ABSTRACT
Split‐thickness skin grafting (STSG) is a widely used method in reconstructive surgery, but donor site wounds (DSWs) are often slow healing and painful. This prospective study evaluated the performance of a composite wound dressing containing collagen/oxidised regenerated cellulose in the treatment of medium‐depth (0·4 mm) DSWs in 25 multi‐morbid patients with chronic leg ulcers requiring STSG. The range of patients' ages was 44–84 years (mean 71·6 years) with DSW sizes ranging between 12 and 162 cm2 (mean 78 cm2). Comorbidities included anticoagulation therapy (15 patients), anaemia (11 patients), diabetes (6 patients) and methicillin‐resistant Staphylococcus aureus (MRSA) ulcer colonisation (6 patients). The first dressing change was performed after 10 days. Complete reepithelialisation was observed between the 10th and 34th day (mean 17·2, median 14 days). Postoperative medium to strong bleeding occurred in only five patients (four with anticoagulation). Wound pain levels one day after harvesting were only moderate (range 0–1·5, mean 0·5, median 0·5 on a six‐item scale). No wound infection was observed during the first dressing. The composite dressing used allowed for the fast healing of medium‐depth DSWs with minimal or no postoperative pain and bleeding in older multi‐morbid patients under anticoagulation treatment.
Keywords: Anticoagulants, Bleeding, Cellulose/collagen dressing, Comorbidities, Donor site wounds
Introduction
In the Western world, chronic venous leg ulcers (VLUs) and mixed arterial‐venous ulcers (AVLUs) are the most common type of chronic leg wounds (70–85% of cases) 1, 2, 3, 4. A tangential ulcer excision combined with autologous split‐thickness skin grafting (STSG) as a one‐step procedure is widely accepted as the treatment of choice if combined with long‐term maintenance afterwards 5, 6, 7. STSG is also considered the method of choice for the treatment of burn wounds and other cutaneous injuries in plastic reconstructive surgery and dermatology. However, harvesting of the grafts usually results in a large superficial, often slowly healing donor site wound (DSW), which often causes more pain than the site receiving the graft. STSG are categorised as thin (up to 0·3 mm), medium (0·4–0·5 mm) and thick (0·6–0·7 mm) 8, 9. STSGs of medium thickness are often the best treatment option regarding mechanical graft stability and DSW healing time 7, 9. Healing time of DSWs depends on their depth 10, patient age 11 and wound infections as well as comorbidities such as diabetes 4, 5.
Postoperative diffuse bleeding in DSWs is an important complication, especially in patients receiving systemic anticoagulation because of underlying diseases. For the treatment of diffuse larger haemorrhages, biological substances promoting cell aggregation, such as microfibrillar cellulose, collagen, gelatin and poly‐N‐acetylglucosamine, are used 12, 13, 14.
Effective antimicrobial wound treatment can be achieved using modern topical antiseptics, particularly polyhexanide and octenidine but also nanocrystalline silver preparations. Silver demonstrates a broad antimicrobial 15, 16 and anti‐fungal 17 activity. The systemic incorporation of silver is reduced considerably by avoiding long‐term application on wound areas larger than 10% of the body surface area 18.
Wound dressings providing a continuous moist wound environment (MWE) are recognised as the treatment of choice as it speeds up epithelialisation and reduces pain 5, 19, 20, 21, 22. A broad range of wound dressings is available, providing clinical advantages in DSW treatment, such as prevention of wound infection, reduction of postoperative bleeding, leakage, pain, shear forces, friction and frequency of dressing changes, but no product appears to meet all of the requirements at once 5.
The aim of this prospective, non‐comparative study was to evaluate the efficacy of a composite wound dressing containing a non‐adherent silicone layer, a matrix made of oxidised regenerated cellulose (ORC), collagen and silver‐ORC and finally a hydropolymer foam, thus allowing a reduction of postoperative bleeding, pain and frequency of dressing changes of medium‐depth DSWs (0·4 mm) in multi‐morbid patients undergoing grafting of VLUs and AVLUs. This particular composite wound dressing was chosen to maximise the effectiveness of the individual properties of each of the dressing components for optimal wound management and accelerated epithelialisation.
Materials and methods
Study objectives and outcome measures
The primary objective of this study was to determine the DSW healing time defined as the duration from skin harvesting until complete wound epithelialisation. The secondary objective of this study was to evaluate the incidence and extent of postoperative DSW bleeding and pain.
Patient recruitment
This study was designed as a non‐randomised, prospective, controlled, single‐centre study and was performed at the Department of Dermatology and Allergology Biederstein, Technical University of Munich (Germany). The study was conducted in accordance with both the Good Clinical Practice and the ethical guidelines of the 1975 Declaration of Helsinki and the revised version from Seoul in 2008. The study protocol was approved by the Ethical Committee of the Medical Board of the Technical University of Munich. All patients provided written informed consent prior to study enrollment.
Inclusion criteria
Eligible study participants included multi‐morbid patients diagnosed with chronic VLUs and UM and required STSG. Ulcer size was ≥10 cm2 and involved chronic wound persistence without improvement in healing for at least 6 months despite appropriate conservative treatment, including compression and negative pressure therapy. Patients with infected ulcers stages 2 and 3 according to the criteria of the European Wound Management Association [stage 2: increased signs of infection, low or moderate exudation; stage 3: signs of local infection and increased wound exudate, with normal CRP and leucocyte levels 23] were included. Furthermore, patients taking anticoagulants and platelet aggregation inhibitors as well as patients with methicillin‐resistant Staphylococcus aureus (MRSA) and Gram‐negative species with extended‐spectrum‐beta‐lactamase (ESBL) resistance were also included in this study.
Exclusion criteria
Exclusion criteria included age under 18 years, preoperative INR values above 3·0, uncontrolled diabetes (HbA1c > 10%), ankle to brachial systolic pressure index (ABI) ≤ 0·5, hypersensitivity to silver‐containing products and other dressing components, drug abuse, excessive alcohol consumption, malignant or autoimmune disease, chemo‐ and immunosuppressive therapy, reduced compliance, decreased serum levels of factor XIII and wound infection stages 1 and 4 (stage 1: few subtle signs of infection, normal healing progress; stage 4: overt signs of local infection and signs of systemic infection 23).
Preoperative procedures
All preoperative and postoperative measurements, including wound documentation, surgery and postoperative dressing changes, were performed solely by one dermatosurgeon (first author).
Upon admission, general data on patients' clinical history were noted, and an extensive physical examination was performed. The status of venous and arterial vessels was analysed clinically and by extensive diagnostic measures using Doppler and colour duplex sonography. The status of venous vessels was evaluated in accordance with the CEAP classification 24. Peripheral artery occlusive disease (PAOD) was diagnosed using patient history and clinical examination, including Doppler sonography, to determine the ABI 25. In patients with diabetes, the transcutaneous oxygen tension (TcPO2) and the Toe‐Brachial‐Pressure Index were additionally measured because of potential media calcinosis 25, 26, 27.
Lymphoedema was classified into four stages (0–3) according to Földi et al. 28. The bioburden level was determined through microbiological wound specimens using the Levine technique 29, 30.
Ulcer and DSW size were determined pre‐ and intraoperatively by placing a sterile transparent film with an integrated metric grid (OpSite® Flexigrid, Smith and Nephew, London, UK) on top of the wound and tracing the outlines with a permanent marker. The wound area was determined by counting the number of squares (1 cm2, ¼ cm2) on the grid within the traced area 31, 32.
Prophylactic perioperative antibiotic treatment (cefuroxime 3 × 500 mg/day p. o., or in cases of penicillin allergy clindamycine 4 × 300 mg/day p.o.) was given to all patients for 3 days, starting 1 hour before surgery. Anticoagulation was not interrupted or changed perioperatively.
Surgical procedures
All donor sites had not previously been harvested for skin grafts. For graft harvesting, the donor area, which was always the ipsilateral proximal anterolateral thigh, was disinfected with an octenidine dihydrochloride/phenoxyethanol solution (Octenisept®, Schülke, Norderstedt, Germany), marked with a sterile permanent skin marker and infiltrated with a modified tumescent local anaesthetic solution (0·15% prilocaine with epinephrine 1:200·000; 0·5 ml/cm2 donor area) until the area assumed a plane state. After anaesthesia set in, the donor site was lubricated with a fusidic acid ointment for better gliding of the dermatome while harvesting. Fusidic acid ointment has been used by the first author for more than 25 years because of its additional bacteriostatic effect on the Staphylococcus species as well as a very low risk of resistance and contact sensitisation 33. The skin was stretched, and an STSG of 0·4 mm thickness was harvested with a battery‐powered dermatome (ACCULAN® 3Ti dermatome, Aesculap, Tuttlingen, Germany). To achieve a standardisation of the harvesting procedure, the same dermatosurgeon harvested all skin grafts using the same dermatome in all patients. The downward pressure and angle of approach between the dermatome and donor skin as well as the velocity of harvesting were maintained the same in each patient. The same physician also stretched the convex donor surface in all cases. Immediately after harvesting, DSWs were covered with saline‐soaked gauze pads for 10–15 minutes, followed by a non‐adhesive, soft, silicone‐coated polyamide mesh (Mepitel®, Mölnlycke, Germany). A biodegradable, freeze‐dried, ORC/collagen/silver‐ORC dressing (Promogran Prisma®, Systagenix, Gatwick, UK) moisturised with isotonic saline solution served as the second layer. The third dressing layer was an absorbing hydropolymer foam with a 2‐cm adhesive border (Tielle®Adhesive dressing, Systagenix, Gatwick, UK), (Figure 1). Then, two layers of cotton gauze pads were placed in an overlapping manner onto the composite dressing and fixated with retention tape (Fixomull®stretch, BSN Medical, Hamburg, Germany). Finally, long‐stretch bandaging was performed for 4 hours.
Figure 1.
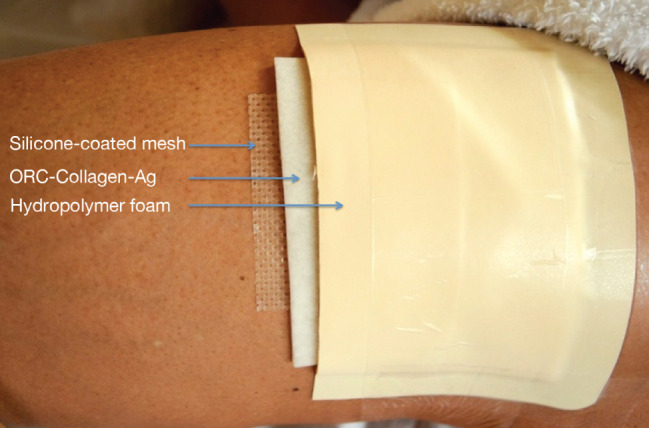
Arrangement of the first three dressing layers. The hydropolymer foam at the top was covered with cotton gauze pads and fixated with retention tape (not shown). Dressings were applied to cover the entire surface of the donor site wound (DSW), with Promogran Prisma overlapping the wound by 1 cm and Tielle® foam area by 1–2 cm onto the surrounding skin. In case of DSW size >80 cm2, Tielle® 18 cm × 18 cm was used in an overlapping manner in order to cover the entire wound area.
Postoperative procedures
Because of the grafted ulcers, patients were asked to take strict bed rest for one day. Patients were monitored daily and discharged 7–10 days after grafting. Adequate analgesia was provided to avoid postoperative pain. For an objective evaluation of postoperative pain in the donor area as well as in the grafted area, both a six‐item scoring scale ranging from 0 to 5 as well as a visual scale with faces ranging from a smiling face (no pain) to a tearful face (severe pain) were used daily.
Estimation of the degree of postoperative bleeding was assessed as follows: ‘little’: no visible blood absorption through the fixating tape, ‘medium’: partial blood absorption through the fixating tape, ‘strong’: extensive blood absorption through the fixating tape and/or leakage of blood from the dressing. The absorptive capacity of the composite dressing was tested prior to the study using a 0·1% eosine solution. Using both a 18 cm × 18 cm Tielle (contains 10 g hydropolymer foam) and 123‐cm2 Promogran Prisma dressing, about 40 ml of liquid were fully absorbed without leakage after the fixating tape was applied.
The first DSW dressing was left intact for 10 days. At dressing replacement, a bacterial swab of each wound was performed immediately after removing the wound dressing to avoid contamination. In case of heavy exudation and/or blood leakage through the dressing within the first 10 days, only the outer absorbent dressing layer (cotton gauze pads and retention tape) was changed, while the three‐component dressing (including Tielle) beneath was left in place. Early removal of all layers was avoided in order to prevent potential damage to the regenerating tissue (Figures 2 and 3).
Figure 2.
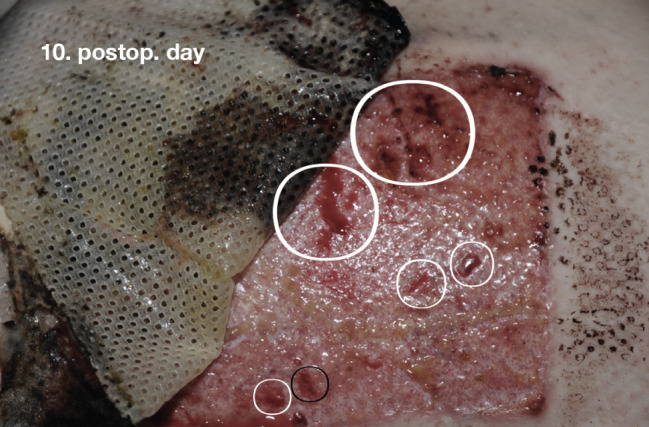
Reepithelialisation of a typical donor site wound (DSW) (patient 11) at first dressing change 10 days after surgery. Removal of the partially adherent dressing may artificially damage the regenerating epithelium, causing acute bleeding (circles).
Figure 3.
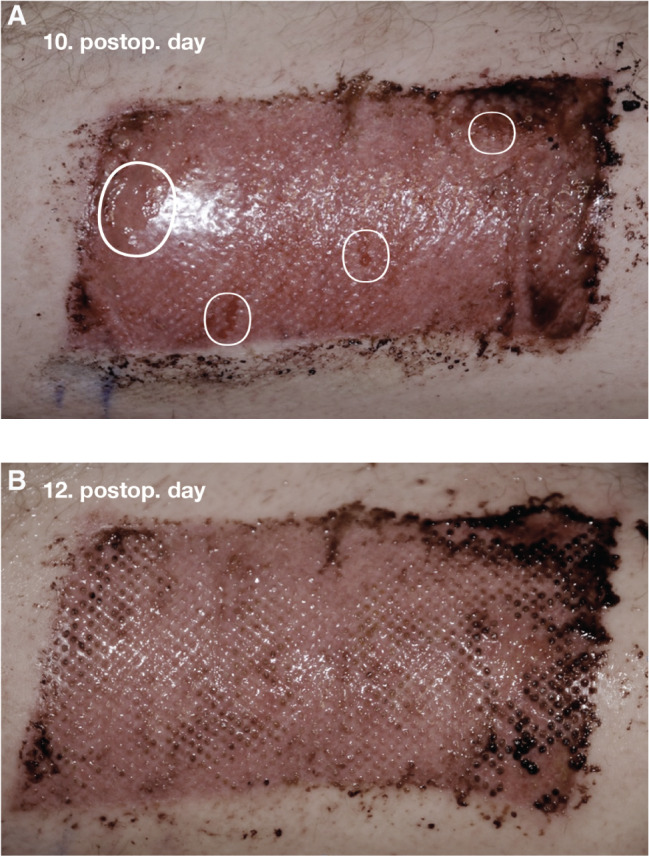
(A) Donor site wound (DSW) (112 cm2) of patient 8 (a 72‐year‐old male, arterial‐venous ulcer (AVLU), acetylsalicylic acid (ASA), methicillin‐resistant Staphylococcus aureus (MRSA), nicotine abuse, hypertension). Immediately after removal of the first dressing (10 postoperative day), small denuded areas (circles) are seen. (b) DSW (112 cm2) of patient 8 (a 72‐year‐old male, AVLU, ASA, MRSA, nicotine abuse, hypertension). Complete homogenous reepithelialisation of DSW on postoperative day 12 after removal of the second dressing is seen.
Extent of epithelialisation at dressing change was determined according to the macroscopic wound appearance. Each layer of the dressing was gently lifted, and a direct visual inspection of the wound was performed. Documentation was completed by digital photographs. Wounds were considered to have been completely (100%) reepithelialised when there were no macroscopically denuded areas with wound exudate or bleeding (Figures 2 and 3).
After being discharged from the hospital, all patients were followed‐up in our outpatient clinic once a week during the first 5 postoperative weeks and then 6 months after surgery.
After 1 week of complete epithelialisation of the DSWs, patients were asked to apply either a silicone gel or a panthenol ointment to the affected area daily for at least 6 months.
Results
A total of 25 patients with leg ulcers (11 male and 14 female; 13 patients with AVLUs, 12 patients with VLUs) were enrolled and grafted with medium thick STSGs (0·4 mm) (Table 1; a complete list of patient data is provided in the Appendix). DSWs were covered with the composite dressing in accordance with the study protocol. All patients completed the study. There were no patients who discontinued treatment prematurely, and there were no severe adverse events or dropouts recorded. The dressing was not removed completely before the 10th postoperative day in any of the cases.
Table 1.
Clinical data of study patients
| Pat no | Age/gender | Ulcer duration (months) | Ulcer size (cm2) | Ulcer microbiome | Anticoagulation | DSW size (cm2) | DSW complete epithelialization (days) | DSW pain score 1. postoperative day (0–5) | DSW adverse events | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82, M | 96 | 30 | SA, EK | PPC | 28 | 12 | 0 | / | Diabetes, hepatopathy GAOD |
| 2 | 81, F | 6 | 48 | MRSA | PPC | 56 | 17 | 1 | Bleeding (strong) 1. postoperative day | Diabetes, CA, hypertension |
| 3 | 76, M | 9 | 80 | PSA, EC | / | 84 | 16 | 0 | Bleeding (medium) | Diabetes, hypertension |
| 4 | 71, M | 9 | 54 | SA, EC, EK | PPC | 58 | 14 | 1–0 | / | Diabetes, hypertension |
| 5 | 62, M | 12 | 78 | SA, CB | Enoxaparin | 75 | 10 | 0 | / | Hepatic cirrhosis child A, anaemia |
| 6 | 72, M | 17 | 36/27 | PSA, EK | ASA | 84 | 12 | 0 | / | Hypertonus, hepatopathy nicotine abuse |
| 7 | 80, F | 120 | 112 | PSA, PM, CB | PPC | 119 | 14 | 2–1 | / | Anaemia, CA |
| 8 | 72, M | 23 | 77 | MRSA, PSA | ASA | 112 | 12 | 1·5 | / | Hypertension nicotine abuse |
| 9 | 62, M | 11 | 141 | SA | / | 135 | 15 | 0 | / | Hepatic cirrhosis child A, anaemia |
| 10 | 67, F | 120 | 69 | Staph. epid. | ASA | 70 | 14 | 0 | / | Diabetes, hepatopathy, CKD nicotine abuse |
| 11 | 59, M | 6 | 81 | PSA | / | 90 | 14 | 0 | / | Hypertension adipositas permagna |
| 12 | 83, F | 24 | 104 | PSA, PM, EC | ASA | 108 | 30 | 1–0 | Hypergranulation (17. postoperative day) | Hypertension |
| 13 | 54, M | 14 | 48 | PSA, EC | / | 48 | 12 | 0 | / | Nicotine abuse |
| 14 | 84, F | 9 | 180 | MRSA, PAS | ASA | 162 | 34 | 1 | Bleeding (strong) MRSA 2. dr.change | Anaemia, hypertension, CVD heart failure NYHA II b |
| 15 | 75, F | 120 | 108 | MRSA, SA, PSA, PM | / | 119 | 18 | 1–0 | MRSA 2. dr.change | Anaemia, hypertension |
| 16 | 44, M | 120 | 37 | PSA, EC | / | 40 | 12 | 1–0 | EC 2. dr. change | Hypotension, acrocyanosis nicotine abuse |
| 17 | 82, F | 12 | 12 | SA, K | PPC | 16 | 21 | 1·5 | CVD, CKD, anaemia, hypertension | |
| 18 | 76, F | 12 | 117 | MRSA | / | 119 | 26 | 0 | MRSA 3. dr.change | Anaemia, hypertension |
| 19 | 80, F | 8 | 34 | SA | PPC | 32 | 16 | 0 | / | GAOD, pulmonary embolism |
| 20 | 78, F | 7 | 117 | PSA, PM | PPC, ASA | 102 | 28 | 1–0 | Bleeding (medium) | Anaemia, CVD, hypercholesterolaemia |
| 21 | 61, F | 84 | 45 | SA, EK, K | / | 65 | 10 | 1–0 | / | Diabetes, COPD nicotine abuse |
| 22 | 76, F | 16 | 78 | MRSA, PAS, PM, EC (ESBL) | / | 82 | 14 | 1–0 | / | Anaemia, hypertension, adipositas magna |
| 23 | 80, M | 18 | 35 | PSA, PM, EK, SER | PPC | 37 | 19 | 1–0 | / | Hypertension, CA |
| 24 | 82, F | 28 | 38 | PSA | PPC | 39 | 26 | 1–0 | Bleeding (medium) | Anaemia |
| 25 | 51, M | 23 | 141 | PSA, SA, PM, K, CB | / | 136 | 14 | 1–0 | Anaemia compulsive hand washing |
ASA, acetylsalicylic acid; CA, cardiac arrhythmia; CB, Citrobacter; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; EC, Escherichia coli; EK, Enterococcus; ESBL, extended‐spectrum‐beta‐lactamase resistance; GAOD, general arterial occlusive disease; K, Klebsiella; MRSA, methicillin‐resistant Staphylococcus aureus; PM, Proteus mirabilis; PPC, phenprocoumon; PSA, Pseudomonas aeruginosa; SA, Staphylococcus aureus.
Patients' ages ranged from 44 to 84 years (median 76 years, mean 71·6 years), with ulcer size ranging from 12 to 180 cm2 (median 77 cm2, mean 77·1 cm2) and DSW size ranging from 12 to 162 cm2 (median 66 cm2, mean 78 cm2). A total of 15 patients were treated with anticoagulants and/or platelet aggregation inhibitors, 6 patients suffered from diabetes mellitus, 11 patients had anaemia with Hb < 10 g/dl and 6 patients showed MRSA colonisation of the ulcers.
Complete DSW epithelialisation was observed between the 10th and 34th postoperative day (median 14 days, mean 17·2 days).
Postoperative medium to strong bleeding at the DSWs occurred in five patients (two taking phenprocoumon, one ASA and one phenprocoumon plus ASA) within the first 2 postoperative days. The amount of bleeding in only two of these patients [No 2 (phenprocoumon) and 14 (ASA)] required a superficial dressing change. The other three patients did not require a dressing change as the dressings were only slightly blood stained.
Postoperative DSW pain levels were only moderate. Subjective pain evaluation by means of a six‐item scale resulted in a mean value of 0·5 (range 0–1·5, median 0·5) on the first postoperative day. Patients received ibuprofen (up to 3 × 400 mg/day) or metamizole (up to 3 × 500 mg/day) as needed, primarily because of pain in the grafted areas. No further analgesia was needed in any of the cases.
Only two patients (No 14, 18) developed clinical signs of DSW infection (stage 1–2) after the first dressing change. However, no patient required treatment with antibiotics.
No maceration of the wound surrounding was observed in any patient.
In order to estimate the influence of comorbidities in healing of DSWs, all 25 patients were divided into four groups (A–D) in accordance to patient comorbidities (anticoagulation, diabetes, anaemia, MRSA).
Group A: patients with anticoagulant/platelet aggregation inhibitor medication
A total of 15 patients aged 62–84 years (mean 77·1 years, median 80 years) were included in this group. Anticoagulation included phenprocoumon (eight patients), acetylsalicylic acid (ASA) (five patients), phenprocoumon and ASA (one patient) and enoxaparin (one patient). Perioperative INR values of the phenprocoumon patients ranged between 1·9 and 2·9 (mean 2·3, median 2·5). MRSA at the ulcer site was confirmed in three patients. Six patients were diagnosed to be anaemic with haemoglobin levels below 10 g/dl; four patients suffered from diabetes mellitus, and three patients were smokers. DSW sizes ranged from 16 cm to 162 cm2 (mean 73·2 cm2, median 64 cm2). Complete epithelialisation was observed between the 10th and 34th postoperative day (mean 18·6 days, median 15 days). Strong postoperative bleeding occurred in two patients (No 2, 14) within the first 2 days, making superficial dressing change necessary.
Group B: patients with diabetes mellitus
A total of six patients aged 61–82 years (median 73·5 years) were included in this group. Four of six patients had additional anticoagulant therapy with phenprocoumon (three patients) and ASA (one patient). One patient was confirmed with MRSA at the ulcer site, and one patient was a smoker. DSW sizes ranged from 28 to 84 cm2 (median 61·5 cm2). Complete epithelialisation was observed between the 10th and 17th postoperative day (median 14 days).
Group C: patients with anaemia
A total of 11 patients aged 51–84 years (median 75·5 years) were included in this group. Seven patients had additional anticoagulant therapy with phenprocoumon (four patients), ASA (two patients) and enoxaparin (one patient). MRSA at the ulcer site was confirmed in four patients. The size of the DSWs ranged from 16 to 162 cm2 (median 118 cm2). Complete epithelialisation was observed between the 10th and 34th postoperative day (median 16·5 days).
Group D: patients with MRSA at the ulcer site
A total of six patients aged 72–84 years (median 76 years) were included in this group. Three patients had additional anticoagulant therapy with phenprocoumon (one patient) and ASA (two patients). Four of six patients were diagnosed as anaemic. DSW sizes ranged from 48 to 162 cm2 (median 115·5 cm2). Complete reepithelialisation was observed between the 12th and 34th postoperative day (median 17·5 days). MRSA at DSWs was postoperatively confirmed in three patients (No 14, 15, 18) at the second and third dressing change. In two of these patients (No 14, 18), epithelialisation was delayed [34 days (No 14) and 26 days (No 18)].
Discussion
In the present study, the duration of healing, occurrence of postoperative wound bleeding and pain intensity of medium depth (0·4 mm) DSWs were evaluated in 25 multi‐morbid patients with simultaneously grafted chronic leg ulcers. The majority of patients received anticoagulants or platelet aggregation inhibitors perioperatively. The combination of three dressing components resulted in a non‐adherent, hemostyptic antiseptic dressing with a high fluid absorptive capacity while at the same time providing a moist healing environment and minimising shear and friction force. Application of a non‐adherent silicone film as first layer was necessary because the cellulose‐collagen matrix tends to dry out, and the dressing thus could partially adhere to the healing wound and damage the new epithelium during the first dressing change.
Complete epithelialisation was observed after an average of 17·2 days (median 14 days), which is remarkable in spite of the depth of the DSW and the unfavourable comorbidities of the patients in our study. For a majority of patients, pain is their most important concern. In this study, patients reported very low pain levels at the donor site. Already, on the first postoperative day, 10 patients reported no pain (0), and another 10 patients reported pain levels between 0 and 1 on a six‐item scale. Only 5 of 25 patients developed medium 3 and strong 2 postoperative bleeding within the first 2 postoperative days. The two heavier bleedings required only one superficial dressing change of the upper layers (gauze pads and retention tape). The first complete dressing change was performed without exception on the 10th postoperative day according to the study protocol.
The incidence of positive bacterial cultures was very low. None of the 25 treated patients showed a DSW contamination or infection within the first 10 days before the first dressing change. MRSA in the DSWs was found in three, and E. coli in one, patient only at the second and third dressing change. Two of the three MRSA patients showed delayed epithelialisation.
The positive experiences obtained using our composite wound dressing system are of particular value when compared to studies in the literature (Table 2). In almost all of these studies, very superficial DSWs (0·15–0·32 mm) were treated, and even then, some studies report reepithelialisation periods of significantly more than 30 days. In some studies, wound depth and postoperative pain intensity are not indicated. In most studies, no information regarding perioperative anticoagulation and comorbidities of patients is given.
Table 2.
DSW treatment in the literature: comparison of existing data with study findings
| Study, year | Number of patients | Age range (years) | Mean | Median | Donor site localization | Depth (mm) | Size (cm2) | Dressing | Reepithelialization (days) | Median | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | ||||||||||
| Konstantinow et al., 2016 | 25 | 44–84 | 71·5 | 76 | Thigh | 0·4 | 16–162 (mean: 78·4; median: 78) | Mepitel/PRISMA/TIELLE | 10–34 | 19·8 | 18·5 |
| Konstantinow et al., 1991 40 | 8 | 16–43 | 29 | 28 | Thigh | 0·3 | 100–480 (mean: 285; median: 270) | CEA | 6–8 | 7·5 | 8 |
| 8 | 16–43 | 29 | 28 | Thigh | 0·3 | 100–540 (mean: 362·5; median: 345) | Biobrane | 9–18 | 13·4 | 12·5 | |
| Phillips et al., 1993 42 | 9 | 63–87 | 71 | n.i. | 0·2–0·42 | 50–220 | CEA | 5–14 | 8·4 | ||
| N‐terface | 11–21 | 15·3 | |||||||||
| Innes et al., 2001 50 | 17 | 15–64 | 40·6 | Thigh (15), div. | 0·34 | 40 | Acticoat | 14·5 (>90%) | |||
| Thigh (15), div. | 0·34 | 40 | Allevyn | 9·1 (>90%) | |||||||
| Malpass et al., 2003 51 | 19 | 13–67 | 33 | Thigh | 0·2 (−0·3) | ≥21 inch2 | Xeroform | 5–18 | 10·5 | ||
| Jelonet | 5–18 | 10·5 | |||||||||
| Barnea et al., 2004 52 | 23 | 19–86 | 51 | Thigh | 0·3 | Aquacel | 7–90 (?) | 7–10 (?) | |||
| Paraffin gauze | 10–90 (?) | 10–14 (?) | |||||||||
| Uysal et al., 2006 47 | 40 | 8–67 | 45·3 | Thigh | ? | n.i.a. | Surgical (ORC) | (5·4 ± 0·5) | |||
| 40 | 8–67 | 45·3 | Thigh | ? | n.i.a. | Furacin gauze | |||||
| Argirova et al., 2007 53 | 15 | 10–18 | 14·7 | Thigh | 0·15 | mean: 400 | Acticoat | 8·3 | |||
| 12 | 10–18 | 13·6 | Thigh | 0·15 | mean: 400 | Allevyn | 10 | ||||
| Lohsiriwat et al., 2009 54 | 18 | 22–73 | 47·2 | Thigh | n.i.a. | mean: 145·5 | Aquacel | 4–13 | 7·9 | ||
| 21–72 | 49 | Thigh | n.i.a. | mean: 135·8 | paraffin gauze | 4–19 | 11·2 | ||||
| Vandenberg et al., 2010 55 | 22 | n.d. | Thigh, leg | n.i.a. | n.i.a. | AWBAT‐D | 8–15 | 11·3 | 11 | ||
| Kaartinen et al., 2011 56 | 14 | 16–78 | 60 | Thigh | 0·25 | n.i.a. | Suprathel | ≥14 | |||
| 14 | 16–78 | 60 | Thigh | 0·25 | n.i.a. | Mepilex | ≥14 | ||||
| Dornseifer et al., 2011 48 | 50 | 18–95 | 62·6 | Thigh | 0·3 | 90–732 (mean: 233) | Aquacel | (10) | |||
| 50 | 18–95 | 62·6 | Thigh | 0·3 | 90–732 (mean: 233) | Opsite | (10) | ||||
| Higgins et al., 2012 57 | 18 | n.d. | Thigh | 0·25 | n.i.a. | Kaltostat | n.i.a. | ||||
| 18 | n.d. | Thigh | 0·25 | n.i.a. | Allevyn | n.i.a. | |||||
| Davidson et al., 2013 11 | 26 | 15 > 50 years | 73 | Thigh, arm (1×) | 0·25 | n.i.a. | Keramatrix | 7 | |||
| 26 | 11 > 50 years | 35 | Thigh, arm (1×) | 0·25 | n.i.a. | Algisite | 7 | ||||
| Assadian et al., 2013 58 | 17 | 5–76 | 36·6 | 36 | Thigh, leg | 0·25–0·3 | mean: 264; median: 154 | Aquacel Ag | 9–20 | 13·20 | |
| 17 | Thigh, leg | 0·25–0·3 | mean: 229; median: 197 | TMD | 10–23 | 14·20 | |||||
| Heavy et al., 2013 59 | 20 | 20–88 | 60 | Thigh | 0·2–0·25 | mean: 80 | Fibrin spray + Mefix | 13–34 | 23·7 | ||
| 20 | 20–88 | 60 | Thigh | 0·2–0·25 | mean: 80 | Mefix | 16–54 | 35 | |||
| Brölmann et al., 2013 49 | 45 | 60 | Thigh | 0·3 | 10–240; median; 50 | Kaltostat, Algisite, Melgisorb | 19–29 | 27·1 | 22 | ||
| 49 | 61 | Thigh | 0·3 | 10–600; median: 49 | Tegaderm, Opsite | 14–36 | 32·9 | 23 | |||
| 50 | 62 | Thigh | 0·3 | 10–450; median: 50 | Adaptic, Jelonet | 18–33 | 27·9 | 22 | |||
| 49 | 61 | Thigh | 0·3 | 10–800; median: 49 | Duoderm E | 12–21 | 19·4 | 16 | |||
| 47 | 60 | Thigh | 0·3 | 10–750; median: 37·5 | Aquacel | 15–27 | 26 | 22 | |||
| 48 | 62 | Thigh | 0·3 | 10–760; median: 40 | Mepitel | 18–33 | 29·2 | 26 | |||
| Blome‐Eberwein et al., 2013 60 | 10 | 19–72 | 49·1 | Thighs, arms, fl. | 0·2–0·3 | n.i.a. | Tegaderm | 10–28 | 16·1 | 13·5 | |
| Läuchli et al., 2013 61 | 19 | 35–95 | 72·1 | Thigh | 0·2 | 12–300 (mean: 47·2) | Kaltostat | 13–36 | 18·8 | 16·5 | |
| 19 | 46–96 | 78·6 | Thigh | 0·2 | 12–300 (mean: 68·8) | Opsite Flexigrid | 14–41 | 21·9 | 20 | ||
| Weyandt et al., 2009 38 | 166 | 26–93 | 71 | 78 | Sclap | 0·2–0·3 | 4·5–434; mean: 42·1; median: 17·75 | Adaptic + PVP ointment | 3–9 | 5·4 | 5 |
CEA, cultured epidermal allograft; ORC, oxidised regenerated cellulose; TMD, transforming methacrylate dressing; PVP, povidone‐iodine.
?, inconsistent data.
Healing time of DSWs is largely dependent on the wound depth 10 and extent of postoperative bleeding. In this context, there is a considerable anatomical difference between a 0.‐ mm and a 0·4‐mm deep wound (and all the more between a 0·15–0·2 mm and a 0·4‐mm deep one). The dermal vascular plexus consists of a superficial and a deep glomerulus, which are connected with each other by relatively straight vertical vessel sections. The very fine superficial vascular plexus with all its partly non‐muscular capillaries lies directly under the epidermis in the dermal papillae at a depth of 0·1–0·3 mm. This is also the length of the epidermal rete ridges that, depending on the body region, have a length of approximately 0·1–0·3 mm 34. A 0·15–0·3‐mm deep acute wound shows only very small bleeding foci, which, as a rule, soon cease spontaneously if there is no anticoagulation. When a 0·4‐mm deep wound is generated, connecting vessels with larger lumens are separated under the fine capillary plexus, which leads to substantially heavier bleedings of the wound surface, especially under accompanying medication with anticoagulants and platelet aggregation inhibitors. Skin ageing in older patients leads to a severe decrease of epidermal and dermal thickness as well as the vascular plexuses and adnexal structures 35. Therefore, in elderly patients, even the harvesting of STSGs of medium thickness can reach deeper dermal layers, thus causing stronger bleeding and delayed healing of the DSWs. Davidson et al. 11 also observed a slower DSW epithelialisation rate in patients older than 50 years compared to patients younger than 50 years (Table 2).
After the harvesting of only epidermal grafts with an epidermal graft‐harvesting device, the mean DSW healing time was 5·5 days 36. After acute superficial epidermo‐dermal injury, epithelial stem cells from the wound margins, the hair follicles as well as sebaceous and sweat gland appendages migrate into the denuded area 37, 38. Therefore, DSWs located on the scalp show the fastest epithelialisation rates because of the high density of hair follicles and accompanying glands and thus the highest concentration of stem cells 39.
Fast‐healing rates of superficial DSWs outside the scalp region were observed after application of cultured epidermal allografts (CEA). In a small, self‐conducted, prospective clinical trial with eight patients, half of each DSW area (0·3 mm thickness) was covered with Biobrane® (Smith and Nephew, London, UK) and the other half with allogeneic cryopreserved keratinocytes. In the Biobrane‐covered areas, complete epithelialisation was observed after a mean of 13·4 days and in the wounds covered with CEA after a mean of 7·5 days (Table 2) 40, 41. CEA were also used by Phillips et al. in the treatment of superficial DSWs in nine patients 42. Half of the DSW area was treated with CEA, while the other half was covered with a non‐adherent dressing. The DSW areas treated with CEA healed in a mean of 8·4 days, while the areas treated with non‐adherent dressings in the control healed in a mean of 15·3 days.
Local haemostasis is the first step in the successful treatment of an acutely generated wound like DSWs. For decades, ORC has been widely used as local hemostyptic 12, 13, 14, 43, 44. Within 24–48 hours, a gelatinous coagulum consisting of cellulosic acid and haemoglobin is generated through clot formation and is completely resorbed or broken down after 1–6 weeks. Besides its local haemostatic effect, OCR has bactericidal properties that inhibit the growth of a wide range of Gram‐positive and Gram‐negative organisms 45. Microfibrillar collagen is another fully resorbable haemostatic agent 12, 13, 14. Furthermore, ORC/collagen dressings induce the production of higher levels of several growth factors in acute and chronic wounds, leading to accelerated wound healing 46.
Experiences with DSW treatment with an ORC dressing have been reported by Uysal et al. 47. However, no information about DSW size or underlying diseases and anticoagulation status is provided. Moreover, the information about the thickness of the harvested split skin grafts and thus the indicated wound depth of the DSW of 0·015 μm appears implausible. Supposing the authors meant 0·015 inches (approximately 0·4 mm, comparable with the study presented here), the observed complete epithelialisation in the ORC group within a mean of only 5·4 days appears surprisingly fast. Patients reported a minimal periodic pain on the ORC‐covered area and only few minimal bleedings.
Dornseifer et al. 48 compared a polyurethane dressing with a carboxymethylcellulose hydrocolloid polymer in a prospective randomised control trial (RCT) including 50 patients with 0·3 mm DSWs. The primary outcome was the reepithelialisation rate 10 days postoperatively. Secondary outcome variables were pain, cost and scarring. Complete reepithelialisation on the 10th postoperative day was observed in 86·4% of the donor sites treated with the perforated polyurethane foil, compared to 54·5% of the carboxymethylcellulose‐treated sites. However, there was no further evaluation of the incompletely epithelialised wounds. Pain at the donor site was mild in both groups during the postoperative period, that is, 90% of the six‐item pain scale values were less than or equal to 1. The study, however, includes no information about accompanying diseases or anticoagulation status.
Infection is a major risk in DSW treatment as shown by Brölmann et al. 49 who compared different wound dressings (alginates, semipermeable films, gauze dressings, hydrocolloids, hydrofibers and silicone dressings) in a 14‐centre RCT with 288 adult patients with DSWs larger than 10 cm2. The standardised graft thickness was 0·3 mm in all patients. Patients with diabetes and nicotine abusers were included, but there was no reference on the perioperative anticoagulant status. The time to complete reepithelialisation with hydrocolloid dressings was 7 days shorter than with any other dressing (median 16 versus 23 days), and infection rates in patients treated with gauze was twice as high as in those who had other dressings.
Regarding the small number of patients in this non‐comparative study, statistical analysis was limited to frequencies, mean and median values. Given the non‐comparative nature of this study, larger, randomised controlled clinical studies should be performed in the future.
This study demonstrates that the ORC/collagen/Ag‐ORC containing composite dressing allows for the rapid epithelialisation of medium‐depth (0·4 mm) DSWs in older multi‐morbid patients with chronic lower leg ulcers while reducing postoperative complications such as bleeding and pain. Although the costs for a single dressing are higher (about 45·00 Euro for a DSW measuring 120 cm2) compared to standard basic hydrocolloid and foam dressings, rapid healing as well as a decrease of postoperative morbidity and frequency of dressing changes consequently reduce the overall treatment costs. All of these benefits led to implementation of this composite dressing in our daily clinical practice. To avoid early damage of the new epithelium, we adjusted the wound management plan so that the first complete DSW dressing change is now performed between the 10th and 14th postoperative day depending on dressing appearance.
Supporting information
Appendix. Clinical data of study patients.
References
- 1. Heyer K, Herberger K, Protz K, Glaeske G, Augustin M. Epidemiology of chronic wounds in Germany: analysis of statutory health insurance data. Wound Repair Regen 2016;24:434–42. [DOI] [PubMed] [Google Scholar]
- 2. Jöckenhöfer G, Gollnick H, Herberger K, Isbary G, Renner R, Stücker M, Valesky E, Wollina U, Weichenthal M, Karrer S, Kuepper B, Roesch A, Dissemond J. Aetiology, comorbidities, and cofactors of chronic leg ulcers: retrospective evaluation of 1000 patients from 10 specialised dermatologic wound care centers in Germany. Int Wound J 2016;13(5):821–8. DOI: 10.1111/iwj.12387. Epub 2014 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Donnell TF, Passmann MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, Lurie F, Henke PK, Gloviczki ML, Eklöf BG, Stoughton J, Raju S, Shortell CK, Raffetto JD, Partsch H, Pounds LC, Cummings ME, Gillespie DL, McLafferty RB, Murad MH, Wakefield TW, Gloviczki P. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg 2014;60(Suppl 2):3S–59. [DOI] [PubMed] [Google Scholar]
- 4. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marston W, Tang J, Kirsner RS, Ennis W. Wound healing society 2015 update on guidelines for venous ulcers. Wound Repair Regen 2016;24:136–44. [DOI] [PubMed] [Google Scholar]
- 6. Schmeller W, Gaber Y, Gehl HB. Shave therapy is a simple, effective treatment of persistent venous leg ulcers. J Am Acad Dermatol 1998;39:232–8. [DOI] [PubMed] [Google Scholar]
- 7. Serra R, Rizzuto A, Rossi A, Perri P, Barbetta A, Abdalla K, Caroleo S, LOngo C, Amantea B, Sammarco G, de Franciscis S. Skin grafting for the treatment of chronic leg ulcers—a systemic review in evidence-based medicine. Int Wound J 2016. DOI: 10.1111/iwj.12575. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirsner RS, Eaglstein WH, Kerdel FA. Split‐thickness skin grafting for lower extremity ulcerations. Dermatol Surg 1997;23:85–91. [DOI] [PubMed] [Google Scholar]
- 9. Rompel R. Dermatological surgery. In: Burgdorf WHC, Plewig G, Wolff HH, Landthaler M, editors. Braun‐Falco's dermatology, 5th edn. Heidelberg: Springer Medizin, 2009:1633–4. [Google Scholar]
- 10. Converse JM, Robb‐Smith AHT. The healing of surface cutaneous wounds: its analogy with the healing of superficial burns. Ann Surg 1944;120:873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davidson A, Jina NH, Marsh C, Than M, Simcock JW. Do functional keratin dressings accelerate epithelialization in human partial thickness wounds? A randomized controlled trial on skin graft donor sites. Eplasty 2013;13:e45. [PMC free article] [PubMed] [Google Scholar]
- 12. Abbott WM, Austen WG. The effectiveness and mechanism of collagen induced topical hemostasis. J Cardiovasc Surg (Torino) 1976;17:95–6. [PubMed] [Google Scholar]
- 13. Chen DL, Carlson EO, Fathi R, Brown MR. Undermining and hemostasis. Dermatol Surg 2015;41:S201–15. [DOI] [PubMed] [Google Scholar]
- 14. Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg 2008;34:431–45. [DOI] [PubMed] [Google Scholar]
- 15. Lin Y‐H, Hsu W‐S, Chung W‐Y, Ko T‐H, Lin J‐H. Silver‐based wound dressings reduce bacterial burden and promote wound healing. Int Wound J 2016;13:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Percival SL, Slone W, Linton S, Okel T, Corum L, Thomas JG. The antimicrobial efficacy of a silver alginate dressing against a broad spectrum of clinically relevant wound isolates. Int Wound J 2011;8:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowler PG, Jones SA, Walker M, Parsons D. Microbicidal properties of a silver‐containing hydrofiber® dressing against a variety of burn wound pathogens. J Burn Care Rehabil 2005;25:192–6. [DOI] [PubMed] [Google Scholar]
- 18. Pfurtscheller K, Petnehazy T, Goessler W, Bubalo V, Kamolz LP, Trop M. Transdermal uptake and organ distribution of silver from two different wound dressings in rats after a burn trauma. Wound Repair Regen 2014;22:654–9. [DOI] [PubMed] [Google Scholar]
- 19. Chaby G, Senet P, Vaneau M, Martel P, Guillaume JC, Meaume S, Teot L, Debure C, Dompmartin A, Bachelet H, Carsin H, Matz V, Richard JL, Rochet JM, Sales‐Aussias N, Zagnoli A, Denis C, Guillot B, Chosidow O. Dressings for acute and chronic wounds. Arch Dermatol 2007;143:1297–304. [DOI] [PubMed] [Google Scholar]
- 20. Diehm C, Lawall H. Evaluation of Tielle hydropolymer dressings in the management of chronic exuding wounds in primary care. Int Wound J 2005;2:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaneau M, Chaby G, Guillot B, Martel P, Senet P, Teot L, Chosidow O. Consensus panel recommendations for chronic and acute wound dressing. Arch Dermatol 2007;143:1291–4. [DOI] [PubMed] [Google Scholar]
- 22. Voineskos SH, Ayeni OA, McKnight LM, Thoma A. Systematic review of skin graft donor‐site dressings. Plast Reconstr Surg 2009;124:298–306. [DOI] [PubMed] [Google Scholar]
- 23. Vowden P, Cooper RA. An integrated approach to managing wound infection. In: Calne S, editor. European Wound Management Association (EWMA). Position document: management of wound infection. London: Medical Education Partnership Ltd, 2006:2–3. [Google Scholar]
- 24. Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW. American Venous Forum International Ad Hoc Committee for revision of the CEAP classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248–52. [DOI] [PubMed] [Google Scholar]
- 25. Kullo IJ, Rooke TW. Peripheral artery disease. N Engl J Med 2016;374:861–71. [DOI] [PubMed] [Google Scholar]
- 26. Federmann DG, Ladiiznski B, Dardik A, Kelly M, Shapshak D, Ueno CM, Mostow EN, Richmond NA, Hopf HW. Wound healing society update on guidelines for arterial ulcers. Wound Repair Regen 2016;24:127–35. [DOI] [PubMed] [Google Scholar]
- 27. Høyer C, Sandermann J, Petersen LG. The toe‐brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg 2013;58:231–8. [DOI] [PubMed] [Google Scholar]
- 28. Földi E, Baumeister RGH, Bräutigam P, Tiedjen KU. Zur diagnostik und therapie des lymphödems. Dtsch Ärzteblatt 1998;95:A‐740–7. [Google Scholar]
- 29. Harding KD, editor. Wound infection in clinical practice. An international consensus. Int Wound J 2008;5(Suppl 3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levine NS, Lindberg RB, Mason AD, Pruitt BA. The quantitative swab culture and smear: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma 1976;16:89–94. [PubMed] [Google Scholar]
- 31. Jørgensen LB, Sørensen JA, Jemec GBE, Yderstraede KB. Methods to assess area and volume of wounds – a systematic review. Int Wound J 2016;13:540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaw J, Bell PM. Wound measurement in diabetic foot ulceration. In: Dinh T, editor. Global perspective on diabetic foot ulcerations. Rijeka: In Tech, 2011:71–82. [Google Scholar]
- 33. Bonamonte D, Belloni F, Neri L, Patrizi A. Fusidic acid in skin infections and infected atopic eczema. G Ital Dermatol Venereol 2014;149:453–9. [PubMed] [Google Scholar]
- 34. Huzaira M, Rius F, Rajadhyaksha M, Anderson RR, Gonzalez S. Topographic variation in normal skin as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol 2001;116:846–52. [DOI] [PubMed] [Google Scholar]
- 35. Scharffetter‐Kochanek K, Wlaschek M. Cutaneous aging. In: Burgdorf WHC, Plewig G, Wolff HH, Landthaler M, editors. Braun‐Falco's dermatology, 5th edn. Heidelberg: Springer Medizin, 2009:1170–1. [Google Scholar]
- 36. Harach‐Haram N, Bystrzonowski N, Kanapathy M, Smith O, Harding K, Mosahebi A, Richards T. A prospective, multicenter study on the use of epidermal grafts to optimize outpatient wound management. Int Wound J 2016. Mar 20. DOI: 10.1111/iwj.12595. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 2015;173:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nie J, Fu X, Han W. Microenvironment‐dependent homeostasis and differentiation of epidermal basal undifferentiated keratinocytes and their clinical application in skin repair. J Eur Acad Dermatol Venereol 2013;27:531–5. [DOI] [PubMed] [Google Scholar]
- 39. Weyandt GH, Bauer B, Berens N, Hamm H, Broecker EB. Split‐skin grafting from the scalp: the hidden advantage. Dermatol Surg 2009;35:1873–9. [DOI] [PubMed] [Google Scholar]
- 40. Konstantinow A, Mühlbauer W, Hartinger A, von Donnersmarck GG. Skin banking: a simple method for cryopreservation of split‐thickness skin and cultured human epidermal keratinocytes. Ann Plast Surg 1991;26:89–97. [DOI] [PubMed] [Google Scholar]
- 41. Konstantinow A, von Donnersmarck GH, Hartinger A, Mühlbauer W. Cryopreserved epidermal allografts as skin substitute. [German]. Ninth Annual Meeting of the German Society for Burn Surgery. 1991; Bad Hofgastein/Austria. Meeting report 1991, 32.
- 42. Phillips TJ, Provan A, Colbert D, Easley KW. A randomized single‐blind controlled study of cultured epidermal allografts in the treatment of split‐thickness skin graft donor sites. Arch Dermatol 1993;129:879–82. [PubMed] [Google Scholar]
- 43. Hazarika EZ. Oxidised regenerated cellulose: an effective emergency haemostatic in burns surgery. Br J Plast Surg 1985;38:419–21. [DOI] [PubMed] [Google Scholar]
- 44. Lebendiger A, Gitlitz GF, Hurwitt ES, Lord GH, Henderson J. Laboratory and clinical evaluation of a new absorbable hemostatic material prepared from oxidized regenerated cellulose. Surg Forum 1960;10:440–3. [PubMed] [Google Scholar]
- 45. Spangler D, Rothenburger S, Nguyen K, Jampani H, Weiss S, Bhende S. In vitro antimicrobial activity of oxidized regenerated cellulose against antibiotic‐resistant microorganisms. Surg Infect (Larchmt) 2003;4:255–62. [DOI] [PubMed] [Google Scholar]
- 46. Jeschke MG, Sandmann G, Schubert T, Klein D. Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair Regen 2005;13:324–31. [DOI] [PubMed] [Google Scholar]
- 47. Uysal AC, Alagoz MS, Orbay H, Sensoz Q. An alternative dressing material for the split‐thickness skin graft donor site: oxidized regenerated cellulose. Ann Plast Surg 2006;57:60–4. [DOI] [PubMed] [Google Scholar]
- 48. Dornseifer U, Lonic D, Gerstung TI, Herter F, Fichter AM, Holm C, Schuster T, Ninkovic M. The ideal split‐thickness skin graft donor‐site dressing: a clinical comparative trial of a modified polyurethane dressing and aquacel. Plast Reconstr Surg 2011;128:918–24. [DOI] [PubMed] [Google Scholar]
- 49. Brölmann FE, Eskes AM, Goslings JC, Niessen FB, de Bree R, Vahl AC, Pierik EG, Vermeulen H, Ubbing DT. Randomized clinical trial of donor‐site wound dressings after split‐skin grafting. Br J Surg 2013;100:619–27. [DOI] [PubMed] [Google Scholar]
- 50. Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns 2001;27:621–7. [DOI] [PubMed] [Google Scholar]
- 51. Malpass KG, Snelling CFT, Tron V. Comparison of donor‐site healing under xeroform and jelonet dressings: unexpected findings. Plast Reconstr Surg 2003;112:430–9. [DOI] [PubMed] [Google Scholar]
- 52. Barnea Y, Amir A, Leshem D, Zaretski A, Weiss J, Shafir R, Gur E. Clinical comparative study of aquacel and paraffin gauze dressing for split‐skin donor site treatment. Ann Plast Surg 2004;53:132–6. [DOI] [PubMed] [Google Scholar]
- 53. Argirova M, Hajiski O, Victorova A. Acticoat versus allevyn as a split‐thickness skin graft donor‐site dressing. Ann Plast Surg 2007;59:415–22. [DOI] [PubMed] [Google Scholar]
- 54. Lohsiriwat V, Chuangsuwanich A. Comparison of the ionic silver‐containing hydrofiber and paraffin gauze dressing on split‐thickness skin graft donor sites. Ann Plast Surg 2009;62:421–2. [DOI] [PubMed] [Google Scholar]
- 55. Vandenberg VB. AWBAT: an early clinical experience. Eplasty 2010;10:e23. [PMC free article] [PubMed] [Google Scholar]
- 56. Kaartinen IS, Kuokkanen HO. Suprathel® causes less bleeding and scarring than Mepilex® Transfer in the treatment of the donor sites of split‐thickness skin grafts. J Plast Surg Hand Surg 2001;45:200–3. [DOI] [PubMed] [Google Scholar]
- 57. Higgins L, Wasiak J, Spinks A, Cleland H. Split‐thickness skin graft donor site management: a randomized controlled trial comparing polyurethane with calcium alginate dressings. Int Wound J 2012;9:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Assadian O, Arnoldo B, Purdue G, Burris A, Skrinjar E, Duschek N, Leaper DJ. A prospective, randomised study of a novel transforming methacrylate dressing compared with a silver‐containing sodium carboxymethylcellulose dressing on partial‐thickness skin graft donor sites in burn patients. Int Wound J 2015;12:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Healy C, Greig AV, Murphy AD, Powell C, Pinder RJ, Saour S, Abela C, Knight W, Geh J. Prospective randomized controlled trial: fibrin sealant reduces split skin graft donor‐site pain. Plast Reconstr Surg 2013;132:139e–46. [DOI] [PubMed] [Google Scholar]
- 60. Blome‐Eberwein S, Abboud M, Lozano DD, Sharma R, Eid S, Gogal C. Effect of subcutaneous epinephrine/saline/local anesthetic versus saline‐only injection on split‐thickness skin graft donor site perfusion, healing, and pain. J Burn Care Res 2013;34:e80–6. [DOI] [PubMed] [Google Scholar]
- 61. Läuchli S, Hafner J, Ostheeren S, Mayer D, Barysch MJ, French LE. Management of split‐thickness skin graft donor sites: a randomized controlled trial of calcium alginate versus polyurethane film dressing. Dermatology 2013;227:361–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix. Clinical data of study patients.


