Abstract
This study aimed to evaluate and compare the effects of extracorporeal shock wave therapy (ESWT) and conventional wound therapy (CWT) for acute and chronic soft tissue wounds. All English‐language articles on ESWT for acute and chronic soft tissue wounds indexed in PubMed, Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Library, Physiotherapy Evidence Database, and HealthSTAR published prior to June 2017 were included, as well as corresponding articles cited in reference lists of related review articles. The methodological quality of the selected studies was assessed with the Cochrane Collaboration's “risk of bias” tool. Study design, subject demographics, wound aetiology, treatment protocols, assessment indexes, and follow‐up duration were extracted. The fixed or random‐effects model was used to calculate the pooled effect sizes according to studies’ heterogeneity. Ten randomised controlled trials (RCTs) involving 473 patients were included in this systematic review and meta‐analysis. The meta‐analysis showed that ESWT statistically significantly increased the healing rate of acute and chronic soft tissue wounds 2.73‐fold (odds ratio, OR = 3.73, 95% confidence interval, CI: 2.30‐6.04, P < .001) and improved wound‐healing area percentage by 30.45% (Standardized Mean Difference (SMD) = 30.45; 95% CI: 23.79‐37.12; P < .001). ESWT reduced wound‐healing time by 3 days (SMD = −2.86, 95% CI:‐3.78 to −1.95, P < .001) for acute soft tissue wounds and 19 days (SMD = −19.11, 95% CI: −23.74 to −14.47, P < .001) for chronic soft tissue wounds and the risk of wound infection by 53% (OR = 0.47, 95% CI: 0.24‐0.92, P = .03) when compared with CWT alone. Serious adverse effects were not reported. ESWT showed better therapeutic effects on acute and chronic soft tissue wounds compared with CWT alone. However, higher‐quality and well‐controlled RCTs are needed to further assess the role of ESWT for acute and chronic soft tissue wounds.
Keywords: meta‐analysis, rehabilitation, shock wave therapy, wound healing, wounds
1. INTRODUCTION
Wound healing is a complex sequence of events on multiple biological levels involving systemic, cellular, and molecular signals and is a common and major medical problem today.1 Soft tissue wounds (including burn wounds, diabetic foot ulcers [DFU], venous leg ulcers, and pressure ulcers) often show delayed or disturbed healing processes.2 Recently, the overall incidence of acute and chronic soft tissue wounds has continued to increase because of various conditions caused by trauma, disease, and old age.3 For example, 15% to 20% of diabetics are likely to develop chronic foot wounds.4 Soft tissue wounds are a major, functionality‐limiting problem causing great discomfort to patients, impairing quality of life, and imposing a substantial financial burden on the health care system.2
The management of soft tissue wounds requires a multidisciplinary approach. Conventional wound therapy (CWT) involves controlling the underlying causes, such as infection, ischaemia, and diabetes; optimising nutrition; debridement to remove devitalised tissue; moistening dressings to maintain a clean, moist bed of granulation tissue; compression; and treatment to resolve infection.2 However, these therapies have shown inconsistent outcomes over the years.5 Poor response to or failure of these treatments places a substantial burden on patients, their families, and the whole health care system in general. Therefore, efficacious and non‐invasive treatments to improve or accelerate the healing of soft tissue wounds are imperative.
Recently, alternative physical therapy, including ultrasound therapy, phototherapy, negative pressure therapy, hyperbaric oxygen therapy (HBOT), and extracorporeal shock wave therapy (ESWT), has offered a potential solution for improving the wound‐healing process.6 ESWT was originally used for stone management in urology and was subsequently introduced as treatment for various musculoskeletal disorders since the 1990s.7, 8 Today, the application of ESWT has been expanded to new therapeutic fields of myocardial infarction,9 wound healing,10, 11 and erectile dysfunction.12
Recent clinical studies demonstrated the efficacy of ESWT for accelerating tissue repair and regeneration in various wounds.10 Schaden et al13 found that 75% of wounds had complete epithelialisation after ESWT, and ESWT was feasible and well tolerated by patients with complicated, non‐healing, acute and chronic wounds. According to a study by Wolff et al14 on ESWT for wounds previously treated unsuccessfully, the wound‐healing rate was 74.03% after ESWT, and no wound reappeared at the same location. Furthermore, comorbidities and wound aetiologies had no significant influence on the effects of ESWT. Fioramonti et al15 reported the application of ESWT to chronic venous ulcers in the lower limbs and concluded that ESWT was a feasible and cost‐effective treatment for venous ulcers. Saggini et al16 investigated the effects of ESWT as an alternative treatment for chronic post‐traumatic venous and diabetic ulcers unresponsive to conservative treatments and observed a significant decrease in pain and exudates and improvement in the wound‐healing process.
To date, the mechanisms underlying the effects of ESWT for acute and chronic soft tissue wounds remain unclear.10 Extracorporeal shock waves (ESWs) could have a direct and indirect effect, producing a relative biological response in treated tissues.7 Mechanobiologically, ESWs increase tissue density and transmit direct mechanical perturbations, with effects on cell membrane polarisation and radical formation.17, 18 Based on this, ESWs could produce therapeutic benefits through cell proliferation and tissue regeneration in the therapeutic target.19 It has been demonstrated that ESWT could improve impaired healing of soft tissue wounds by increasing the expression of angiogenesis‐related growth and proliferation factors, inducing the production of collagen, fibroblastic proliferation, neovascularisation, and reducing the inflammatory phase and wound infection risk—all factors that may accelerate repair.20, 21, 22, 23, 24, 25 In addition, ESWT was also found to considerably alleviate pain around wounds by regulating substance‐P positive sensory nerve fibres and calcitonin gene‐related peptide.26
Thus, published data suggest that ESWT is an effective treatment for acute and chronic soft tissue wounds, especially when other conventional therapies fail. This systematic review and meta‐analysis aimed to evaluate the effects of ESWT on acute and chronic soft tissue wounds compared with CWT and to provide clinicians with an evidence base for decision‐making.
2. METHODS
2.1. Study search and selection
We conducted a systematic review of all English‐language articles indexed in PubMed, Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Library, Physiotherapy Evidence Database (PEDro) and Health Services, Technology, Administration, and Research (HealthSTAR), as well as articles cited in reference lists of related review articles and systematic reviews, prior to June 2017.
Medical subject heading terms included “randomized‐controlled trial,” “controlled clinical trial,” “random allocation,” “double‐blind method,” “single‐blind method,” “uncontrolled trials with shock waves,” “extracorporeal shock wave therapy,” “shock wave treatment,” “focused shock wave therapy,” “defocused shock wave therapy,” “radial shock wave therapy,” “wound healing,” “diabetic foot ulcer,” “post‐traumatic wound,” “skin ulcer,” “venous leg ulcer,” “press ulcers,” “decubitus ulcer,” “arterial insufficiency ulcer,” “postsurgical wound,” “burn wound,” “chronic wounds,” and “ulcer healing,” in relation to human subjects.
2.2. Inclusion criteria
Studies were included in our meta‐analysis when the following criteria were fulfilled1: participants randomly allocated to intervention and control groups,2 any kind of shock wave therapy compared with CWT,3 wound‐healing indexes monitored and recorded in terms of shock wave efficacy compared with CWT during the entire trial,4 ≥80% of participants completed the trial, and5 study published in the English language prior to June 2017.
All literature reviews, editorial comments, animal models, case reports, and articles published in non‐English languages were excluded.
2.3. Data extraction
The following data were recorded for each study: first author, year of publication, mean age of subjects, sample size in the ESWT and control groups, wound aetiology, ESWT treatment protocols, assessment indexes, and duration of follow up.
The literature search, assessment for inclusion, and data extraction were conducted independently by 2 reviewers (ZL, ZZB), and any disagreements were resolved by consensus.
2.4. Quality assessment
The methodological quality of the selected studies was assessed by 2 reviewers (ZL, ZZB) using the Cochrane Collaboration's “risk of bias” tool.27 Any inconsistencies in the results were verified by FXB and WCS.
2.5. Statistical analysis
Proper effect sizes and statistical analysis methods were chosen according to different data types and for different evaluation purposes. For continuous variables, the standardized mean difference (SMD) and a 95% confidence interval (CI) were used. For discontinuous variables, the odds ratio (OR) and 95% CI were used. For the heterogeneity test between studies, the χ 2 test (significant if P < .05) and I 2 test (with substantial heterogeneity defined as values >50%) were used. The fixed‐effects model was used to calculate the pooled effect sizes when studies did not show heterogeneity (P > .05, I 2 ≤ 50%). The random‐effects model was used when studies showed significant heterogeneity (P < .05, I2 ≥ 50%) and could not be explained. The cumulative effect on each outcome was illustrated with forest plots. Subgroup analysis was performed on acute vs chronic wounds. A funnel plot was applied to evaluate potential publication bias, and the significance level was set at .05. REVIEW MANAGER (version 5.3.5, The Cochrane Collaboration 2014, The Nordic Cochrane Center, Copenhagen, Denmark) was used for data analysis.
3. RESULTS
The flow chart for the screening and selection results according to the PRISMA (preferred reporting items for systematic reviews and meta‐analyses) guidelines28 is shown in Figure 1. After reviewing the information in the titles and abstracts, 20 articles were considered for review. After detailed reading, 10 articles were excluded from further meta‐analysis; only 10 articles met the inclusion criteria. Of these, Ottomann et al29, 30 and Wang et al26, 31 each produced 2 articles, deriving from their different randomised controlled trials (RCTs). In summary, 10 RCTs involving 473 patients were included in our systematic review and meta‐analysis.26, 29, 30, 31, 32, 33, 34, 35, 36, 37
Figure 1.
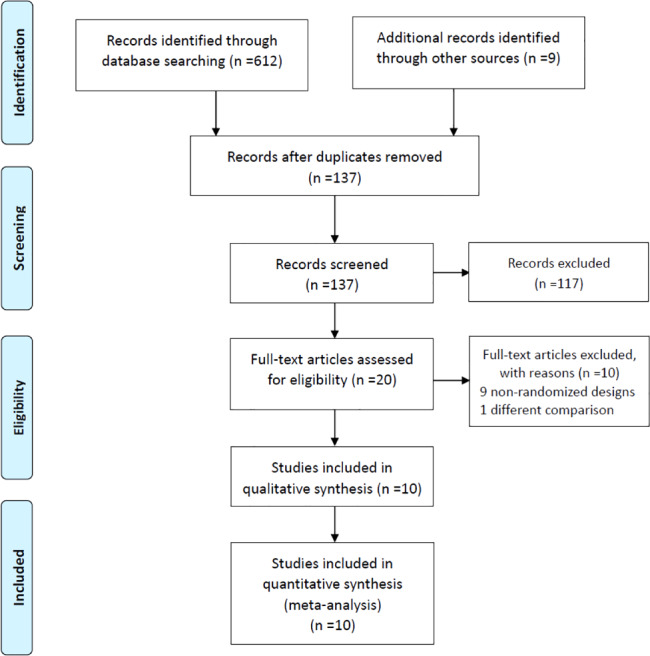
PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow diagram and exclusion criteria
The major subject characteristics of the 10 selected studies are outlined in Table 1. All of the studies were published prior to June 2017 and were performed by different medical centres in different countries. The mean age of subjects included in the selected trials ranged from 45 to 69 years.
Table 1.
Study design and patient characteristics of included studies
| Study/year | Country | Mean age (y) | Subjects | Intervention | Time of follow up | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aetiology | ESWT (n) | Control (n) | ESWT | Control | ||||||
| 1 | Dumfarth et al32 | Austria | 69.0 | Vein harvesting wounds for coronary artery bypass graft surgery | 50 | 50 | ESWT and CWT | CWT | Postoperative 7 d | ①⑧⑫ |
| 2 | Moretti et al33 | Italy | 56.5 | Neuropathic DFU | 15 | 15 | ESWT and CWT | CWT | 20 wk | ①②⑧ |
| 3 | Wang et al26 | China (Taiwan) | 61.1 | Chronic DFU | 36 | 36 | ESWT and CWT | CWT and HBOT | 6‐14 mo | ①④⑥⑦⑧⑨ |
| 4 | Larking et al34 | England | 63.3 | Chronic decubitus ulceration | 4 | 5 | ESWT and CWT | CWT | 6 wk | ①②③ |
| 5 | Ottomann et al30 | Germany | 48.8 | Skin graft donor site wounds after the acute traumatic wounds and burns | 13 | 15 | ESWT and CWT | CWT | 12 wk after hospital discharge | ①②⑧⑪ |
| 6 | Wang et al31 | China (Taiwan) | 61.5 | Chronic DFU | 44 | 40 | ESWT and CWT | CWT and HBOT | 3‐18mo | ①④⑥⑦⑪ |
| 7 | Ottomann et al29 | Germany | 45.0 | Acute second‐degree burns | 22 | 22 | ESWT and CWT | CWT | 12wk after hospital discharge | ①②⑧⑪ |
| 8 | Nossair et al35 | Egypt | 55.9 | Chronic DFU | 20 | 20 | ESWT and CWT | CWT | 12 wk | ③⑪ |
| 9 | Omar et al36 | Egypt | 56.8 | Chronic DFU | 24 | 21 | ESWT and CWT | CWT | 20 wk | ①②③④⑪ |
| 10 | Jeppesen et al37 | Denmark | 66.6 | Chronic DFU | 10 | 11 | ESWT and CWT | CWT | 7 wk | ③④⑤⑥⑧⑩⑪ |
Abbreviations: CWT, conventional wound therapy; DFU, diabetic foot ulcers; ESWT, extracorporeal shock wave therapy; HBOT, hyperbaric oxygen therapy; n, number of subjects; ①wound‐healing rate; ②wound‐healing time (d); ③percentage of the wounds areas (%); ④wounds status; ⑤transcutaneous oxygen tension (TcPO2); ⑥blood flow perfusion; ⑦histopathological examination; ⑧bacteriological examination; ⑨immunohistochemical analysis; ⑩pain score; ⑪adverse effects; ⑫ASEPSIS score (additional treatment, presence of serous discharge, erythema, purulent exudate, separation of the deep tissue, isolation of bacteria, and duration of inpatient stay).
Some differences existed in the ESWT protocols adopted by the selected studies (Table 2). All studies applied standard care of wounds as the control, except for Wang et al,26, 31 where both HBOT and standard care were used in the control group. With regards to the type of ESWs, focused ESWs were applied in 5 studies, defocused ESWs in 4 studies, and radial ESWs in 1 study. The frequency of ESWT varied from 0.5 to 2 sessions per week for a duration of 1 to 8 weeks. The ESWT impulses used ranged from 25 to 500 pulses/cm2 (wound area) or pulses/cm (wound length), with energy density (ED) between 0.03 and 0.23 mJ/mm2.
Table 2.
Protocol of ESWT and control treatments in the included studies
| Study/year | ESWT protocol | Protocol of CWT | ||||||
|---|---|---|---|---|---|---|---|---|
| Type of ESWT | ED (mJ/mm2) | Frequency (pulses/cm2) | No. of treatment sessions per week | Total treatment course | Total number of treatment sessions | |||
| 1 | Dumfarth et al32 | fESWT | 0.1 | 25 | 1 | 1 wk | 1 | Non‐occlusive surgical dressing, absorbable sutures, staples, drains, debridement, and antibiotic treatment |
| 2 | Moretti et al33 | fESWT | 0.03 | 100 | 2 | 1.5 wk | 3 | Therapeutic footwear, debridement, and dressing |
| 3 | Wang et al26 | fESWT | 0.11 | 300 + 100 | 1/2 wk | 6 wk | 3 | 1. HBOT daily for 20 treatments; 2. CWT: offloading on the affected foot, wound cleansing with sterile normal saline solution, and application of silver sulfadiazine cream |
| 4 | Larking et al34 | dESWT | 0.1 | 200 + 100 | 1 | 4 wk | 4 | Debridement and dressing |
| 5 | Ottomann et al30 | dESWT | 0.1 | 100 | 1 | 1 wk | 1 | Non‐adherent silicone mesh and antiseptic gel (polyhexanide/octenidine) |
| 6 | Wang et al31 | fESWT | 0.23 | 500 | 2 | 3 wk | 6 | 1. HBOT daily for 20 treatments; 2. CWT: offloading on the affected foot, wound cleansing with sterile normal saline solution, and application of silver sulfadiazine cream |
| 7 | Ottomann et al29 | dESWT | 0.1 | 100 | 1 | 1 wk | 1 | Burn wound debridement and topical antiseptic therapy |
| 8 | Nossair et al35 | rESWT | 0.1 | 500 | 1 | 3 wk | 3 | Debridement, adequate pressure relief, and treatment of infection |
| 9 | Omar et al36 | dESWT | 0.11 | 100 | 2 (with 1wk interval) | 8 wk | 8 | Debridement, blood‐glucose control agents, and footwear modification for pressure reduction |
| 10 | Jeppesen et al37 | fESWT | 0.2 | 250‐500 | 2 | 3 wk | 6 | Standard wound care (Danish national clinical guidelines) |
Abbreviations: CWT, conventional wound therapy; ED, energy density; ESWT, extracorporeal shock wave therapy; dESWT, defocused extracorporeal shock wave therapy; fESWT, focused extracorporeal shock wave therapy; HBOT, hyperbaric oxygen therapy; rESWT, radial extracorporeal shock wave therapy.
CWT in all studies is summarised in Table 2. CWT alone was used as the control in 8 studies.29, 30, 32, 33, 34, 35, 36, 37 The CWT protocol varied among different studies and included debridement, dressing, pressure reduction, blood glucose control agents, and topical antiseptic therapy. In 2 studies, CWT combined with HBOT was used as the control.26, 31
Quality evaluations of the selected studies are shown in Figure 2. According to the Cochrane Collaboration's tool, 6 RCTs reported randomisation methods.29, 30, 31, 34, 36, 37 Treatment allocation was specifically concealed from participants and investigators in 5 studies.29, 30, 34, 36, 37 Six studies reported appropriate blinding of outcomes assessments.26, 29, 30, 32, 34, 36 However, most studies did not describe whether the physicians were blinded to the study participants because it would be difficult to blind the physician to ESWT with CWT or CWT alone. Only the studies conducted by Ottomann et al29, 30 reported the blinding of patients to treatment allocation.
Figure 2.
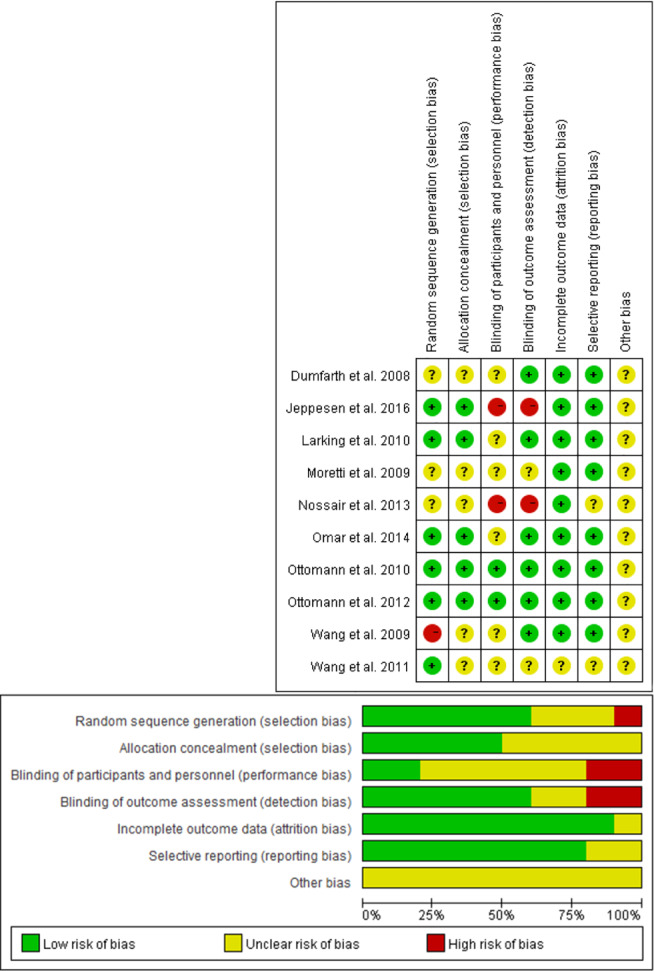
The quality evaluation and risk of bias in included studies
Six studies26, 31, 32, 33, 34, 36 used wound‐healing rate as outcome measure (Table 1). Five33, 34, 35, 36, 37 used the percentage (%) of the wound area to compare the effects of ESWT and CWT. Four studies29, 30, 33, 36 evaluated wound‐healing time (in days), and 6 studies26, 29, 30, 32, 33, 37 specifically reported wound infection after ESWT or CWT. Meta‐analysis was feasible for the above 4 indexes.
Six studies involving 340 subjects measured the wound‐healing rate.26, 31, 32, 33, 34, 36 The meta‐analysis demonstrated that ESWT was more effective than CWT alone in the treatment of various wounds (OR = 3.73, 95% CI: 2.30‐6.04, P < .0001; Figure 3). Minimal evidence of heterogeneity between studies was obtained (P = .57, I 2 = 0%), indicating that the effects of ESWT on wounds with different aetiologies were not statistically significantly different. The potential influence of publication bias was visually represented using a funnel plot, and all of the studies were closely distributed within the 95% CI axis (Figure 4).
Figure 3.
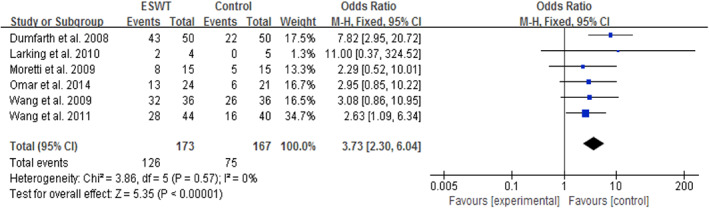
Forest plot of the wound‐healing rate between extracorporeal shock wave therapy (experimental) and control wound therapy for acute and chronic soft tissue wounds
Figure 4.
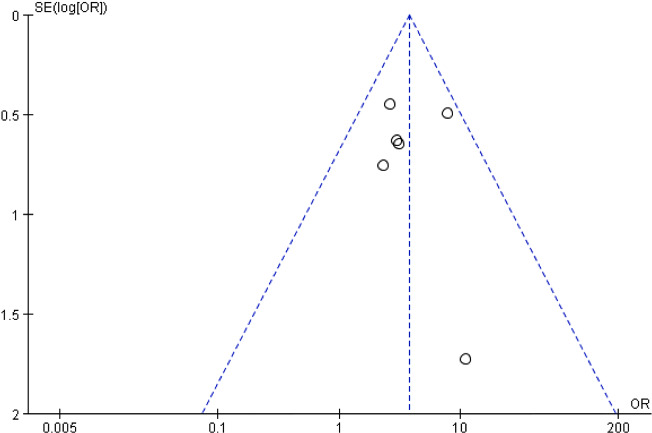
Funnel plot of the wound‐healing rate
Five studies (128 subjects) compared the effects of ESWT and CWT on the percentage (%) of the wound‐healing area.33, 34, 35, 36, 37 The data included the mean and SD of the percentage of the wound‐healing area and the number of subjects in the treatment and control groups. There was little heterogeneity among these studies (P = .45, I 2 = 0%). Meta‐analysis with a fixed‐effects model showed that ESWT statistically significantly increased the wound‐healing area and had a more significant treatment effect when compared with CWT (SMD = 30.45, 95% CI: 23.79‐37.12, P < .0001; Figure 5).
Figure 5.
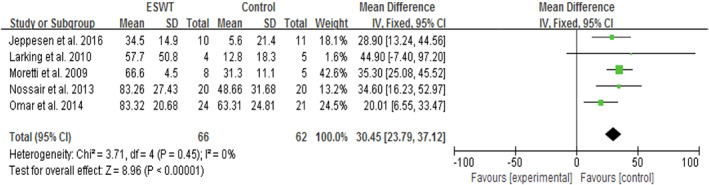
Forest plot of the percentage (%) of the wound‐healing area between extracorporeal shock wave therapy (experimental) and control wound therapy for acute and chronic soft tissue wounds
Wound‐healing time was available from 4 RCTs,29, 30, 33, 36 with high heterogeneity (P < .0001, I 2 = 98%) across studies. The random‐effects model showed that, on average, the healing time was 11 days shorter in the ESWT groups than in the CWT groups (SMD = −10.72, 95% CI: −17.68 to −3.77, P = .003; Figure 6A). With further subgroup analysis based on wound duration, the results showed that ESWT statistically significantly shortened the healing time of acute wounds by 3 days (SMD = −2.86, 95% CI: −3.78 to −1.95, P < .0001; Figure 6B) and that of chronic wounds by 19 days (SMD = −19.11, 95% CI: −23.74 to −14.47, P < .0001; Figure 6C) compared with CWT. No statistically significant heterogeneity (P = .92, I 2 = 0%) was noted among patients with acute wounds.
Figure 6.
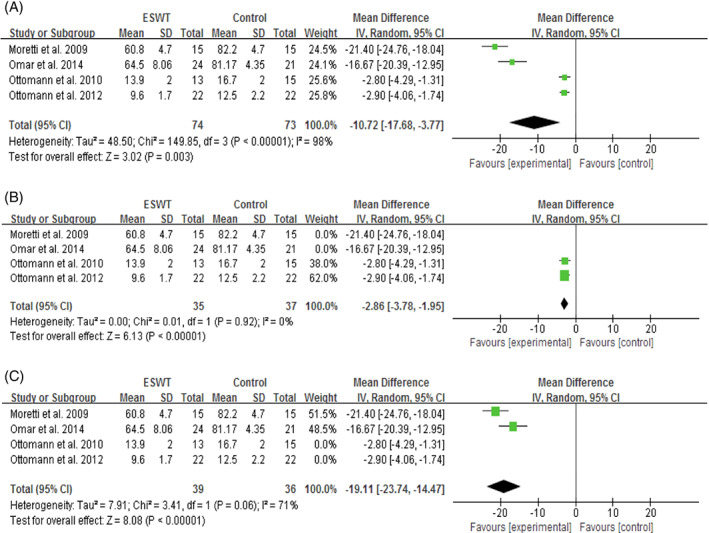
Forest plot of the wound‐healing time (d) between extracorporeal shock wave therapy (experimental) and control wound therapy for acute and chronic soft tissue wounds. A, wound‐healing time of both acute and chronic wounds; B, wound‐healing time of acute wounds; C; wound‐healing time of chronic wounds
Six studies involving 295 subjects reported wound infection or bacteriological contamination.26, 29, 30, 32, 33, 37 The meta‐analysis showed a statistically significantly lower incidence of wound infection after ESWT compared with CWT (OR = 0.47, 95% CI: 0.24‐0.92, P = .03; Figure 7), which indicated a 53% reduction in the risk of wound infection after ESWT. Considerable heterogeneity among studies was not observed (P = .34, I 2 = 11%).
Figure 7.
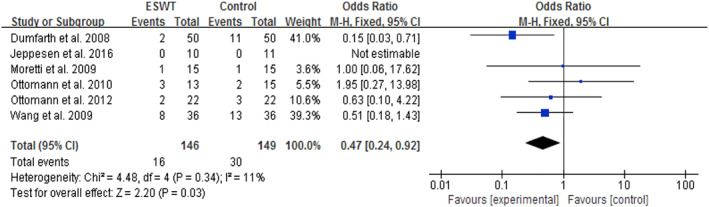
Forest plot of the wound infection rate between extracorporeal shock wave therapy (experimental) and control wound therapy for acute and chronic soft tissue wounds
Some additional outcome measures related to wound healing were reported but did not undergo further meta‐analysis. With histopathological examination and immunohistochemical staining, Wang et al found a more significant increase in proliferation, concentration, cell activity, and angiogenesis‐related growth and proliferating indicator expressions, including endothelial nitric oxide synthase (eNOS), vessel endothelial growth factor (VEGF), and proliferation cell nuclear antigen (PCNA), in the ESWT group compared with the control group.26, 31 Local blood flow perfusion and transcutaneous oxygen tension (TcPO2) also showed marked improvement after ESWT compared with CWT.26, 31, 37 Decrease in wound pain was observed, although there was no statistically significant difference between the intervention and control group.37
Six studies reported complications or side effects secondary to the application of ESWT.29, 30, 31, 35, 36, 37 The most common complications after ESWT intervention included transitory reddening of the skin, slight pain, and small haematomas. Serious adverse events, such as cardiac, neurological adverse reactions, muscle damage, haemorrhage, or thrombosis, were not reported in these above studies,29, 30, 31, 35, 36, 37 which suggests that ESWT is a safe adjunct treatment method for different kinds of wounds.
4. DISCUSSION
Skin soft tissue repair and wound healing are complex processes that involve a series of dynamic events. Thus, therapeutic interventions and approaches that efficiently accelerate healing should be implemented. ESWT and various new technologies were proposed in the biomedical sciences to promote many kinds of tissue regeneration. Over the years, clinical evidence of wound healing with ESWT has been accumulating. However, to date, the effectiveness of ESWT is equivocal because of the lack of convergence of findings from RCTs of ESWT for acute and chronic wounds. Consequently, the present systematic review and meta‐analysis aimed to evaluate the impact and usefulness of ESWT on wounds with different aetiologies in clinical practice.
The results of the present study showed that ESWT, as an adjunct to wound treatment, efficiently accelerated the impaired wound‐healing process compared with CWT alone. Specifically, ESWT markedly increased the wound‐healing rate by 2.73‐fold, improved the mean percentage change in wound area by 30.45%, reduced the mean wound‐healing time by 3 days for acute wounds and 19 days for chronic wounds, and reduced the risk of wound infection by 53% when compared with CWT alone. These data suggest that ESWT is more effective than CWT alone in the management of acute and chronic soft tissue wounds.
The results of the present systematic review and meta‐analysis agree with the findings of previous systematic reviews without meta‐analysis conducted by Butterworth et al., Dymarek et al., and Omar et al.38, 39, 40 Nevertheless, the present meta‐analysis only included clinical RCTs, which are regarded as the strongest experimental design for evaluating therapeutic effects. The previous systematic reviews included randomised and non‐RCTs as well as cohort studies, which might have masked the actual treatment effect of ESWT on acute and chronic soft tissue wounds.
Heterogeneity among some of the RCTs was evaluated in the present meta‐analysis. Less heterogeneity was noted among these studies except for the assessment index of wound‐healing time, which might be explained by the different aetiologies and duration of wounds among the included subjects because chronic wounds do not adhere to the standard time course that leads to healing of acute wounds.41
In the present meta‐analysis, the clinical effect on wound healing was closely related to the protocols of ESWT, including the types of ESWs applied, ED, the number of ESWs per ESWT session, and the number of ESWT sessions. The ED used in the RCTs included in the present analysis varied between 0.03 and 0.23 mJ/mm2, the frequency of ESWT varied from once every 2 weeks to twice every week (with ESWT sessions for a duration of 1 to 8 weeks), and the number of ESWs per ESWT session varied between 25 and 500 pulses/cm2 (wound area) or pulses/cm (wound length). According to a previous study, 200 to 300 ESWs per unit area can enhance cell proliferation and clinical efficacy.18 Although most studies presented results that suggest the effectiveness of ESWT, whether the protocols described in these studies are the optimal ones has remained unknown, necessitating further investigations.
The mechanisms underlying the effects of ESWT for acute and chronic soft tissue wounds were also preliminarily discussed in the included studies. For example, Wang et al observed that ESWT can increase proliferation and cell density as well as activate angiogenesis‐related growth factors, including eNOS, VEGF, and PCNA in chronic DFU wounds.26, 31 Based on laser Doppler imaging and TcPO2 measurements, these authors also found that local blood flow perfusion and TcPO2 were markedly enhanced after ESWT compared with HBOT.26, 31, 37 Wang et al hypothesised that the effects of ESWT were primarily related to the stimulation of cell proliferation, tissue regeneration, and angiogenesis.26, 31, 37
The predominant limitations of the present meta‐analysis should be noted. First, although the present meta‐analysis performed funnel plotting, the power of the test was too low to distinguish chance from real asymmetry as there were not enough studies included. Therefore, the risk of publication bias could not be excluded. Second, confounding bias might exist because of the different conditions of wounds in the included studies. The treatment strategy and wound‐healing process of various wounds are notably different. Third, the measurements of wound healing used in different studies were markedly different, partly explaining why a meta‐analysis could not be performed for all outcomes. Furthermore, few studies have examined the cost‐effectiveness of ESWT compared with CWT for acute and chronic soft tissue wounds. The costs of ESWT depended on the number of required treatment sessions and ESWs per treatment session and varied greatly between studies. Finally, the present meta‐analysis only included English‐language articles for better understandability and consistency of the studies’ result. However, the exclusion of articles written in other languages may have introduced bias into the results of the review.
In summary, the available data consistently suggested that ESWT was easily applied, with little obvious discomfort to the patient and complications, and had good therapeutic effects on acute and chronic soft tissue wounds of different aetiologies. However, the effectiveness of ESWT for acute and chronic soft tissue wounds still requires further high quality, well‐controlled RCTs with an adequate sample size because the existing clinical and experimental evidence has been limited. Furthermore, optimal ESWT regimens and dosages are required to provide evidence‐based therapeutic guidance.
ACKNOWLEDGEMENTS
This work was funded by the National Natural Science Foundation of China (grant no. 81421064 and 81230041) and China Postdoctoral Science Foundation (grant no. 2015M572764).
Zhang L, Fu X, Chen S, Zhao Z, Schmitz C, Weng C. Efficacy and safety of extracorporeal shock wave therapy for acute and chronic soft tissue wounds: A systematic review and meta‐analysis. Int Wound J. 2018;15:590–599. 10.1111/iwj.12902
REFERENCES
- 1. Demidova‐Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25(7):304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greer N, Foman NA, MacDonald R, et al. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med. 2013;159(8):532‐542. [DOI] [PubMed] [Google Scholar]
- 3. Jiang Y, Huang S, Fu X, et al. Epidemiology of chronic cutaneous wounds in China. Wound Repair Regen. 2011;19(2):181‐188. [DOI] [PubMed] [Google Scholar]
- 4. Turner NJ, Badylak SF. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv Wound Care (New Rochelle). 2015;4(8):490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonic V, Mittermayr R, Schaden W, Stojadinovic A. Evidence supporting extracorporeal shock wave therapy for acute and chronic soft tissue wounds. Wounds. 2011;23(7):204‐215. [PubMed] [Google Scholar]
- 6. Vélez‐Díaz‐Pallarés M, Lozano‐Montoya I, Abraha I, et al. Nonpharmacologic interventions to heal pressure ulcers in older patients: an overview of systematic reviews (the SENATOR‐ONTOP series). J Am Med Dir Assoc. 2015;16(6):448‐469. [DOI] [PubMed] [Google Scholar]
- 7. Speed C. A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med. 2014;48:1538‐1542. [DOI] [PubMed] [Google Scholar]
- 8. Schmitz C, Császár NB, Milz S, et al. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br Med Bull. 2015;116:115‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cassar A, Prasad M, Rodriguez‐Porcel M, et al. Safety and efficacy of extracorporeal shock wave myocardial revascularization therapy for refractory angina pectoris. Mayo Clin Proc. 2014;89(3):346‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittermayr R, Antonic V, Hartinger J, et al. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012;20(4):456‐465. [DOI] [PubMed] [Google Scholar]
- 11. Qureshi AA, Ross KM, Ogawa R, Orgill DP. Shock wave therapy in wound healing. Plast Reconstr Surg. 2011;128(6):721e‐727e. [DOI] [PubMed] [Google Scholar]
- 12. Lu Z, Lin G, Reed‐Maldonado A, Wang C, Lee YC, Lue TF. Low‐intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta‐analysis. Eur Urol. 2017;71(2):223‐233. [DOI] [PubMed] [Google Scholar]
- 13. Schaden W, Thiele R, Kölpl C, et al. Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res. 2007;143(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 14. Wolff KS, Wibmer A, Pusch M, et al. The influence of comorbidities and etiologies on the success of extracorporeal shock wave therapy for chronic soft tissue wounds: midterm results. Ultrasound Med Biol. 2011;37(7):1111‐1119. [DOI] [PubMed] [Google Scholar]
- 15. Fioramonti P, Onesti MG, Fino P, Fallico N, Scuderi N. Extracorporeal shock wave therapy for the treatment of venous ulcers in the lower limbs. Ann Ital Chir. 2012;83(1):41‐44. [PubMed] [Google Scholar]
- 16. Saggini R, Figus A, Troccola A, Cocco V, Saggini A, Scuderi N. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med Biol. 2008;34(8):1261‐1271. [DOI] [PubMed] [Google Scholar]
- 17. d'Agostino MC, Craig K, Tibalt E, Respizzi S. Shock wave as biological therapeutic tool: from mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. 2015;24(pt B):147‐153. [DOI] [PubMed] [Google Scholar]
- 18. Rosso F, Bonasia DE, Marmotti A, Cottino U, Rossi R. Mechanical stimulation (pulsed electromagnetic fields “PEMF” and extracorporeal shock wave therapy “ESWT”) and tendon regeneration: a possible alternative. Front Aging Neurosci. 2015;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuo YR, Huang YT, Wang CT, Wang CJ. Proteome analysis the differences of the mesenchymal stem cells and extracorporeal shock wave therapy enhancing diabetic wound healing. Plast Reconstr Surg. 2015;136(4 suppl):76‐77.26111315 [Google Scholar]
- 20. Kuo YR, Wang CT, Wang FS, Chiang YC, Wang CJ. Extracorporeal shock‐wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ‐induced diabetes. Wound Repair Regen. 2009;17(4):522‐530. [DOI] [PubMed] [Google Scholar]
- 21. Stojadinovic A, Elster EA, Anam K, et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis. 2008;11(4):369‐380. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Wang J, Wang M, et al. Activation of bone marrow‐derived mesenchymal stromal cells‐a new mechanism of defocused low‐energy shock wave in regenerative medicine. Cytotherapy. 2013;15(12):1449‐1457. [DOI] [PubMed] [Google Scholar]
- 23. Vetrano M, d'Alessandro F, Torrisi MR, Ferretti A, Vulpiani MC, Visco V. Extracorporeal shock wave therapy promotes cell proliferation and collagen synthesis of primary cultured human tenocytes. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2159‐2168. [DOI] [PubMed] [Google Scholar]
- 24. Yang MY, Chiang YC, Huang YT, et al. Serum proteomic analysis of extracorporeal shock wave therapy‐enhanced diabetic wound healing in a streptozotocin‐induced diabetes model. Plast Reconstr Surg. 2014;133(1):59‐68. [DOI] [PubMed] [Google Scholar]
- 25. Contaldo C, Högger DC, Khorrami BM, et al. Radial pressure waves mediate apoptosis and functional angiogenesis during wound repair in ApoE deficient mice. Microvasc Res. 2012;84(1):24‐33. [DOI] [PubMed] [Google Scholar]
- 26. Wang CJ, Kuo YR, Wu RW, et al. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J Surg Res. 2009;152(1):96‐103. [DOI] [PubMed] [Google Scholar]
- 27. Turner L, Boutron I, Hróbjartsson A, Altman DG, Moher D. The evolution of assessing bias in Cochrane systematic reviews of interventions: celebrating methodological contributions of the Cochrane collaboration. Syst Rev. 2013;2:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ottomann C, Stojadinovic A, Lavin PT, et al. Prospective randomized phase II trial of accelerated reepithelialization of superficial second‐degree burn wounds using extracorporeal shock wave therapy. Ann Surg. 2012;255(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 30. Ottomann C, Hartmann B, Tyler J, et al. Prospective randomized trial of accelerated re‐epithelialization of skin graft donor sites using extracorporeal shock wave therapy. J Am Coll Surg. 2010;211(3):361‐367. [DOI] [PubMed] [Google Scholar]
- 31. Wang CJ, Wu RW, Yang YJ. Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res Clin Pract. 2011;92(2):187‐193. [DOI] [PubMed] [Google Scholar]
- 32. Dumfarth J, Zimpfer D, Vögele‐Kadletz M, et al. Prophylactic low‐energy shock wave therapy improves wound healing after vein harvesting for coronary artery bypass graft surgery: a prospective, randomized trial. Ann Thorac Surg. 2008;86(6):1909‐1913. [DOI] [PubMed] [Google Scholar]
- 33. Moretti B, Notarnicola A, Maggio G, et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskelet Disord. 2009;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larking AM, Duport S, Clinton M, Hardy M, Andrews K. Randomized control of extracorporeal shock wave therapy versus placebo for chronic decubitus ulceration. Clin Rehabil. 2010;24(3):222‐229. [DOI] [PubMed] [Google Scholar]
- 35. Nossair AA, Eid MM, Salama AB. Advanced protocol of shock wave therapy for diabetic foot ulcer. J Am Sci. 2013;9:633‐638. [Google Scholar]
- 36. Omar MT, Alghadir A, Al‐Wahhabi KK, Al‐Askar AB. Efficacy of shock wave therapy on chronic diabetic foot ulcer: a single‐blinded randomized controlled clinical trial. Diabetes Res Clin Pract. 2014;106(3):548‐554. [DOI] [PubMed] [Google Scholar]
- 37. Jeppesen SM, Yderstraede KB, Rasmussen BS, Hanna M, Lund L. Extracorporeal shockwave therapy in the treatment of chronic diabetic foot ulcers: a prospective randomised trial. J Wound Care. 2016;25(11):641‐649. [DOI] [PubMed] [Google Scholar]
- 38. Dymarek R, Halski T, Ptaszkowski K, Slupska L, Rosinczuk J, Taradaj J. Extracorporeal shock wave therapy as an adjunct wound treatment: a systematic review of the literature. Ostomy Wound Manage. 2014;60(7):26‐39. [PubMed] [Google Scholar]
- 39. Omar MT, Gwada RF, Shaheen AA, Saggini R. Extracorporeal shockwave therapy for the treatment of chronic wound of lower extremity: current perspective and systematic review. Int Wound J. 2017;14:898‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butterworth PA, Walsh TP, Pennisi YD, Chesne AD, Schmitz C, Nancarrow SA. The effectiveness of extracorporeal shock wave therapy for the treatment of lower limb ulceration: a systematic review. J Foot Ankle Res. 2015;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]


