Abstract
The identification of appropriate skin tear prevention guidelines for the elderly requires clinicians to focus on local risk factors such as structural alterations of the epidermis and dermis related to skin tears. The aim of this cross‐sectional study is to explore the prevalence of skin tears and to explore skin properties related to skin tears in elderly Japanese patients at a long‐term medical facility. After doing the prevalence study, 18 participants with skin tears and 18 without were recruited and an evaluation of their skin properties using 20‐MHz ultrasonography, skin blotting and also Corneometer CM‐825, Skin‐pH‐meterPH905, VapoMeter, Moisture Meter‐D and CutometerMPA580 was undertaken. A total of 410 patients were examined, the median age was 87 years and 73·2% were women. The prevalence of skin tears was 3·9%, and 50% of skin tears occurred on the dorsal forearm. The changes in skin properties associated with skin tears included increased low‐echogenic pixels (LEP) by 20‐MHz ultrasonography, decreased type IV collagen and matrix metalloproteinase‐2, and increased tumour necrosis factor‐α by skin blotting. In conclusion, this study suggests that increased dermal LEP, including solar elastosis, may represent a risk factor for skin tears; this indicates that skin tear risk factors might not only represent chronological ageing but also photoageing.
Keywords: Ageing, Elderly, Skin blotting, Skin properties, Skin tears
Introduction
Skin tears are wounds caused by shearing, friction, and/or blunt forces that result in the separation of skin layers 1. Depending on the level of tissue damage, they can be classified as partial or full‐thickness wounds 1. Skin tears occur more commonly among frail, aged or disabled populations and neonates and are more likely to occur on fragile exposed skin surfaces such as the extremities or on skin lesions such as purpura and ecchymoses. They are painful and can ultimately reduce the quality of patient life 1, 2, 3, 4, 5. Unfortunately, skin tears can also occur when health life 1, 2, 3, 4, 5. Skin tears can also occur when health care practitioners handle the patients or remove surgical tapes as these interventions apply external forces to the skin surface in the elderly 1, 2, 3, 6. Therefore, skin tears can be a constant stress for health care practitioners if families and residents incorrectly interpret the occurrence of skin tears as the evidence of poor care 2.
The development of a skin tear prevention protocol requires an investigation of risk factors related to why skin tears occur. Previous studies have reported an association between skin tears and both external force and systemic factors such as age, dependency in activities of daily living, sensory loss, limited mobility, use of assistive devices, impaired cognition and medication 1, 2, 3, 4, although existing prevention protocols have only focused on external forces and systemic factors. However, these efforts do not always ameliorate skin tears sufficiently 1.
The skin of the elderly is predisposed to skin tears because of a series of changes that occurs during the ageing process 7, 8, 9. Skin properties are determined by systemic factors such as age, gender, use of medications and other local factors that influence the structural alterations of the epidermis, dermis and constitutive proteins. Changes that occur during ageing include flattening of dermal–epidermal junctions, which impairs the resistance of skin to shear forces 7, 8, 9. However, clinical research has not showed a direct relationship between these ageing‐related skin properties and skin tears.
In a rapidly ageing society, it is important to note the gravity of skin tears 1, 4, and the determination of their prevalence is essential to understand the magnitude. Incident reports that have investigated the epidemiology of skin tears are limited 2, 6 and to date there are no studies that focus on their epidemiology in Japan.
One of the reasons for the lack of research in this area may be the requirement for an invasive biopsy to examine the skin histologically to identify damage. High‐frequency ultrasonography has been used for the non‐invasive visualisation and quantification of age‐related dermal changes 10, and in this study this was used in combination with skin blotting. This is a newly developed non‐invasive technique for the transepidermal collection of secreted proteins based on the principle that large soluble proteins pass through the barrier of hydrated skin 11, 12. This approach was used to assess skin inner tissue factors related to the dermal–epidermal junction. The aim of this study, therefore, was to explore both the prevalence of skin tears in elderly patients at a long‐term medical facility in Japan and the status of skin properties in relation to skin tear risk factors.
Methods
Study design and setting
This cross‐sectional study was conducted at a long‐term medical facility with 500 beds in Ishikawa, Japan, between August and November 2012. This is a specialised facility for older adults who require chronic medical care. It was selected because almost all patients equally receive daily care and support according to their individual physical condition during their hospital stay, which was sufficient for a primary researcher to evaluate aged skin conditions related to skin tear. The study consisted of two stages. The first stage involved collection of data related to prevalence of skin tears (study 1). The second stage consisted of measuring the skin properties related to skin tears (study 2). Both studies were undertaken in the same medical facility. A case–control study was carried out to compare skin properties in study 2 patients with and without skin tears in a similar environment. This study was approved by the Ethical Committee of the Graduate School of Medicine, The University of Tokyo, Tokyo, Japan, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Study 1: prevalence of skin tears
Participants
Patients were included in this study if they were 65 years or older and if they or their proxies gave informed written consent to participate in this study. However, there is a possibility that critically ill patients are at high risk for development of skin tear 1, the exclusion criteria disallowed those patients who had a risk of worsening their symptoms or pain by handling patient's positions in consultation with primary physicians and chief nurses.
Measurements and instruments
The data collected included age, gender, length of hospitalisation, Braden Scale score 13, body mass index, immobility, paralysis, articular contracture, medication (polypharmacy, corticosteroid use, anticoagulant agents) and diagnosis 1, 2, 3, 4, 5, 6. These data were obtained from medical and nursing records and were examined for their associations with increased risk of skin tears. The Braden Scale for predicting pressure ulcer risk requires the examination of six criteria including sensory perception, moisture, activity, mobility, nutrition and friction and shear 13. This gives a maximum total score of 23 points, with a lower score indicating a higher risk of developing a pressure ulcer 13. The systemic factors associated with increased risk of skin tears, including sensory perception as neuropathy, activity, mobility and nutrition status are similar to those of pressure ulcer development 1, 13. There is no appropriate parameter to assess systemic factors for increased risk of skin tears, such as sensory perception as neuropathy and nutrition status in elderly individuals 1. Therefore, all six criteria of Braden Scale were used in this study to assess systemic factors associated with increased risk of skin tears, including sensory perception as neuropathy, activity, mobility and nutrition status in elderly individuals. Immobility was defined according to the level of independent living and included measurement of parameters of self‐care such as transfer, locomotion and communication using examples from judgment criteria of elderly activities and independence level established by the Ministry of Health, Labour and Welfare in Japan 14. These criteria must be determined before applying for long‐term care insurance. A score at the B or C level in this judgment criteria represents immobility and complete dependence; B level score represents those who receive assistance for daily living indoors and can be kept in sitting position but spend most of the day in bed; and C level score represents those who spend almost all day in bed and receive assistance only for toileting, meals and change of clothes 14. The patients judged as B or C level were regarded as immobile.
Two researchers conducted a direct inspection of patients to determine the presence of skin tears. One of the researchers, a fully‐trained research nurse from the Department of Wound Care Management, inspected the skin of all patients except for the perineal area and recorded the findings. We excluded the perineal area from inspection to avoid overestimation of skin tear prevalence because it is quite challenging to correctly diagnose the skin lesions around the perineal area, which include incontinence‐associated dermatitis (IAD), intertriginous dermatitis and pressure ulcers that are similar to skin tears 15. A primary researcher collected information about the likely cause of the skin tear from the attending nursing staff. All patient extremities and the areas with skin tear were photographed to confirm whether patients had skin tears on their extremities by a wound, ostomy, and continence nurse and two researchers including the primary researcher in order to identify reliable prevalence. Each skin tear was classified by a wound, ostomy, and continence nurse and two researchers including primary researcher according to the Skin Tear Audit Research (STAR), which is one of the skin tear classification system used in clinical practice 16. The STAR Classification System includes three categories and two subcategories of skin tears 16. Finally, the prevalence of skin tears was calculated as the number of patients with skin tears divided by the total number of inpatients at the facility who gave their informed consent to participate in the study.
Study 2: the skin properties related to skin tears
Participants
Patients with skin tears were included in the study if they had at least one skin tear, were 65 years or older and gave their informed consent to participate in the study.
Individuals with no skin tears, aged 65 years and older, were included in the group of patients without skin tears. The exclusion criteria disallowed those patients who had a risk of worsening their symptoms or pain by our measurement survey, who died or were transferred to another facility during the study period, who had scar formation at more than two extremity sites indicating a potential history of previous skin tears, who had evidence of cutaneous disease at the measurement site or who had articular contractures at the extremities, which hampered measurements. Finally, the exclusion criteria related to patient status was made as an informed decision after getting inputs from the primary physician and chief nurse.
The without skin tear group comprised the same number of individuals as the skin tear group. The primary researcher randomly sampled the patients without skin tear by using random number table from individuals who met these inclusion and exclusion criteria and who were age‐ and gender‐matched with the patients in the skin tear group (error range is 3 years).
Measurements and instruments
Patients were assessed in the supine position. Measurements were not taken on the day a patient was given a bath, in order to keep the measurement bias to a minimum because the devices used to measure the skin properties related to skin tears are sensitive to the effect of moisture. Skin properties were measured at the visually uninjured side on the dorsal forearm (midway between the wrist and elbow), which represents the region of the body most susceptible to skin tearing according to the prevalence of skin tears in study 1. If participants displayed skin tears on their dorsal forearm, we then evaluated the contralateral dorsal forearm. All measurements were performed by the primary researcher.
High‐frequency skin ultrasonography
In aged skin, high‐frequency skin ultrasonography using a 20‐MHz ultrasound scanner showed a subepidermal low‐echogenic band (SLEB) that is probably an ultrasound manifestation of solar elastosis and oedema in the papillary dermis. Use of these parameters was recommended to quantify skin photoageing 10, 17, 18. A 20‐MHz ultrasound scanner (Dermascan C; Cortex Technology, Hadsund, Denmark) was used to obtain cross‐sectional, two‐dimensional, B‐mode images of the subepidermal structure. Captured ultrasound images were standardised twice (gain level 3) by moving the probe 1 cm intervals in the direction of the long axis designed to avoid measuring the same area.
To quantify the area of the SLEB, the number of low‐echogenic pixels (LEP) in the area that extended 0·75 mm under the skin surface was calculated using MATLAB2012a software (MathWorks Inc., Natick, MA; 17). For the ultrasound scanner, echo amplitudes were ascribed to a numerical pixel scale (range of pixel, 0–255); a low‐echogenic amplitude extended from 0 to 30 19.
Skin blotting
In principle, the combination of moisture and polarity of a nitrocellulose membrane temporally disrupt skin barrier functions 11, with the nitrocellulose membrane attracting and absorbing proteins from deep and shallow layers of the skin through the transfollicular and transepidermal route, respectively 11. Skin blotting is therefore useful to non‐invasively evaluate skin inner tissue factors, as carried out previously in the detection of inflammation‐enhanced nerve growth factor, which induces hypersensitivity for itching and is related to the invasion of C‐fibres into the epidermis of experimental animals 12.
Four types of soluble proteins related to dermal–epidermal junctions and inflammation were selected. Type IV collagen and fibronectin represent the major matrices of the basal membrane 20, whereas matrix metalloproteinase‐2 (MMP‐2) is a proteolytic enzyme that degrades type IV collagen and fibronectin and is involved in basal membrane remodelling 21. Tumour necrosis factor‐alpha (TNF‐α) is a proinflammatory cytokine that mediates several inflammatory events 22.
Nitrocellulose membranes (8 mm in diameter) were moistened with 2 µl of normal saline and attached with medical adhesive tape (Mepitac®; Mölnlycke Health Care, Goteborg, Sweden) on the dorsal forearm skin of patients for 10 minutes to collect samples. To inactivate peroxidase, the membranes were incubated with 20% methanol supplemented with 0·3% hydrogen peroxide at room temperature for 30 minutes. To inactivate alkaline phosphatase, the membranes were incubated with blocking buffer (Blocking One; Nacalai Tesque, Kyoto, Japan) supplemented with 500 µg/ml of levamisole hydrochloride at room temperature for 20 minutes. Antigens were detected by incubating with goat polyclonal antibody against TNF‐α (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal antibody against type IV collagen (Merck Millipore, Billerica, MA), rabbit polyclonal antibody against MMP‐2 (Thermo Fisher Scientific, Tokyo, Japan) and rabbit polyclonal antibody against fibronectin (Cosmo Bio, Tokyo, Japan).
Immunoreactivity was detected using alkaline phosphatase‐conjugated anti‐goat IgG (Rockland Immunochemicals, Gilbertsville, PA) and horseradish peroxidase‐conjugated anti‐rabbit IgG (Thermo Fisher Scientific), visualised using chemiluminescent substrates for alkaline phosphatase (DuoLuX, Vector Laboratories, Burlingame, CA) and peroxidase (Luminata Forte, Merck Millipore), and recorded using a membrane imager (Liponics, Tokyo, Japan). To detect each protein marker on the membrane images, MATLAB2012a was used to calculate the median brightness of the luminescent region based on the membrane locations specified on the original image.
Epidermal function
Stratum corneum hydration was measured using a capacitive method (Corneometer CM‐825; Courage and Khazaka, Electronic GmbH, Cologne, Germany; 23). Skin pH was determined using the glass electrode method (Skin‐pH‐meter PH905; Courage and Khazaka; 24). Transepidermal water loss (TEWL), which represents skin dryness 25, was measured using a closed chamber vaporimeter (VapoMeter; Delphin Technologies Ltd., Kuopio, Finland; 25). Higher TEWL values indicate increased evaporation of water throughout the epidermis. Each parameter was measured three times and the two most reliable measurements that minimised the coefficient of variance were used.
Dermal components
Dermal hydration was measured dielectrically with an open‐ended coaxial probe that was layered in structure (Moisture Meter‐D; Delphin Technologies Ltd.; 26). We used a type S15 probe, which is capable of measuring the skin surface to a depth of 1·5 mm 26, 27. To evaluate skin elasticity parameters, we used a commercially available instrument (Cutometer® MPA 580; Courage and Khazaka; 28), which measures the vertical deformation of skin when it is pulled into the probe via a controlled vacuum of 450 mbar 28, 29. Time/strain mode was used in three consecutive 2‐second cycles of suction followed by a 2‐second relaxation period 28, 29. Before starting the main survey, we conducted a preliminary test to determine vacuum pressure among a few patients with skin tear by confirming whether the vacuum of 450 mbar induced erythema or pain at the dorsal forearm after measurement the next day. We kept vacuum level of 450 mbar during the period of survey because no one described any erythema or pain after measurement. Two parameters were used to evaluate skin elasticity: R0 indicates the vertical deformation of the skin and R2 indicates the ratio of elastic recovery after suspension of the vacuum 28, 29. Lower R0 values indicate decreased deforming forces of the skin and lower R2 values indicate decreased skin elasticity 28, 29.
Statistics
Each set of values represent the median and top 25th percentile (first quartiles) and bottom 25th percentile (third quartiles). Different proportions of each categorical variable between patients with and without skin tears were determined by the chi‐square test or Fisher's exact probability test. Differences in interval variables were tested using a Wilcoxon rank sum test. In this study, P < 0·05 was considered statistically significant. All analyses were performed using Statistical Analysis System Ver. 9·3 software (SAS Institute Inc., Cary, NC).
Results
Study 1: prevalence of skin tears
A complete census of individuals with skin tears was conducted because this is the first prevalence study of skin tears to be conducted in Japan. A total of 488 patients who were hospitalised in this medical facility were recruited. Of these, 12 critically ill patients with pneumonia, respiratory failure or a decreased level of consciousness and 64 who did not provide consent were excluded from the analysis. Therefore, a total of 412 patients comprised the participant pool for this study, although an additional two were excluded because of insufficient data (Figure 1).
Figure 1.
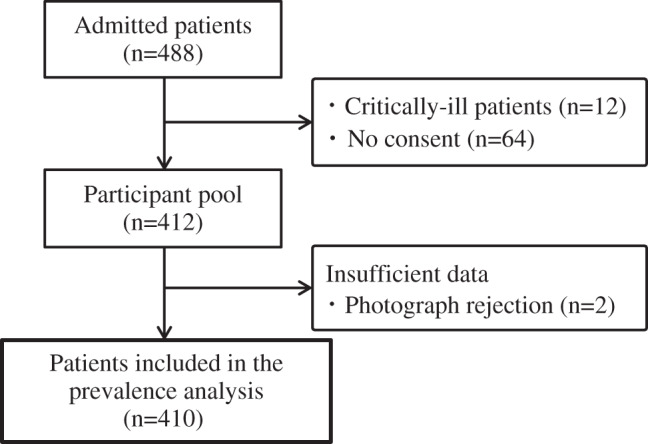
Flow diagram of participants in determining the prevalence of skin tears.
The median age (first and third quartiles) of patients was 87 years (range, 81–92 years), and 73·2% of the patients studied were women. Physical immobility was identified in 84·9% of patients, and cerebrovascular disease was noted in 73·7%. A total of 16 patients had one skin tear, and the prevalence of skin tears was 3·9%. As shown in Table 1, the proportion of tears was highest on the dorsal forearms (50%). Of the patients with tears, 68·8% and 6·3% of the tears were classified as Category 1b and Category 3, respectively, based on the STAR Classification System. Patient characteristics with regard to skin properties are summarised in Table 2. No significant differences were observed between patients with and without skin tears.
Table 1.
The number of skin tears in each site and its STAR Classification
| STAR Classification | ||||||
|---|---|---|---|---|---|---|
| Site | 1a | 1b | 2a | 2b | 3 | |
| Around the collarbone | 1 (6·3) | 1 | ||||
| Ventral upper arm | 1 (6·3) | 1 | ||||
| Dorsal upper arm | 1 (6·3) | 1 | ||||
| Ventral forearm | 1 (6·3) | 1 | ||||
| Dorsal forearm | 8 (50·1) | 7 | 1 | |||
| Dorsal hand | 1 (6·3) | 1 | ||||
| Ventral leg | 1 (6·3) | 1 | ||||
| Dorsal leg | 2 (12·3) | 1 | 1 | |||
| Total | 16 (100) | 2 (12·3) | 11 (68·8) | 1 (6·3) | 1 (6·3) | 1 (6·3) |
Table 2.
Patient characteristics and risk factors related to skin tears in the prevalence study
| Patients with skin tear (n = 16) | Patients without skin tear (n = 394) | P value | |
|---|---|---|---|
| Age (years) | 92·5 (84·0–94·5) | 87 (81·0–92·0) | 0·085 |
| Gender | |||
| Male | 4 (25.0) | 106 (26·9) | 1 |
| Female | 12 (75.0) | 288 (73·1) | |
| Hospitalisation (years) | 3 (0–5) | 2 (0–4) | 0·260 |
| Braden Scale | |||
| Total score | 11·5 (10·5–12) | 12 (11–13) | 0·331 |
| Sensory perception | 3 (2·5–3) | 3 (2–3) | 0·756 |
| Moisture | 2 (1·5–3·0) | 2 (2–3) | 0·874 |
| Activity | 1 (1–2) | 1 (1–2) | 0·918 |
| Mobility | 2 (2–2) | 2 (1–2) | 0·949 |
| Nutrition | 3 (1·5–3) | 3 (3–3) | 0·066 |
| Friction and shear | 1 (1–1) | 1 (1–1) | 0·087 |
| Body mass index | 17·5 (16·3–19·4) | 17·4 (15·0–19·6) | 0·828 |
| Immobility | 15 (93·8) | 333 (84·5) | 0·485 |
| Paralysis | 4 (25.0) | 104 (26·4) | 1.000 |
| Contracture of the arm | 11 (68·8) | 266 (67·5) | 1.000 |
| Contracture of the leg | 12 (75.0) | 296 (75·1) | 1.000 |
| Polypharmacy* | 10 (62·5) | 170 (43·2) | 0·198 |
| Corticosteroid use | 1 (6·3) | 10 (2·5) | 0·358 |
| Anticoagulant agent | 7 (43·8) | 90 (22·8) | 0·070 |
| Underlying disease | |||
| Cerebrovascular disease | 12 (75.0) | 290 (73·6) | 0·432 |
| Cardiac disease | 3 (18·7) | 26 (6·6) | |
| Dementia | 0 (0·0) | 31 (7·9) | |
| Parkinson disease | 0 (0·0) | 14 (3·6) | |
| Disuse syndrome | 0 (0·0) | 11 (2·8) | |
| Malignant neoplasm | 0 (0·0) | 7 (1·8) | |
| Others | 1 (6·3) | 15 (3·7) |
Values are medians (first quartiles and third quartiles) or number of patients (%). Differences in interval variables were assessed using a Wilcoxon rank sum test. Differences in categorical variables were assessed using a chi‐square test or Fisher's exact probability test.
Polypharmacy; over five medicines of internal use per oral or gastrosoma, nasal tube.
Study 2: measuring the skin properties related to skin tears
A total of 18 patients with skin tears were recruited; 16 patients from study 1 who had skin tears were included. However, one patient subsequently died and three more patients who developed new skin tears before the evaluation of skin properties were then added. Three of the 18 participants (16·6%) who had skin tears on their dorsal forearm were evaluated on the contralateral dorsal forearm.
As a control group, 18 patients of the 410 remaining participants from study 1 without skin tears were recruited; we excluded 219 because of skin tears (n = 18); critical illness such as pneumonia, respiratory failure or a decreased level of consciousness (n = 11); death or transfer to another facility during the study period (n = 25); scar formation at more than two sites (n = 124); evidence of cutaneous disease at the measurement site (n = 7); and hard articular contractures in the extremities (n = 34). Of the remaining 191 patients, 18 were selected randomly as noted in the methods (Figure 2). Patient characteristics with regard to measuring skin properties are summarised in Table 3. No significant differences were observed between patients with and without skin tears.
Figure 2.
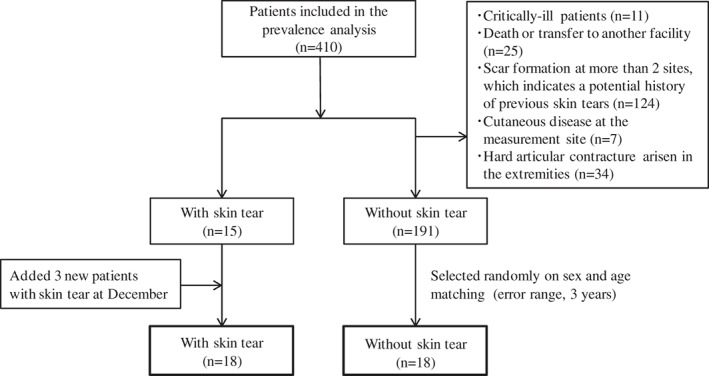
Flow diagram of participants in measuring the skin properties.
Table 3.
Patient characteristics and risk factors related to skin tears in measuring the skin properties
| Patients with skin tear (n = 18) | Patients without skin tear (n = 18) | P value | |
|---|---|---|---|
| Age (years) | 90 (84–94) | 90·5 (85–94) | 1·000 |
| Gender | |||
| Male | 5 (27·8) | 5 (27·8) | 1·000 |
| Female | 13 (72·2) | 13 (72·2) | |
| Hospitalisation (years) | 3 (1–5) | 2 (0–4) | 0·522 |
| Braden Scale | |||
| Total score | 11·5 (11–12) | 12 (11–13) | 0·332 |
| Sensory perception | 3 (3–3) | 3 (3–3) | 0·818 |
| Moisture | 2 (1–3) | 2 (2–3) | 0·342 |
| Activity | 1 (1–2) | 1 (1–2) | 0·667 |
| Mobility | 2 (2–2) | 2 (2–2) | 0·364 |
| Nutrition | 3 (2–3) | 3 (3–3) | 0·397 |
| Friction and shear | 1 (1–1) | 1 (1–1) | 0·172 |
| Body mass index | 17·7 (16·3–19·7) | 17·8 (15·8–19·7) | 0·754 |
| Immobility | 16 (88·9) | 15 (83·3) | 1·000 |
| Paralysis | 5 (27·8) | 4 (22·2) | 1·000 |
| Contracture of the arm | 13 (72·2) | 10 (55·6) | 0·489 |
| Contracture of the leg | 14 (77·8) | 13 (72·2) | 1·000 |
| Polypharmacy* | 12 (66·7) | 8 (44·4) | 0·315 |
| Corticosteroid use | 2 (11·1) | 1 (5·6) | 1·000 |
| Anticoagulant agent | 6 (33·3) | 5 (27·8) | 1·000 |
| Underlying disease | |||
| Cerebrovascular disease | 14 (77·8) | 13 (72·0) | 0·498 |
| Cardiac disease | 3 (16·7) | 1 (5·6) | |
| Dementia | 0 (0·0) | 1 (5·6) | |
| Parkinson disease | 0 (0·0) | 1 (5·6) | |
| Disuse syndrome | 0 (0·0) | 0 (0·0) | |
| Malignant neoplasm | 0 (0·0) | 1 (5·6) | |
| Others | 1 (5·5) | 1 (5·6) |
Values are medians (first quartiles and third quartiles) or number of patients (%). Differences in interval variables were assessed using a Wilcoxon rank sum test. Differences in categorical variables were assessed using a chi‐square test or Fisher's exact probability test.
Polypharmacy; over five medicines of internal use per oral or gastrosoma, nasal tube.
High‐frequency skin ultrasonography
Images of three patients were excluded from LEP analysis because of too bright gain level of ultrasound scanner images. As shown in Figure 3, the SLEBs in patients with skin tears were larger in area than in those without skin tears. LEP is a parameter measured in the SLEB of patients with skin tears and was statistically significantly greater in these patients compared with those without skin tears (P = 0·045, Table 4).
Figure 3.
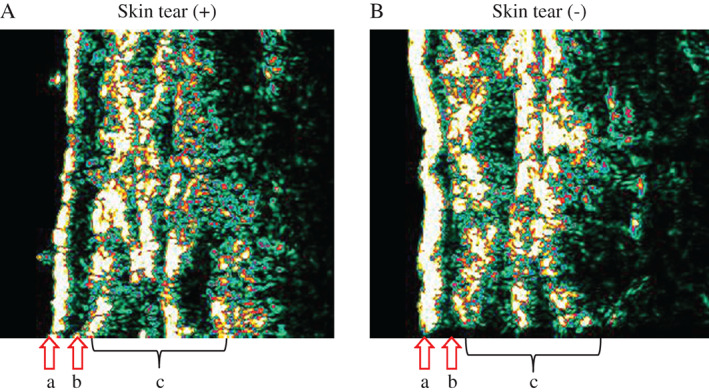
Representative 20‐MHz ultrasound images. (A) Elderly with skin tear (84‐year‐old), (B) elderly without skin tear (90‐year‐old) on dorsal forearm skin. Epidermis is marked with arrows (a). Subepidermal low‐echogenic band (SLEB) is marked with arrows (b). Dermis and subcutaneous layer is marked (c). Images (A) and (B) were used for results of each skin property presented in Table 4. SLEB was obtained from the numbers of low‐echogenic pixels (LEP: 0–30) extending 0·75 mm under skin surface.
Table 4.
Levels of skin properties measured by 20‐MHz ultrasound scanner and skin blotting
| Patients with skin tear (n = 18) | Patients without skin tear (n = 18) | P value | |
|---|---|---|---|
| LEP (n = 33) | 2329 (1575–3234) | 1258·5 (702·0–2036·5) | 0·045 |
| Type IV collagen | 13 (11–14) | 16·5 (12–48) | 0·042 |
| MMP‐2 | 12 (11–13) | 13·5 (12–23) | 0·028 |
| Fibronectin | 13 (12–14) | 12·5 (11–15) | 0·506 |
| TNF‐α | 31·5 (23–42) | 16 (13–25) | <0·001 |
Values are medians (first quartiles and third quartiles). Differences in interval variables were assessed using a Wilcoxon rank sum test. Categorical variables were assessed using a chi‐square test or Fisher's exact probability test.
Skin blotting
The expression levels of four proteins were measured as indicators of skin properties (Table 4). The levels of type IV collagen and MMP‐2 were statistically significantly lower in patients with tears than in those without skin tears (P = 0·042 and P = 0·028, respectively). Significantly higher levels of TNF‐α were also found in patients with skin tears compared with those without (P < 0·001). No significant differences in fibronectin levels were found between the two groups (P = 0·506). Membranes containing fibronectin were non‐luminescent.
Epidermal function and dermal components
There were no significant differences in the parameters of epidermal functions (stratum corneum hydration, skin pH and TEWL) and dermal hydration between patients with and without skin tears (Table 5). With regard to skin elasticity, only the R2 value differed significantly between the two groups (P = 0·020).
Table 5.
Levels of skin properties related to epidermal function and dermal components
| Patients with skin tear (n = 18) | Patients without skin tear (n = 18) | P value | |
|---|---|---|---|
| Stratum corneum hydration | 46·1 (34·6–50·9) | 37·6 (35·2–48·1) | 0·577 |
| Skin pH | 6 (5·3–6·1) | 5·5 (5·3–5·9) | 0·229 |
| Transepidermal water loss (TEWL) | 4·2 (3·6–4·9) | 4·7 (3·7–5·2) | 0·411 |
| Dermal hydration | 42·5 (37·5–46·2) | 47·3(40–52·5) | 0·254 |
| Cutometer (R0) | 0·08 (0·04–0·11) | 0·07 (0·05–0·11) | 0·843 |
| Cutometer (R2) | 0·83 (0·74–0·88) | 0·73 (0·65–0·78) | 0·020 |
Values are medians (first quartiles and third quartiles). Differences in interval variables were assessed using a Wilcoxon rank sum test. Categorical variables were assessed using a chi‐square test or Fisher's exact probability test.
Discussion
This clinical study represents the first effort to determine the prevalence of skin tears by direct inspection of elderly patients at a long‐term medical facility in Japan. Several findings about skin properties associated with skin tears were also demonstrated.
The skin tear prevalence in this study was 3·9% and the proportion of category 3 skin tears was 6·3%. A previous study in western Australia showed that skin tears comprised 20% of known wounds in the community in a population predominantly over 70 years of age 30, whereas the prevalence of skin tears with a severity level corresponding to category 3 of the STAR Classification System was 16% in a second Australian study 5. A retrospective review of all incident reports involving skin tears for the preceding 6 months was conducted in the United States 6. This study showed that an average of 14% of the patients with a mean age of 85 years sustained a skin tear each month 6. Thus, the prevalence and severity of tears in our study were lower than in those of Caucasian elderly patients from other countries. This could be explained by direct inspection to determine the most reliable prevalence of skin tears after excluding the wounds sharing similarity with skin tears, such as pressure ulcer, IAD.
Physical immobility, observed in 84·9% of patients in this study, was higher than in previous studies 2, 6. Thus, the lower prevalence and severity of skin tears in our analysis could indicate that the institution we studied provides a high quality of care. Alternatively, Caucasian elderly patients may have specific skin properties or be exposed to environmental factors that predispose them to skin tears compared with Japanese patients. Responses against environmental stresses, including ultraviolet (UV) radiation, may reflect the photoprotective differences of melanin as a UV filter between diverse human skin phenotypes in various racial/ethnic groups 31, 32. Indeed, the prevalence of actinic keratosis was greater in Caucasian Australians than in Japanese with yellow skin tones 33, 34. Hence, we consider that the combination of environmental factors (e.g. cumulative sun exposure), occupational exposure and race‐based sun sensitivity may explain the varied prevalence and severity of skin tears between this and other studies.
As shown in Table 1, the proportion of skin tears was highest on the dorsal forearms and lowest on the legs. This pattern of skin tear is relatively consistent with the findings of Malone et al. 2 and White et al. 6. Totally dependent residents who require complete care for all activities of daily living were previously shown to have the most frequent incidents of skin tears, and these skin tears were located primarily on the arms 6. The lower incidence of leg tears compared with arm tears could reflect the reduced exposure to external forces in these areas, for example, when transferring from a bed to the wheelchair.
Chronic use of corticosteroids can be considered a risk factor for skin tears due to potential side effects of the altered collagen synthesis 1, 2, 3, 4, 5, 6, 35. It may be considered that only a few people have chronic corticosteroids as the reason for identifying no association between skin tears and chronic use of corticosteroids in this study (prevalent in 2·6% of overall participants). Although anticoagulant agents can cause dermatologic changes such as senile purpura and ecchymosis 1, 2, 3, 36, they might not have been associated with skin tears in elderly patients because anticoagulant agents were required by many participants regardless of whether they suffered skin tears. This suggests that it is necessary to explore the epidemiology of skin tears across different health care settings in which patients are target group for chronic corticosteroid or anticoagulant agent usage.
We identified three skin properties associated with skin tears in elderly patients, including a higher LEP measurement on the dorsal forearm (Table 5), which may reflect alterations in collagen and elastin fibres and the accumulation of glycosaminoglycan, indicating oedema in the subepidermal region 10, 17, 18, 19. Solar elastosis, or changes in collagen structure, was previously suggested to be partially responsible for echolucent material formation 10, 18. The results of this study infer the possibility that the areas of SLEB, including solar elastosis in the dermis, represent a risk factor for skin tears associated with lifestyle and occupational backgrounds caused by photoageing.
The in vitro effects of TNF‐α on extracellular matrix (ECM) remodelling have been analysed previously 37, 38, 39. In this study, increased TNF‐α was positively associated with skin tears. MMPs, primarily MMP‐2 and MMP‐9, are involved in this type of ECM remodelling 22, and in vitro studies have shown that TNF regulates collagenase in fibroblasts by suppressing the production of tissue inhibitors of metalloproteinases and accelerating MMP production 37. In this study, however, decreased secretion of both MMP‐2 and type IV collagen was positively associated with skin tears. It is possible that increased TNF‐α suppresses ECM remodelling, which may disrupt the remodelling balance between basal membrane protein degradation and synthesis. Patients with this condition are predisposed to skin damage and evidence has demonstrated the vulnerability of dermal–epidermal junctions to skin tears. Fibronectin, another ECM protein, usually exists as a 440 kDa dimer comprising two nearly identical∼ 250 kDa subunits linked covalently near their C‐termini by a pair of disulphide bonds. Our analysis of fibronectin in this study showed non‐luminescent skin membranes, which could be explained by the molecular mass of fibronectin being too large to penetrate through the intact skin barrier of elderly patients 40, 41.
A high R2 value usually indicates increased skin elasticity 28, 29. However, our results do not support necessarily this interpretation, as the suction power may have not penetrated to deeper tissues in the SLEB area because of its high water volume. We believe that the suction device was insufficient to obtain precise measurements of skin elasticity on the dorsal forearm of elderly patients.
In clinical settings, a moisturiser is the main strategy used to prevent skin tears as it decreases the magnitude of external forces such as friction and shear forces by relieving the itching caused by dry skin and reducing susceptibility to irritation on the skin surface 42, 43. However, Hunter et al. reported that 42% of patients developed skin tears despite management with moisturisers 43. It is possible that a functional improvement of the skin barrier itself did not contribute to reduced skin tear prevalence in this study. Indeed, we found no significant difference between epidermal functions such as stratum corneum hydration, skin pH and TEWL between patients with and without skin tears. Additionally, Japanese subjects have higher water content and lower TEWL in their stratum corneum compared with Caucasian subjects 44, 45. By combining our results with those of previous studies 44, 45, we estimate that the skin hydration and barrier functions of Japanese subjects are greater than Caucasian subjects caused by different environment factors and skin properties. Therefore, it might be advisable to devise a skin tear prevention protocol specific to the Japanese elderly population that is not limited to using a moisturiser and protective garments for reducing external force on the extremities. Moreover, as over 75% of skin tears occurred in the upper extremities in our study, we suggest that the establishment of a new skin tear prevention protocol remains indispensable for identifying both factors of external force and skin properties being predisposed towards skin tears, and not only moisturiser and protective garments.
Our study has a number of limitations. First, the small sample size could have decreased the statistical power in stratified analyses; thus larger samples are required to confirm our results in Japanese elderly patients, especially with respect to the effects of photoageing. Other settings such as nursing homes or acute care hospitals should also be investigated. Second, our study was conducted in the summer and early autumn. As skin conditions vary according to season, the prevalence of skin tears could also differ. Finally, it is difficult to interpret secreted protein levels accurately by using the brightness of skin blotting luminescent regions as threshold levels of brightness were difficult to establish based on protein markers related to skin tear development. Therefore, prospective studies are required to explore the accuracy of this assessment in predicting skin tear development.
In conclusion, this study demonstrated a 3·9% prevalence of skin tears, identified common sites of skin tears and showed skin properties related to skin tears in elderly patients at a long‐term medical facility in Japan. The results obtained from our clinical data indicate that risk factors for skin tear prevalence are not only chronological ageing involving the effects of senescence, but also photoageing. The development of skin tear prevention guidelines and a skin tear prediction assessment tool based on skin properties will elucidate risk factors and contribute to improving the quality of life for the elderly and reduce the stress placed on health care practitioners.
Acknowledgements
The authors are thankful for the generous support received from the participants and staff members of Sengi Hospital, Ishikawa Prefecture, Japan. The authors state no conflict of interest.
References
- 1. LeBlanc K, Baranoski S. Skin tear: state of the science: consensus statements for the prevention, prediction, assessment, and treatment of skin tear. Adv Skin Wound Care 2011;24:2–15. [DOI] [PubMed] [Google Scholar]
- 2. Malone ML, Rozario N, Gavinski M, Goodwin J. The epidemiology of skin tear in the institutionalized elderly. J Am Geriatr Soc 1991;39:591–5. [DOI] [PubMed] [Google Scholar]
- 3. Payne RL, Martin ML. Defining and classifying skin tear: need for a common language. Ostomy Wound Manage 1993;39:16–26. [PubMed] [Google Scholar]
- 4. Stephen‐Haynes J, Callaghan R, Bethell E, Greenwood M. The assessment and management of skin tear in care homes. Br J Nurs 2011;20:12–6. [DOI] [PubMed] [Google Scholar]
- 5. Lopez V, Dunk AM, Cubit K, Parke J, Larkin D, Trudinger M, Stuart M. Skin tear prevention and management among patients in the acute aged care and rehabilitation units in the Australian Capital Territory: a best practice implementation project. Int J Evid Based Healthc 2011;9:429–34. [DOI] [PubMed] [Google Scholar]
- 6. White W, Karam S, Cowell B. Skin tear in frail elders: a practical approach to prevention. Geriatr Nurs 1994;15:95–9. [DOI] [PubMed] [Google Scholar]
- 7. Baranoski S. Skin tear: staying on guard against the enemy of frail skin. Nursing 2000;30:41–6. [DOI] [PubMed] [Google Scholar]
- 8. Desai H. Ageing and wounds. Part 2: healing in old age. J Wound Care 1997;6:237–9. [DOI] [PubMed] [Google Scholar]
- 9. Richey ML, Richey HK, Fenske NA. Aging‐related skin changes: development and clinical meaning. Geriatrics 1988;43:49–64. [PubMed] [Google Scholar]
- 10. Gniadecka M, Jemec GB. Quantitative evaluation of chronological ageing and photoageing in vivo: studies on skin echogenicity and thickness. Br J Dermatol 1998;139:815–21. [DOI] [PubMed] [Google Scholar]
- 11. Minematsu T, Horii M, Oe M, Sugama J, Mugita Y, Huang L, Nakagami G, Sanada H. Skin blotting: a noninvasive technique for evaluating physiological skin status. Adv Skin Wound Care 2014. (In press). [DOI] [PubMed] [Google Scholar]
- 12. Kishi C, Minematsu T, Huang L, Mugita Y, Kitamura A, Nakagami G, Yamane T, Yoshida M, Noguchi H, Funakubo M, Mori T, Sanada H, Hypo‐osmotic shock‐ induced subclinical inflammation of skin in rat model of disrupted skin barrier function. Biol Res Nurs 2014. (In press). [DOI] [PubMed] [Google Scholar]
- 13. Braden BJ, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil Nurs 1987;12:8–12. [DOI] [PubMed] [Google Scholar]
- 14.Available at: http://www.mhlw.go.jp/english/index.html
- 15. Mahoney M, Rozenboom B, Doughty D, Smith H. Issues related to accurate classification of buttocks wounds. J Wound Ostomy Continence Nurs 2011;38:635–42. [DOI] [PubMed] [Google Scholar]
- 16. Carville K, Lewin G. STAR: a consensus for skin tear classification. Prim Intent 2007;15:18–29. [Google Scholar]
- 17. Gniadecka M, Gniadecki R, Serup J, Søndergaard J. Ultrasound structure and digital image analysis of the subepidermal low echogenic band in aged human skin: diurnal changes and interindividual variability. J Invest Dermatol 1994;102:362–5. [DOI] [PubMed] [Google Scholar]
- 18. De Rigal J, Escoffier C. Assessment of aging of the human skin by in vivo ultrasonic imaging. J Invest Dermatol 1989;93:621–5. [DOI] [PubMed] [Google Scholar]
- 19. Seidenari S, Di Nardo A. B scanning evaluation of allergic reactions with binary transformation and image analysis. Acta Derm Venereol 1992;175:3–7. [PubMed] [Google Scholar]
- 20. Gelse K, Pöschl E, Aigner T. Collagens‐structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531–46. [DOI] [PubMed] [Google Scholar]
- 21. Birkedal‐Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal‐Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993;4:197–250. [DOI] [PubMed] [Google Scholar]
- 22. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001;344:907–16. [DOI] [PubMed] [Google Scholar]
- 23. Wendling P, Dell'Acqua G. Skin biophysical properties of a population living in Valais, Switzerland. Skin Res Technol 2003;9:331–8. [DOI] [PubMed] [Google Scholar]
- 24. Ehlers C, Ivens UI, Møller ML, Senderovitz T, Serup J. Comparison of two pH meters used for skin surface pH measurement: the pH meter 'pH900' from Courage & Khazaka versus the pH meter '1140' from Mettler Toledo. Skin Res Technol 2001;7:84–9. [DOI] [PubMed] [Google Scholar]
- 25. Steiner M, Aikman‐Green S, Prescott GJ, Dick FD. Side‐by‐side comparison of an open‐chamber (TM 300) and a closed‐chamber (vapometer) transepidermal water loss meter. Skin Res Technol 2011;17:366–72. [DOI] [PubMed] [Google Scholar]
- 26. Nuutinen J, Lahtinen T, Turunen M, Alanen E, Tenhunen M, Usenius T, Kolle R. A dielectric method for measuring early and late reactions in irradiated human skin. Radiother Oncol 1998;47:249–54. [DOI] [PubMed] [Google Scholar]
- 27. Nuutinen J, Ikaheimo R, Lahtinen T. Validation of a new dielectric device to assess changes of tissue water in skin and subcutaneous fat. Physiol Meas 2004;25:447–54. [DOI] [PubMed] [Google Scholar]
- 28. Takema Y, Yorimoto Y, Kawai M, Imokawa G. Age‐related changes in the elastic properties and thickness of human facial skin. Br J Dermatol 1994;131:641–8. [DOI] [PubMed] [Google Scholar]
- 29. Krueger N, Luebberding S, Oltmer M, Streker M, Kerscher M. Age‐related changes in skin mechanical properties: a quantitative evaluation of 120 female subjects. Skin Res Technol 2011;17:141–8. [DOI] [PubMed] [Google Scholar]
- 30. Carville K, Smith JA. Report on the effectiveness of comprehensive wound assessment and documentation in the community. Prim Intent 2004;12:41–8. [Google Scholar]
- 31. Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin‐a comparison of black and Caucasian skin. J Am Acad Dermatol 1979;1:249–60. [DOI] [PubMed] [Google Scholar]
- 32. Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV‐induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J 2003;17:1177–9. [DOI] [PubMed] [Google Scholar]
- 33. Salasche SJ. Epidemiology of actinic keratosis and squamous cell carcinoma. J Am Acad Dermatol 2000;42:4–7. [DOI] [PubMed] [Google Scholar]
- 34. Naruse K, Ueda M, Nagano T, Suzuki T, Harada S, Imaizumi K, Watanabe S, Ichihashi M. Prevalence of actinic keratosis in Japan. J Dermatol Sci 1997;15:183–7. [DOI] [PubMed] [Google Scholar]
- 35. Mj B, Riddel JM Jr, Best WR. Cutaneous side effects of ACTH, cortisone and pregnenolone therapy. J Invest Dermatol 1951;16:205–10. [DOI] [PubMed] [Google Scholar]
- 36. Kaya G, Saurat JH. Dermatoporosis: a chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology 2007;215:284–94. [DOI] [PubMed] [Google Scholar]
- 37. So T, Ito A, Sato T, Mori Y, Hirakawa S. Tumor necrosis factor‐alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cell. Biol Reprod 1992;46:772–8. [DOI] [PubMed] [Google Scholar]
- 38. Mauviel A, Heino J, Kähäri VM, Hartmann DJ, Loyau G, Pujol JP, Vuorio E. Comparative effects of interleukin‐1 and tumor necrosis factor‐alpha on collagen production and corresponding procollagen mRNA levels in human dermal fibroblasts. J Invest Dermatol 1991;96:243–9. [DOI] [PubMed] [Google Scholar]
- 39. Distler JH, Schett G, Gay S, Distler O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheum 2008;58:2228–35. [DOI] [PubMed] [Google Scholar]
- 40. Tan G, Xu P, Lawson LB, He J, Freytag LC, Clements JD, John VT. Hydration effects on skin microstructure as probed by high‐Resolution cryo‐scanning electron microscopy and mechanistic implications to enhanced transcutaneous delivery of biomacromolecules. J Pharm Sci 2010;99:730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitragotri S. Breaking the skin barrier. Adv Drug Deliv Rev 2004;56:555–6. [DOI] [PubMed] [Google Scholar]
- 42. Cheong WK. Gentle cleansing and moisturizing for patients with atopic dermatitis and sensitive skin. Am J Clin Dermatol 2009;10:13–7. [DOI] [PubMed] [Google Scholar]
- 43. Hunter S, Anderson J, Hanson D, Thompson P, Langemo D, Klug MG. Clinical trial of a prevention and treatment protocol for skin breakdown in two nursing homes. J Wound Ostomy Continence Nurs 2003;30:250–8. [DOI] [PubMed] [Google Scholar]
- 44. Muizzuddin N, Hellemans L, Van Overloop L, Corstjens H, Declercq L, Maes D. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. J Dermatol Sci 2010;59:123–8. [DOI] [PubMed] [Google Scholar]
- 45. Yamashita Y, Okano Y, Ngo T, Buche P, Sirvent A, Girard F, Masaki H. Differences in susceptibility to oxidative stress in the skin of Japanese and French subjects and physiological characteristics of their skin. Skin Pharmacol Physiol 2012;25:78–85. [DOI] [PubMed] [Google Scholar]


