16 (9·64%) | 19 (10·91%) | 11 (9·57%) | 7 (15·9%) | 4 (21·05%) | 5 (21·74%) | 3 (10·34%) |
| Lipid disorders | 84 (15·52%) | 20 (12·05%) | 24 (13·79%) | 17 (14·78%) | 10 (22·73%) | 6 (31·58%) | 7 (30·44%) | 4 (13·79%) |
| Hypertension | 75 (13·86%) | 20 (12·05%) | 17 (9·77%) | 17 (14·78%) | 9 (20·45%) | 5 (26·31%) | 7 (30·44%) | 3 (10·34%) |
| Corona phlebectatica | 135 (24·95%) | 14 (8·43%) | 23 (13·22%) | 48 (41·74%) | 23 (52·27%) | 12 (63·15%) | 15 (65·22%) | 0 |
| Total | 541 (100%) | 166 (30·68%) | 174 (32·17%) | 115 (21·26%) | 44 (8·13%) | 19 (3·51%) | 23 (4·25%) | 29 (100%) |
C1–C6, clinical stages.
Figures 1 and 2 report the analysis of the levels of MMP‐1, MMP‐2, MMP‐8, MMP‐9, TIMP‐1, TIMP‐2, NGAL, ADAM‐10, ADAM 12, ADAM‐17, ADAMTS‐1, ADAMTS‐4, ADAMTS‐5 and ADAMTS‐7 during all the stages of CVD and in healthy subjects.
Figure 1.
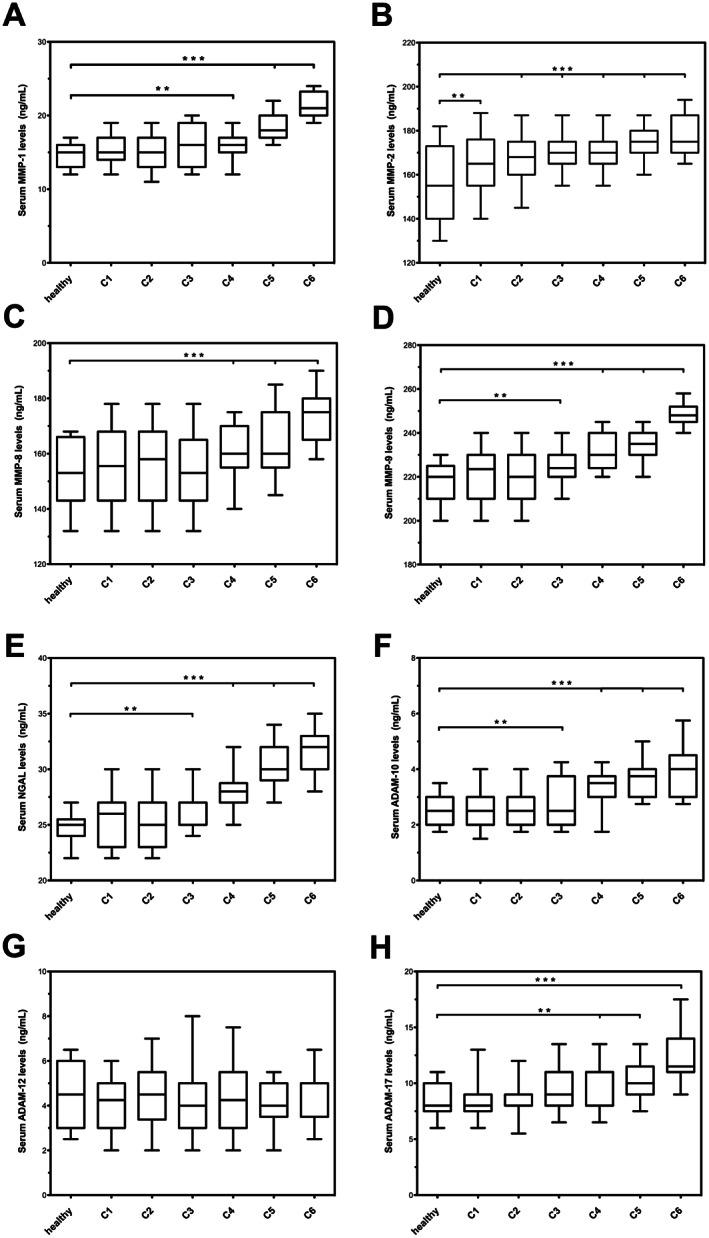
Serum levels of MMP‐1 (matrix metalloproteinases), MMP‐2, MMP‐8, MMP‐9, NGAL (neutrophil gelatinase‐associated lipocalin), ADAM‐10 (a disintegrin and metalloproteinases), ADAM‐12, ADAM‐17. *P < 0·05; **P < 0·01; ***P < 0·001.
Figure 2.
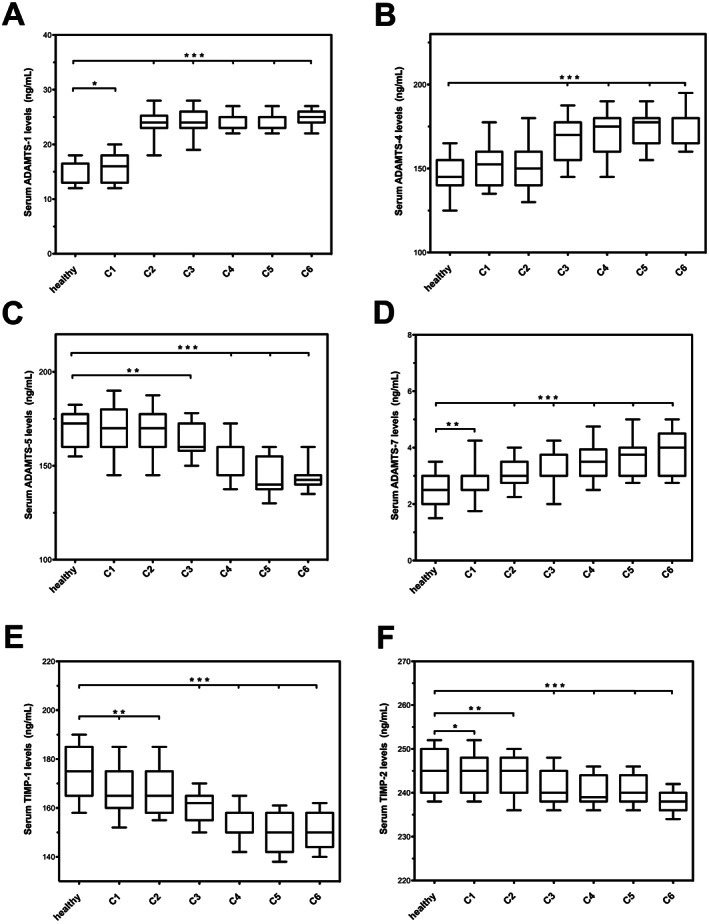
Serum levels of a disintegrin and metalloproteinases with thrombospondin motifs (ADAMTS)‐1, ADAMTS‐4, ADAMTS‐5, ADAMTS‐7, tissue inhibitor of metalloproteinases (TIMP)‐1, TIMP‐2. *P < 0·05; **P < 0·01; ***P < 0·001.
In the mild forms of CVD (C1–C2), we found an initial significant increase of MMP‐2, ADAMTS‐1 and ADAMTS‐7 and an initial decrease of ADAMTS‐5, TIMP‐1 and TIMP‐2 with respect to healthy controls.
Notably, C1–C2 patients with corona phlebectatica [14 (8·43%) for C1 patients and 23 (13·22%) for C2 patients] had higher values of the aforementioned metalloproteinases (MMP‐2, ADAMTS‐1 and ADAMTS‐7) and of MMP‐9 and NGAL and lower values of ADAMTS‐5, TIMP‐1 and TIMP‐2 with respect to patients in the same group without corona phlebectatica. The proteases levels of patients with corona phlebectatica determined, in the C1–C2 groups, the increase of the upper range limit of the aforementioned MMPs, even coinciding with the levels of C3 patients (Figures 1, 2; Table 2).
Table 2.
Synopsis – CVD clinical manifestations and proteases
| Healthy subjects (controls) | C1 patients | C2 patients | C3 patients | C4 patients | C5 patients | C6 patients | Subgroup of C1–C2 patients with corona flebectatica | Subgroup of C6 patients with hard‐to‐heal ulcers | |
|---|---|---|---|---|---|---|---|---|---|
| MMP‐1 | + | + | + | + | ++ | ++ | +++ | + | ++++++ |
| MMP‐2 | + | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| MMP‐8 | + | + | + | + | ++ | ++ | +++ | + | ++++++ |
| MMP‐9 | + | + | + | ++ | +++ | +++ | ++++ | ++ | +++++ |
| NGAL | + | + | + | ++ | +++ | +++ | ++++ | ++ | +++++ |
| ADAM‐10 | + | + | + | ++ | +++ | +++ | ++++ | ++ | +++++ |
| ADAM‐12 | + | + | + | + | + | + | + | + | + |
| ADAM‐17 | + | + | + | ++ | +++ | +++ | ++++ | ++ | ++++++ |
| ADAMTS‐1 | + | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| ADAMTS‐4 | + | + | + | +++ | ++++ | +++++ | +++++ | +++ | +++++++ |
| ADAMTS‐5 | +++++ | ++++ | +++ | ++ | ++ | ++ | + | ++ | + |
| ADAMTS‐7 | + | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| TIMP‐1 | +++++ | ++++ | +++ | ++ | ++ | ++ | + | ++ | + |
| TIMP‐2 | +++++ | ++++ | +++ | ++ | ++ | ++ | + | ++ | + |
ADAMTS, a disintegrin and metalloproteinases with thrombospondin motifs; C1–C6, clinical stages; NGAL, neutrophil gelatinase‐associated lipocalin; MMP, matrix metalloproteinases; TIMP, tissue inhibitor of metalloproteinases.
The C3 patients, with moderate disease and with the initial stage of CVI, had similar protease values as the C1–C2 groups, except for a slightly higher value of ADAM‐10, ADAMTS‐4, MMP‐9 and NGAL that indicates a more intense inflammatory response with respect to the C1–C2 patients (Figures 1, 2; Table 2).
For more advanced stages of CVI (C4–C6), with skin changes, we found higher values of MMP‐1, MMP‐8, ADAM‐10, ADAM 17, MMP‐9, NGAL and a very significant increase of ADAMTS‐4, which reached the maximum level in the ulceration stage (C6) (Figures 1, 2; Table 2).
Among patients with chronic venous ulceration (CVU), we identified a subgroup of five patients (three males and two females) with hard‐to‐heal and long‐lasting ulcers, with an important elevation of MMP‐1, MMP‐8 ADAM‐17 and ADAMTS‐4 and with a marked decrease of ADAMTS‐5, TIMP‐1 and TIMP‐2 (Table 2).
ADAM‐12 does not appear to be involved in any stage of CVD, and its values were always similar to the healthy control group.
ADAMTS‐1 and ADAMTS‐7 were generically elevated in all stages of CVD with respect to the control group, but there was no significant difference between the stages of CVD.
ADAMTS‐5, TIMP‐1 and TIMP‐2 decreased progressively during the worsening of CVD.
Table 2 shows a glance of the full spectrum of clinical manifestations of CVD and their relationship with the proteases investigated.
Discussion
CVD is a common condition in Western countries that has an important socioeconomic impact because of its high prevalence in the general population 1, 2. CVD is a progressive disease that is well and simply described, in its clinical component (C classes), by the CEAP classification 3. Mild forms of CVD are represented by the first two classes: teleangectesias (class C1), that is, the confluence of dilated intradermal venules less than 1 mm in calibre, and reticular veins (class C1) that are dilated, usually tortuous, bluish subdermal veins, usually 1 mm to less than 3 mm in diameter and varicose veins (class C2) that are subcutaneous, dilated and tortuous veins, 3 mm in diameter or larger and may involve saphenous veins, their tributaries or the non‐saphenous superficial veins of lower limbs 1. Moderate to severe forms of CVD refer to CVI, which may include oedema (class C3), skin changes (class C4) such as eczema or pigmentation (C4a) and lipodermatosclerosis or atrophie blanche (white atrophy) (C4b) and, finally, healed venous ulcers (class C5) or active venous ulceration (class C6) 1.
A further sign, that is corona phlebectatica, is not actually included in the CEAP classification, but it is thought to be an early sign of advanced venous disease 4, 5.
Several studies have described defects in the regulation of the composition of the ECM and subsequent wall remodelling in varicose veins, leading to decreased elasticity and increased distensibility of the vessel wall 17, 18. Thus, a genetic defect in the regulation of the composition of the ECM might be involved in the pathogenesis of varicose veins 2. Such a genetic defect and ECM alterations should also affect different connective tissues as seen in several documented experiences patients with concomitant CVD 6, 19, 20.
The ECM provides a structural framework and plays an essential role in the structure and function of vessel walls 21 as well as in the metabolism and homeostasis of skin tissues 22.
In fact, metalloproteinases function not only as regulators of ECM composition and structural integrity but also as important mediators in the control of cellular interactions and response to their environment in conditions that promote tissue turnover and wound healing. In this context, metalloproteinases are involved in the functional regulation of several ECM molecules such as growth factors and their receptors, cytokines, chemokines, adhesion receptors and a variety of related enzymes 23.
There are three main families of metalloproteinases that are involved in cardiovascular disease: MMPs ADAMs and ADAMTSs; and TIMPs regulate their activities 8, 15, 16.
MMPs are a group of several multi‐domain zinc‐dependent enzymes that are involved in the remodelling of several components of ECM 24. In the vasculature, MMPs influence the migration, proliferation and apoptosis of vascular smooth muscle cells, endothelial cells and inflammatory cells, thereby affecting intima formation, atherosclerosis, aneurysms, varicose veins and its complications, such as venous ulceration and post‐thrombotic syndrome 6, 7, 8, 9, 10, 11, 12, 13, 14, 25, 26. Various MMPs patterns have been identified to indicate several stages within the disease. MMP‐2, MMP‐9 and NGAL (which is a protein belonging to the lipocalin family, with the ability to positively modulate the activities of MMP‐9) appear to be mostly involved in the acute phase of vascular disease (such as aneurysm rupture and pulmonary embolism) 27, 28, while MMP‐1 and MMP‐8 appear to be primarily involved with chronic or irreversible complications of vascular disease (such as difficult‐to‐heal or infected venous ulcers and post‐thrombotic syndrome) 13, 14, 26.
The ADAMs are a family of transmembrane and secreted proteins that have functions in cell adhesion and the proteolytic processing of the ectodomains of diverse cell surface receptors and signalling molecules, and they have been identified in many species. ADAMs have been linked functionally in many biological processes as well as in some cardiovascular conditions 15. In fact, ADAM‐12 is expressed above all in cardiomyocytes and fibroblasts, and its inhibition prevents cardiac hypertrophy 29. It has also been identified as an important mediator of vascular smooth muscle cells (VSMCs) hypertrophy and appears to be directly involved in hypertension vascular diseases 30, 31. Both ADAM‐10 and ‐17 appear to be involved in immune and inflammatory responses by regulating pro‐tumour necrosis factor (TNF)‐α activation and other related molecules and cytokines 15.
The ADAMTS members descend from the ADAM family of proteases; they have diverse functions and major roles, including the maturation of proproteins such as procollagen and extracellular matrix remodelling during morphogenesis. ADAMTS deficiencies lead to a variety of congenital anomalies, inherited connective tissue disorders, a haemostatic defect (thrombotic thrombocytopenic purpura) and infertility, and they also appear to be involved in vascular disease. In fact, in this family, we can find that ADAMTS‐1, the founding member of the ADAMTS family, is an angiogenic factor that is induced by inflammatory mediators such as lipopolysaccharide and tumour necrosis factor alpha. In atherosclerotic lesions, it is expressed by smooth muscle cells 32, 33.
ADAMTS‐1 and ADAMTS‐4 protein and mRNA expressions were significantly higher in thoracic aortic aneurysms and dissection tissues than in healthy aortic tissues 34
ADAMTS‐5 has been suggested to be even protective for the vascular wall as it is depleted in atherosclerotic aortas, regulating vascular proteoglycan catabolism and altering lipoprotein retention 35.
ADAMTS‐7 is known to play an important role in vascular wall homeostasis as it contributes directly to neointima formation by mediating vascular smooth muscle cell migration 36, 37, and it may also represent a novel therapeutic target for atherosclerosis and for preventing postangioplasty restenosis 38.
The TIMPs are tissue‐specific, endogenous inhibitors of metalloproteinases, including MMPs as well as the closely related ADAMs and ADAMTS 39, and the most studied are represented by TIMP‐1 and TIMP‐2 26, which are also involved in several vascular diseases 26, 40.
In healthy tissues, TIMPs appear to spare the ECM from degradation, and only the following injury/infection TIMPs appear to indirectly control ECM deposition. However, there is still much to be determined about their roles and functions 39.
This is the first study that considered and analysed a wide range of metalloproteinases (MMPs, ADAMs and ADAMTs), their inhibitors (TIMPs) and NGAL in the context of CVD and its full range of clinical manifestations.
The study found that the elevation of MMP‐2, ADAMTS‐1 and ADAMTS‐7 appears to be correlated with the initial stages of CVD and may represent the start of the disease; aADAM‐10, ADAM‐17, ADAMTS‐4, MMP‐9 and NGAL appear to be particularly active in the inflammatory progression of the disease towards the onset of overt CVI and remained elevated during the more severe clinical manifestations, including CVU.
MMP‐1, MMP‐8, ADAM‐17 and ADAMTS‐4 appear to be primarily involved with chronic or irreversible complications of vascular disease and delayed wound healing.
ADAM‐12 appears not to be involved in venous disease as there was no difference between patients with CVD in all stages and healthy subjects.
ADAMTS‐1 and ADAMTS‐7 were found to be generically elevated in all stages of CVD with respect to the healthy subjects.
ADAMTS‐5, TIMP‐1 and TIMP‐2 were negatively associated with the progression of the disease and decreased progressively during the worsening of CVD.
In this study, patients with corona phlebectatica were found to have a marked increase of the inflammatory MMPs (ADAM‐10, ADAM‐17, ADAMTS‐4 and MMP‐9) and NGAL, even in the first stages of CVD, and this appears to justify the concern that corona phlebectatica may be a good predictor of the advanced forms of CVD with skin changes 4, 5, 41.
This study clearly showed that each stage of CVD may be described by particular patterns of metalloproteinases, and this may be useful for identifying new therapeutic targets in order to prevent or to better treat clinical manifestations related to CVD in the near future.
Acknowledgements
The authors declare that they have no competing interests. This work received no funding.
References
- 1. Serra R, Grande R, Butrico L, Fugetto F, de Franciscis S. Epidemiology, diagnosis and treatment of chronic venous disease: a systematic review. Chirurgia 2016;29. [Google Scholar]
- 2. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Molinari V, Montemurro R, de Franciscis S. A genetic study of chronic venous insufficiency. Ann Vasc Surg 2012;26:636–642. [DOI] [PubMed] [Google Scholar]
- 3. Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification . Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248–1252. [DOI] [PubMed] [Google Scholar]
- 4. Uhl JF, Cornu‐Thénard A, Carpentier PH, Widmer MT, Partsch H, Antignani PL. Clinical and hemodynamic significance of corona phlebectatica in chronic venous disorders. J Vasc Surg 2005;42:1163–1168. [DOI] [PubMed] [Google Scholar]
- 5. Uhl JF, Cornu‐Thenard A, Satger B, Carpentier PH. Clinical analysis of the corona phlebectatica. J Vasc Surg 2012;55:150–153. [DOI] [PubMed] [Google Scholar]
- 6. Serra R, Buffone G, Costanzo G, Montemurro R, Scarcello E, Stillitano DM, Damiano R, de Franciscis S. Altered metalloproteinase‐9 expression as the least common denominator between varicocele, inguinal hernia and chronic venous disorders. Ann Vasc Surg 2014;28:705–709. [DOI] [PubMed] [Google Scholar]
- 7. de Franciscis S, Serra R. Matrix metalloproteinases and endothelial dysfunction: the search for new prognostic markers and for new therapeutic targets for vascular wall imbalance. Thromb Res 2015;136:5–6. [DOI] [PubMed] [Google Scholar]
- 8. de Franciscis S, Butrico L, Settimio UF, Grande R, Serra R. The endothelial dysfunction in chronic venous disease: a systematic review. Acta Phlebol 2015;16:69–76. [Google Scholar]
- 9. Mannello F, Raffetto JD. Matrix metalloproteinase activity and glycosaminoglycans in chronic venous disease: the linkage among cell biology, pathology and translational research. Am J Transl Res 2011;3:149–158. [PMC free article] [PubMed] [Google Scholar]
- 10. Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP‐2 induced vein relaxation via inhibition of [Ca2+]e‐dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res 2010;159:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets 2013;14:287–324. [PMC free article] [PubMed] [Google Scholar]
- 12. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen 2013;21:395–401. [DOI] [PubMed] [Google Scholar]
- 13. Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, Grande R, Serra R, de Franciscis S. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J 2015;12:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J 2016;13:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 2008;29:258–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol 2015;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sansilvestri‐Morel P, Rupin A, Jaisson S, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Synthesis of collagen is dysregulated in cultured fibroblasts derived from skin of subjects with varicose veins as it is in venous smooth muscle cells. Circulation 2002;106:479–483. [DOI] [PubMed] [Google Scholar]
- 18. Sansilvestri‐Morel P, Fioretti F, Rupin A, Senni K, Fabiani JN, Godeau G, Verbeuren TJ. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Sci (Lond) 2007;112:229–239. [DOI] [PubMed] [Google Scholar]
- 19. Serra R, Grande R, Buffone G, Costanzo G, Damiano R, De Franciscis S. Chronic venous disease is more aggressive in patients with varicocele. Acta Phlebol 2013;14:57–60. [Google Scholar]
- 20. Serra R, Buffone G, Costanzo G, Montemurro R, Perri P, Damiano R, de Franciscis S. Varicocele in younger as risk factor for inguinal hernia and for chronic venous disease in older: preliminary results of a prospective cohort study. Ann Vasc Surg 2013;27:329–331. [DOI] [PubMed] [Google Scholar]
- 21. Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother 2003;57:195–202. [DOI] [PubMed] [Google Scholar]
- 22. Dalton SJ, Mitchell DC, Whiting CV, Tarlton JF. Abnormal extracellular matrix metabolism in chronically ischemic skin: a mechanism for dermal failure in leg ulcers. J Invest Dermatol 2005;125:373–379. [DOI] [PubMed] [Google Scholar]
- 23. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 2003;200:448–464. [DOI] [PubMed] [Google Scholar]
- 24. Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 2007;6:480–498. [DOI] [PubMed] [Google Scholar]
- 25. Newby AC. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol 2012;56:232–244. [DOI] [PubMed] [Google Scholar]
- 26. de Franciscis S, Gallelli L, Amato B, Butrico L, Rossi A, Buffone G, Caliò FG, De Caridi G, Grande R, Serra R. Plasma MMP and TIMP evaluation in patients with deep venous thrombosis: could they have a predictive role in the development of post‐thrombotic syndrome? Int Wound J in press. DOI: 10.1111/iwj.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serra R, Grande R, Montemurro R, Butrico L, Caliò FG, Mastrangelo D, Scarcello E, Gallelli L, Buffone G, de Franciscis S. The role of matrix metalloproteinases and neutrophil gelatinase‐associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015;157:155–162. [DOI] [PubMed] [Google Scholar]
- 28. Busceti MT, Grande R, Amato B, Gasbarro V, Buffone G, Amato M, Gallelli L, Serra R, de Franciscis S. Pulmonary embolism, metalloproteinases and neutrophil gelatinase associated lipocalin. Acta Phlebol 2013;14:115–121. [Google Scholar]
- 29. Zhang P, Shen M, Fernandez‐Patron C, Kassiri Z. ADAMs family and relatives in cardiovascular physiology and pathology. J Mol Cell Cardiol 2015. DOI: 10.1016/j.yjmcc.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 30. Smiljanic K, Dobutovic B, Obradovic M, Nikolic D, Marche P, Isenovic ER. Involvement of the ADAM 12 in thrombin‐induced rat's VSMCs proliferation. Curr Med Chem 2011;18:3382–3386. [DOI] [PubMed] [Google Scholar]
- 31. Nyren‐Erickson EK, Jones JM, Srivastava DK, Mallik S. A disintegrin and metalloproteinase‐12 (ADAM12): function, roles in disease progression, and clinical implications. Biochim Biophys Acta 2013;1830:4445–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wight TN. The ADAMTS The ADAMTS proteases, extracellular matrix, and vascular disease: waking the sleeping giant(s)!. Arterioscler Thromb Vasc Biol 2005;25:12–14. [DOI] [PubMed] [Google Scholar]
- 33. Jönsson‐Rylander AC, Nilsson T, Fritsche‐Danielson R, Hammarström A, Behrendt M, Andersson JO, Lindgren K, Andersson AK, Wallbrandt P, Rosengren B, Brodin P, Thelin A, Westin A, Hurt‐Camejo E, Lee‐Søgaard CH. Role of ADAMTS‐1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol 2005;25:180–185. [DOI] [PubMed] [Google Scholar]
- 34. Ren P, Zhang L, Xu G, Palmero LC, Albini PT, Coselli JS, Shen YH, LeMaire SA. ADAMTS‐1 and ADAMTS‐4 levels are elevated in thoracic aortic aneurysms and dissections. Ann Thorac Surg 2013;95:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS‐5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem 2012;287:19341–19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang L, Yu F, Wang L, Zheng J, Du Y, Huang Y, Liu B, Wang X, Kong W. ADAMTS‐7 promotes vascular smooth muscle cells proliferation in vitro and in vivo. Sci China Life Sci 2015;58:674–681. [DOI] [PubMed] [Google Scholar]
- 37. Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, Fu Y, Mayr M, Ge Q, Xu Q, Zhu Y, Wang X, Schmidt K, de Wit C, Erdmann J, Schunkert H, Aherrahrou Z, Kong W. ADAMTS‐7 inhibits re‐endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin‐1. Circulation 2015;131:1191–1201. [DOI] [PubMed] [Google Scholar]
- 38. Wang L, Wang X, Kong W. ADAMTS‐7, a novel proteolytic culprit in vascular remodeling. Sheng Li Xue Bao 2010;62:285–294. [PubMed] [Google Scholar]
- 39. Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015;44–46:247–254. [DOI] [PubMed] [Google Scholar]
- 40. Rabkin SW. Differential expression of MMP‐2, MMP‐9 and TIMP proteins in thoracic aortic aneurysm – comparison with and without bicuspid aortic valve: a meta‐analysis. Vasa 2014;43:433–442. [DOI] [PubMed] [Google Scholar]
- 41. de Franciscis S, Fregola S, Gallo A, Argirò G, Barbetta A, Buffone G, Caliò FG, De Caridi G, Amato B, Serra R. PredyCLU: a prediction system for chronic leg ulcers based on fuzzy logic; part I – exploring the venous side. Int Wound J in press. DOI: 10.1111/iwj.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]


