Abstract
Iodine‐based products are antibacterial. The small iodine molecular size is ideally suited to treat surface critical colonisation. Inadine is a 10% povidone iodine dressing with the equivalent of 1% available iodine that is easily extracted from the viscose backing by serum or exudate. The use of hydrophilic polyethylene glycol tulle dressing delivery vehicle allows the dressing to be easily removed by irrigation with potable water or saline. In this study, we developed a short online survey completed by 23 wound‐care key opinion leaders from the nursing, medical and podiatry professions. A computerised modified Delphi technique was used to achieve 80% consensus on 11 statements related to the utility and everyday topical wound‐care use of this product.
Keywords: Biofilm, Chronic wound, Critical colonisation, Inadine, Iodine
Background
Iodine in wound care
Iodine has been applied topically to treat wounds for centuries 1. The antimicrobial action of iodine, first eluded by Vallin 1882 2, is well established and has developed a scientific foundation for iodine‐based products to treat wound‐associated bacterial burden. Early usage of iodine involved elemental aqueous and alcoholic preparations. Elemental iodine is clinically effective, but these iodine formulations were associated with adverse effects, including local pain, skin irritation and orange‐brown skin surface staining.
Modern iodine formulations were developed in the mid‐20th century. These formulations are safer and less painful than earlier formulations while still retaining the antimicrobial effectiveness of elemental iodine. Today, the use of iodine is widespread, yet the optimal role of iodine in wound care remains the subject of ongoing debate 3.
Inadine is an iodine‐containing tulle dressing consisting of a knitted viscose fabric that is made non‐adherent with a polyethylene glycol base containing 10% povidone iodine (PVP‐I) with an equivalent of 1% available iodine 4. This broad‐spectrum antimicrobial dressing has been available in many countries (except the USA) starting in the 1980s.
Inadine dressings slowly release iodine on contact, with wound exudate providing antimicrobial killing action on the wound surface. This non‐adherent dressing releases iodine at a rate dependent on the amount of wound exudate. As the iodine is released from the dressing onto the surface of the wound, the colour of the dressing turns from the iodine‐related brown‐orange to white, indicating depletion of the iodine content. Polyethylene glycol in the tulle base of the dressing facilitates the sustained iodine release.
Sustained release is preferable to painting a wound with 10% PVP‐I that results in a high‐dose iodine ‘dump’, increasing tissue toxicity without enhancing the antimicrobial action. In addition, a high iodine concentration is more likely to result in an unpleasant stinging or burning sensation that may cause patients to remove the surface antiseptic.
Cadexomer iodine is another effective iodine wound dressing that also provides slow release of iodine to the wound surface, absorbing up to 7× its weight in exudate along with providing autolytic debridement. However, this product can often dry on the wound surface, producing a pseudo‐eschar‐like film that is often difficult to remove. Subsequent applications may be ineffective because the newly applied active cadexomer iodine will not come in direct contact with the wound surface. Cadexomer iodine can also be up to ten times the cost of Inadine.
Wound Bed Preparation and the ability of a wound to heal
Wound Bed Preparation is an organised approach to the person with the chronic wound (Figure 1). Wound Bed Preparation 2015 is built on the premise that holistic care requires treating the ‘whole patient’ through treatment of the cause and patient‐centred concerns before the ‘hole in the patient’ is addressed with local wound care, that is, debridement, infection/inflammation and moisture balance 5.
Figure 1.
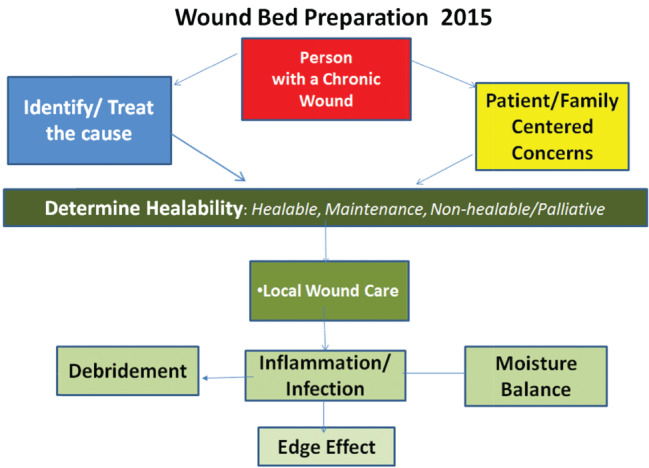
Wound Bed Preparation 2015© Sibbald et al. 2015 5.
For example, a person with a venous leg ulcer often requires compression bandages for healing and support stockings to prevent recurrences. A person with diabetes and a neurotrophic foot ulcer requires the VIPs of care: Vascular supply must be adequate; Infection and prolonged inflammation should be controlled; and Plantar pressure redistribution must be optimised with orthotics and downloading devices.
To determine if the blood supply is adequate to heal, clinicians should palpate for the dorsalis pedis or posterior tibial pulse. If they are palpated, the foot systolic pressure is 80 mmHg or higher, usually indicating there is enough blood supply to heal. A more accurate determination can be made using a hand‐held Doppler and determining the systolic pressure of the foot (ankle) over the brachial systolic pressure. If this is above 0·6, there is generally enough blood supply to heal. Note that non‐compressible vessels can give a falsely high value, especially in persons with diabetes. The audible Doppler signal may also facilitate the determination of adequate vascular supply. A biphasic or triphasic wave sound is usually adequate for healing 6. The large toe arterial pressure is an additional barometer of healing potential and should be above 55 mmHg.
The third component of the healable wound is to examine the person in general. Investigate any factors that could prevent healing. Check for coexisting diseases (e.g. cancer, immunosuppression from inflammatory disorders) or drugs that may inhibit healing. Critical laboratory values may also be important, including albumin below 2 g/dl (20 g/l) or haemoglobin below 8–10 g/dl (80–100 g/l).
Not all wounds are healable. The non‐healable wound has an uncorrectable cause, including non‐treatable cancer, kidney or liver failure and/or inadequate blood supply that cannot be dilated or bypassed. By contrast, a maintenance wound is a healable wound where the patient cannot afford or is unwilling to be adherent to the treatment or the correction of the cause is not provided by the health care system.
Local wound care has three main components: debridement, control of critical colonisation/abnormal prolonged inflammation and moisture balance for the healable wound. Healable wounds require active debridement, bacterial and inflammation reduction and moisture balance. The non‐healable or maintenance wound should only have conservative debridement of slough, bacterial reduction and moisture reduction
Local wound dressings and the use of iodine
There is no wound‐care dressing that is ideal for all patients. Contact‐irritant dermatitis to iodine is often identified with local stinging and burning followed by mild to moderate local erythema and swelling. Contact allergy is very rare but may occur and is a contraindication to the use of iodine dressings. A contact allergy is often accompanied by a more severe and well‐demarcated local erythema and swelling, often mimicking a bacterial infection. Any iodine dressing should be used with caution in the presence of thyroid disease. Thyroid function should be monitored if large areas are being treated for a prolonged period of time.
Inadine has several advantages as a wound‐care dressing. It is inexpensive, tolerated by most patients and does not usually interfere with the wound‐healing process. Although it is pro‐inflammatory, the inflammation is often beneficial, with an influx of macrophages and lymphocytes that can produce growth factors and other beneficial components for the local wound‐healing environment.
It has been suggested that the use of PVP‐I‐containing dressings is especially favourable for the treatment of biofilms. Iodine is a small molecule that easily penetrates the glycocalyx of biofilms in vivo and animal models 7, 8, 9. In comparative studies, iodine often demonstrates superior biofilm eradication compared with silver, PolyHexaMethyleneBiguanide (PHMB), honey and other topical antimicrobial agents 10, 11. Inadine is a suitable primary wound‐care dressing for the healable, non‐healable and maintenance wound to treat surface bacterial critical colonisation in a wet or dry environment. Wet environments often require an absorptive dressing as the second layer. With the advantages outlined, our Delphi survey was designed to obtain expert opinion on the use of this product in everyday clinical practice.
Methods
An online platform (SurveyMonkey®) was utilised to survey a convenience sample of n = 60 wound‐care key opinion leaders (KOLs). A total of 23 (38%) responded. These KOLs included 14 nurses, seven physicians (dermatologists, vascular surgeons, family physicians) and two podiatrists. The responding KOLs were from six countries: Canada: ten KOLs (seven nurses, three physicians), UK: five KOLs (four nurses, one podiatrist), South Africa: four KOLs (one nurse, three physicians) and Singapore: two KOLs (one nurse, one podiatrist) and a single KOL respondent each from Italy (one physician) and Australia (one nurse). The survey was open from 1 July to 30 September 2014.
KOLs ranked 11 statements on a 1–4 scale, where the anchors were defined as: 1, strongly agree; 2, somewhat agree; 3, somewhat disagree; 4, strongly disagree. KOLs had the opportunity to skip each question and provide written commentary on each statement along with a general impression of the Inadine product via open text. See Table 1 for each of the 11 statements. For a statement to appear in the final consensus document, we required 80% of the respondents to strongly agree or somewhat agree.
Table 1.
Consensus statements
| 1 | Wound Bed Preparation is a holistic approach to wound care where the treatment of the cause and patient‐centred concerns must be considered before optimising local wound care. The majority of wounds are healable with the treatment of the cause, but a small subgroup exists of non‐healable wounds where the cause cannot be corrected. A third larger subgroup (maintenance wounds) will have health care system or patient adherence issues, preventing the cause to be corrected, putting those wounds into a maintenance category. In one group of over 100 leg and foot ulcer wounds, 69·9% were healable, 24·9% maintenance and 5·2% non‐healable. |
| 2 | Patient‐centred concerns include:
|
| 3 | Local Wound Care includes:
|
| 4 | Deep and surrounding infection requires systemic antimicrobial agents for treatment |
| 5 | Iodine‐based topical preparations (such as Inadine) have demonstrated a more effective disruption of biofilms than other topical antimicrobial agents (e.g. silver, chlorhexidine derivatives such as PHMB) |
| 6 | Topical antimicrobials (e.g. Inadine) are effective to treat localised infection Features of localised infection may include three or more of the following:
|
| 7 | Iodine dressings today, like Inadine, which have slow and sustained release of iodine bound to carrier agents, are safer than iodine unbound to carrier agents and minimise side effects in patients. Human studies suggest that PVP‐I can help the wound‐healing process by reducing bacterial load and decreasing infection rates |
| 8 | Inadine has been used for most superficial wound types (diabetic neuropathic, neuroischemic, arterial and other causes of leg and foot ulcers, superficial pressure ulcers, post‐surgical wounds, etc.) in healable, non‐healable and maintenance wound scenarios |
| 9 | I would use Inadine in the following situations:
|
| 10 |
Advantages of the Inadine dressing in clinical practice include:
Inadine dressings are more cost‐effective than cadexomer iodine up to 1/10 the cost and with application time of 2–4 days are cost‐effective when considering dressing cost and nursing time
Liquid forms of iodine may have a duration of few hours only |
| 11 |
Advantages of INADINE in reducing patient pain and discomfort are:
The Inadine dressing is easy to remove in a painless fashion. Cadexomer iodine can form an eschar‐like crust on the surface of the wound that may be painful and difficult to remove. If cadexomer is incompletely removed, the pseudo‐eschar can prevent the active cadexomer from interacting with the wound surface |
PHMB, PolyHexaMethyleneBiguanide; PVP‐I, povidone iodine.
Results
The statements are listed in Table 1. We reached 80% consensus (strongly agree and somewhat agree) on all items. The results are presented in Table 2. The lowest strong agreement rating was in Statement 5, concerning the biofilm‐related action of iodine (see Discussion for more details).
Table 2.
Summary of consensus results
| Statement | Strongly agree | Somewhat agree | Somewhat disagree | Strongly disagree | Skipped |
|---|---|---|---|---|---|
| 1 | 22 (96%) | 0 | 1 (4%) | 0 | 0 |
| 2 | 18 (78%) | 5 (22%) | 0 | 0 | 0 |
| 3 | 20 (87%) | 3 (13%) | 0 | 0 | 0 |
| 4 | 21 (91%) | 2 (9%) | 0 | 0 | 0 |
| 5 | 8 (35%) | 12(52%) | 3 (13%) | 0 | 0 |
| 6 | 18 (78%) | 5 (22%) | 0 | 0 | 0 |
| 7 | 20 (87%) | 2 (9%) | 0 | 1 (4%) | 0 |
| 8 | 22 (96%) | 1 (4%) | 0 | 0 | 0 |
| 9 | 17 (74%) | 5 (22%) | 1 (4%) | 0 | 0 |
| 10 | 14 (61%) | 9 (39%) | 0 | 0 | 0 |
| 11 | 17 (74%) | 4 (17%) | 1 (4%) | 0 | 1 (4%) |
Discussion
The panel was in agreement that Inadine provides a useful topical antimicrobial agent for superficial critical colonisation of wounds. It does not treat deep and surrounding infection that requires a systemic antimicrobial agent. There are five clinical signs, the NERDS criteria 12, 13, that define surface critical colonisation of a wound:
Non‐healing – the size does not change over 2–4 weeks (it is also not getting larger).
Exudate – an increase in exudate usually indicates local inflammation, but the cause does not always need to be bacteria.
Red friable granulation – often bleeds easily on dressing removal and can often be hypertrophic or above the wound surface; this type of tissue will never support re‐epithelialisation or healing.
Debris – this is a soft yellow, brown or black material on the wound surface representing cellular or tissue death.
Smell – this is often because of gram‐negative or anaerobic bacteria.
Think of the NERDS criteria as being on the surface of wound‐like soup in a soup bowl, with three or more of the five criteria requiring a topical antimicrobial (iodine, silver, PolyHexaMethyleneBiguanide – PHMB a chlorhexidine derivative, methylene blue‐crystal violet, honey). Topical treatment with antimicrobial dressings should be re‐evaluated every 2–4 weeks, with discontinuation when bacterial balance has been re‐established. At this stage, moisture balance dressings would be indicated for healable wounds, for example, foams, calcium alginates, hydrofibers, hydrogels, hydrocolloids and films.
The deep and surrounding tissue in the soup bowl can be remembered with the seven STONEES criteria 12, 13, where any three or more indicate the need for systemic antimicrobial coverage: Size, Temperature increase, Os = probing to bone, New area of breakdown (i.e. satellites), Erythema/Edema = Cellulitis, Exudate increase, Smell. Any three of the systemic criteria and a systemic antimicrobial would be required. Systemic antimicrobials are often antibiotics, and we use antiseptics topically where they can be combined with agents that facilitate autolytic debridement and moisture balance (see Figure 1)
The topical use of antibiotics is problematic:
Only one mutation is often required for resistance, and this can then eliminate useful systemic agents.
Contact irritant or allergic dermatitis is common.
They often have a narrow therapeutic range.
They cannot be easily combined with autolytic debridement or moisture balance/moisture reduction agents.
Healing of a wound (treatment of the cause) is not always a realistic option. Previous studies of leg and foot ulcer populations have defined approximately 70% of wounds as healable, 25% maintenance and 5% non‐healable 14. With maintenance and non‐healable wounds, patient self‐management can be facilitated with the relatively simple application of Inadine dressings. These dressings can be cut to the size of the wound or have a 1–2 cm overlap onto the surrounding skin. The dressing also has a built‐in colour indicator, with the centre of the dressing changing to white when all the available iodine has been depleted.
Wound‐care patients need a support network for optimal outcomes. A report commissioned by the Government of Ontario, Canada has defined this ‘circle of care’ as the entire team that provides care and services for the individual in need and the family that includes the client/patient, the family and all services providers 15. The circle of care often improves patient outcomes and the capacity to adhere to treatment.
Wound documentation is a key to determining if a patient is on a healing trajectory. In general, a healable wound should be 30% smaller by week 4 to heal by week 12 16, 17. For patients with significant exudate, a primary Inadine dressing can be combined with a foam for moisture balance or a superabsorbent dressing for moisture reduction. Inadine may require daily changes if exudate levels are high and less frequent on drier wounds; however, clinical judgement must be used.
Several in vivo and in vitro studies compared PVP‐I or cadexomer iodine with other topical antimicrobial agents. Leaper and Durani (2008) 18 evaluated PVP‐I and cadexomer iodine products for wound care. The authors also concluded that the products may reduce the need for systemic antibiotics and related antimicrobials. A Cochrane review by O'Meara et al. (2014) 19 examined antibiotics and antiseptics for venous leg ulcers and concluded that there was some evidence to support the use of cadexomer iodine for accelerated healing of venous leg ulcers. No similar studies have been conducted with Inadine, but the same active agent is present.
Many chronic wounds are associated with partial or complete biofilms predominately on the wound surface. Biofilms contain bacterial colonies that have collaborating individual organisms surrounded by a protective glycocalyx. Most human bacterial infections are associated with biofilms (bacterial advantage) that often challenge the host resistance. Biofilm formation often occurs when two surfaces of different viscosity exist, including slough on the surface of a wound that should be debrided to optimise wound healing and decrease the conditions favouring biofilm formation. Several in vitro studies have suggested the positive effects on disrupting or eradicating biofilms from PVP‐I 8, 9, 20 and cadexomer iodine 11, 21.
Selvaggi et al. (2003) 22 reviewed the role of the newer iodine preparation that released lower concentrations of iodine and concluded that these products maintained antimicrobial activity but lowered the local toxicity of the products. A biopsy study by Zhou et al. with cadexomer iodine treating exudative wounds examined fibroblasts in the surface wound compartment and concluded that they had no evidence of fibroblast toxicity, including an absence of cell necrosis. The authors also noted that the re‐epithelialisation process was not inhibited by the iodine dressing. Ohtani et al. linked iodine in vitro to the production of pro‐inflammatory cytokines and the production of vascular endothelial growth factor by macrophages 8. This type of beneficial inflammation often promotes wound healing. The best practices for the use of Inadine in everyday clinical practice are summarised in Table 3.
Table 3.
Concise clinical use of Inadine dressing
| Component | Directions |
|---|---|
| Treat the cause |
|
| Patient‐centred concerns |
|
| Local wound care |
|
| Cleansing |
|
| Debridement |
|
| Critical colonisation, but need systemic antimicrobial if three or more STONEES criteria |
|
| Moisture content |
|
| Change frequency |
|
|
Periwound maceration excess exudate may indicate:
|
|
| Treatment time |
|
In conclusion, this consensus group of clinicians has suggested that Inadine is:
An effective low‐release iodine dressing that is inexpensive, low risk 23 and easy to use.
Ideally suited for surface critical colonisation.
Has the ability to be combined with autolytic debridement and moisture balance/moisture reduction dressings.
Depletion of available iodine is indicated by the dressing surface colour change, from orange to white.
Acknowledgements
We kindly acknowledge the time and contribution of the following consensus panel members: Kumal Rajpaul, Rode Heinz, Karen Witkowski, Kate Arkley, Josée Morin, Leslie Heath, Davide Strippoli, Neil Baker, Robyn Evans, Patricia Liesch, Craig Springate, Adriaan Erasmus, Helen Loudon, Amanda Loney, Anne LeMesurier, Gary Bain, Niamh McLain, Tay Ai Choo, Holly Murray, Fiona Concannon, Michael Marshall, David Keast and Perry Mayer.
References
- 1. Hugo W. A brief history of heat and chemical preservation and disinfection. J Appl Bacteriol 1991;71:9–18. [PubMed] [Google Scholar]
- 2. Vallin E. Traité des désinfectants et de la désinfection. Paris: G. Masson, 1882. [Google Scholar]
- 3. Vermeulen H, Westerbos SJ, Ubbink DT. Benefit and harm of iodine in wound care: a systematic review. J Hosp Infect 2010;76:191–9. [DOI] [PubMed] [Google Scholar]
- 4. Thomas S. Surgical dressings and wound management, 2nd edn. Kestrel Health Information, Meditec: South Wales, 2010. [Google Scholar]
- 5. Sibbald RG, Elliott JA, Ayello EA, Somayaji R. Optimizing the moisture management tightrope with wound bed preparation 2015. Adv Skin Wound Care 2015;28:466–76. [DOI] [PubMed] [Google Scholar]
- 6. Alavi A, Sibbald RG, Nabavizadeh R, Valaei F, Coutts P, Mayer D. Audible handheld Doppler ultrasound determines reliable and inexpensive exclusion of significant peripheral arterial disease. Vascular 2015;23:622–9. URL http://vas.sagepub.com/lookup/doi/10.1177/1708538114568703 [accessed on 9 August 2015]. [DOI] [PubMed] [Google Scholar]
- 7. Suman E, Madhavi R. Role of bacterial biofilms in chronic non‐healing ulcers and effect of subinhibitory concentrations of Betadine and hydrogen peroxide on biofilms. J Hosp Infect 2009;73:87–9. [DOI] [PubMed] [Google Scholar]
- 8. Ohtani T, Mizuashi M, Ito Y, Aiba S. Cadexomer as well as cadexomer iodine induces the production of proinflammatory cytokines and vascular endothelial growth factor by human macrophages. Exp Dermatol 2007;16:318–23. [DOI] [PubMed] [Google Scholar]
- 9. Aparecida Guimarães M, Rocchetto Coelho L, Rodrigues Souza R, Ferreira-Carvalho BT, Marie Sá Figueiredo A. Impact of biocides on biofilm formation by methicillin-resistant Staphylococcus aureus (ST239-SCCmecIII) isolates. Microbiol Immunol 2012;56:203–7. doi: 10.1111/j.1348-0421.2011.00423.x. [DOI] [PubMed] [Google Scholar]
- 10. Thorn RMS, Austin AJ, Greenman J, Wilkins JPG, Davis PJ. In vitro comparison of antimicrobial activity of iodine and silver dressings against biofilms. J Wound Care 2009;18:343–6. [DOI] [PubMed] [Google Scholar]
- 11. Phillips PL, Yang Q, Davis S, Sampson EM, Azeke JI, Hamad A, Schultz GS. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2015;12:469–83. doi: 10.1111/iwj.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sibbald RG, Woo K, Ayello EA. Increased bacterial burden and infection: the story of NERDS and STONEES. Adv Skin Wound Care 2006;19:447–61. [DOI] [PubMed] [Google Scholar]
- 13. Woo K, Sibbald R. A cross‐sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage 2009;55:40–8. [PubMed] [Google Scholar]
- 14. Sibbald RG, Goodman L, Woo KY, Krasner DL, Smart H, Tariq G, Ayello EA, Burrell RE, Keast DH, Mayer D, Norton L, Salcido RS. Special considerations in wound bed preparation 2011: an update. Adv Skin Wound Care 2011;24:415–36. doi: 10.1097/01.ASW.0000405216.27050.97. [DOI] [PubMed] [Google Scholar]
- 15. Donner G, Fooks C, McReynolds J, Sinha S, Smith K, Thomson D. Bringing care home: report of the expert group on home & community care. Toronto: Queen's Printer for Ontario, 2015:1–51. [Google Scholar]
- 16. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 17. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 18. Leaper DJ, Durani P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int Wound J 2008;5:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington L, Martyn‐St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014;1:1–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suman E, Jose S, D'Souza J, Kotian S. Does vancomycin inhibit biofilm production by coagulase‐negative staphylococci? J Hosp Infect 2009;73:86–7. [DOI] [PubMed] [Google Scholar]
- 21. Schultz GS, Phillips PL, Yang QP, Sampson EM. Microbicidal effects of wound dressings on mature bacterial biofilm on porcine skin explants. Poster Present EWMA [Internet]. 2009. URL http://obgyn.ufl.edu/docs/schultz_eurowound.pdf [accessed on 18 January 2014].
- 22. Selvaggi G, Monstrey S, Van Landuyt K, Hamdi M, Blondeel P. The role of iodine in antisepsis and wound management: a reappraisal. Acta Chir Belg 2003;3:241–7. [DOI] [PubMed] [Google Scholar]
- 23. Sibbald RG, Leaper DJ, Queen D. Iodine made easy. Wounds Int 2011;2:1–4. [Google Scholar]


