Abstract
Extensive full‐thickness burns pose a great challenge to the burn surgeon. Lack of autograft donor sites is an important limiting factor to achieving wound closure. To overcome this problem, various methods of treatment have been suggested in the past, including the MEEK technique. This study was carried out at the Bogenhausen Hospital Burn Unit, Munich, Germany from 2006 to 2015. There were a total of 148 skin grafting operations. The modified MEEK technique was performed on 67 patients. Patients included 34 males and 33 females, with an average age of 39·6 years. The mean percentage body surface burned was 65%, and full‐thickness injury occurred in 52%. The mean area graft per procedure was 20%. The viability of the graft as assessed between the 7th and 10th day was generally in the range of 60–90%. The average number of operations required was 2·21. The mean length of stay was 27 days. Infection was documented in five patients, and seven deaths occurred. The mean follow‐up was 3·2 years. When faced with large surface area burns and limited donor sites, the MEEK technique is a satisfactory method for coverage.
Keywords: Burn injury, Burn therapy, Large surface burns, MEEK, Skin graft
Introduction
Extensive full‐thickness burns pose a great challenge to the burn surgeon. In the past, these patients often died early because of a lack of full understanding of the management of shock. With improved knowledge of fluid and electrolyte balance and burn pathophysiology, the mortality of severely burned patients during the early phase of treatment is now a rarity. Early excision of third degree burns and necrotic skin remains paramount in order to avoid septicaemia 1, 2. When attempting to achieve wound closure, the lack of autograft donor sites becomes an important limiting factor 3, 4. To overcome this problem, various methods of treatment have been suggested in the past, such as postage stamp grafting 5, mesh grafting 6, intermingled auto‐ and homograft transplantation 7, 8, alternating strips of auto‐ and homograft transplantation 7, 8, micro‐skin grafting 9 and the MEEK technique 10, 11.
The MEEK technique was first described in 1963 11 and entails using widely expanded postage stamp autografts, in which pre‐folded gauzes are used to achieve a regular distribution of autograft islands cuts with a MEEK–Wall dermatome 10. Maybe because it was more cumbersome, the MEEK technique was used less and less following the introduction of meshed skin grafts in 1964 by Tanner 6.
Meek's original technique was improved by modifications made by Kreis in 1993, who proposed using a different device for skin cutting and using an aluminium foil backing to facilitate the expansion of the skin grafts 12. This modification has helped to make to procedure less elaborate and proved to be a valuable tool when treating extensive burns. Over the past 10 years, we had the opportunity to manage a fair amount of third degree large‐surface burns using this technique. Although the technical details of processing a skin graft have not changed, in order to increase efficiency, we have altered the regimen, that is, the approach to skin graft handling, the order of graft application and the aftercare.
In this paper, we describe our clinical experience using the modified MEEK technique in a series of 67 severely burned patients.
Material and methods
Patients
This retrospective study was carried out at the Bogenhausen Hospital Burn Unit in Munich, Germany from January 2006 to December 2015. There were a total of 148 skin grafting operations. The modified MEEK technique was performed on 67 patients. Patients consisted of 34 males and 33 females, with an average age of 390·6 years (range 18–92 years), including 47 flame burns, 13 inhalation injuries, 4 chemical burns and 3 electrical burns. The mean percentage body surface burned was 65% (range 50–87%), and full‐thickness injury occurred in 52% (range 40–81%) (Table 1).
Table 1.
Patient demographics and outcomes
| Pat. No. | Age (year) | Gender | % TBSA* | % third degree | Cause | Hospital stay (day) | No. of operations | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | 57 | 43 | Flame | 23 | 2 | Survived |
| 2 | 28 | F | 69 | 54 | Flame | 26 | 3 | Survived |
| 3 | 46 | F | 54 | 46 | Flame | 24 | 2 | Survived |
| 4 | 92 | M | 85 | 67 | Inhalation injury | 4 | 1 | Died |
| 5 | 37 | F | 63 | 50 | Flame | 23 | 2 | Survived |
| 6 | 74 | F | 78 | 62 | Flame | 36 | 3 | Survived |
| 7 | 49 | F | 85 | 72 | Flame | 47 | 3 | Survived |
| 8 | 62 | M | 52 | 40 | Flame | 21 | 2 | Survived |
| 9 | 39 | M | 61 | 44 | Electrical | 18 | 2 | Survived |
| 10 | 54 | M | 58 | 47 | Flame | 26 | 2 | Survived |
| 11 | 67 | F | 72 | 41 | Inhalation injury | 42 | 3 | Survived |
| 12 | 83 | F | 50 | 46 | Flame | 13 | 2 | Died |
| 13 | 29 | F | 62 | 42 | Flame | 26 | 2 | Survived |
| 14 | 51 | M | 55 | 47 | Flame | 22 | 2 | Survived |
| 15 | 18 | M | 57 | 50 | chemical | 18 | 2 | Survived |
| 16 | 24 | F | 54 | 48 | Flame | 21 | 2 | Survived |
| 17 | 69 | F | 74 | 63 | Flame | 39 | 3 | Survived |
| 18 | 59 | F | 81 | 74 | Flame | 28 | 2 | Died |
| 19 | 48 | M | 59 | 49 | Flame | 13 | 2 | Survived |
| 20 | 32 | F | 62 | 41 | Flame | 29 | 2 | Survived |
| 21 | 19 | F | 55 | 46 | Flame | 22 | 2 | Survived |
| 22 | 88 | M | 77 | 69 | Flame | 38 | 3 | Survived |
| 23 | 46 | M | 52 | 45 | Inhalation injury | 24 | 2 | Survived |
| 24 | 63 | M | 87 | 74 | Inhalation injury | 23 | 2 | Died |
| 25 | 20 | F | 73 | 64 | Inhalation injury | 37 | 3 | Survived |
| 26 | 69 | M | 55 | 41 | chemical | 18 | 2 | Survived |
| 27 | 37 | M | 57 | 42 | Flame | 26 | 2 | Survived |
| 28 | 29 | F | 62 | 48 | Inhalation injury | 32 | 2 | Survived |
| 29 | 42 | M | 73 | 61 | Flame | 62 | 3 | Survived |
| 30 | 36 | M | 59 | 43 | Flame | 27 | 2 | Survived |
| 31 | 29 | M | 53 | 48 | Flame | 24 | 2 | Survived |
| 32 | 32 | M | 68 | 62 | Flame | 39 | 3 | Survived |
| 33 | 19 | F | 73 | 59 | Inhalation injury | 36 | 3 | Survived |
| 34 | 76 | M | 69 | 42 | Flame | 29 | 2 | Survived |
| 35 | 39 | F | 57 | 49 | Flame | 32 | 2 | Survived |
| 36 | 43 | M | 82 | 73 | Inhalation injury | 54 | 3 | Survived |
| 37 | 27 | M | 67 | 43 | Flame | 24 | 2 | Survived |
| 38 | 25 | M | 59 | 50 | Flame | 27 | 2 | Survived |
| 39 | 21 | F | 62 | 48 | Flame | 26 | 2 | Survived |
| 40 | 34 | F | 68 | 43 | Flame | 23 | 2 | Survived |
| 41 | 38 | M | 63 | 40 | Electrical | 27 | 2 | Survived |
| 42 | 32 | F | 57 | 49 | Flame | 21 | 2 | Survived |
| 43 | 25 | M | 83 | 78 | Flame | 9 | 1 | Died |
| 44 | 47 | M | 61 | 46 | Flame | 19 | 2 | Survived |
| 45 | 24 | M | 59 | 42 | Flame | 25 | 2 | Survived |
| 46 | 31 | F | 65 | 48 | Flame | 23 | 2 | Survived |
| 47 | 28 | F | 81 | 77 | Flame | 43 | 3 | Survived |
| 48 | 26 | F | 64 | 47 | Flame | 23 | 2 | Survived |
| 49 | 39 | F | 53 | 40 | Flame | 18 | 2 | Survived |
| 50 | 19 | F | 73 | 55 | Inhalation injury | 37 | 3 | Survived |
| 51 | 27 | M | 71 | 64 | Inhalation injury | 53 | 4 | Survived |
| 52 | 33 | F | 57 | 46 | Flame | 21 | 2 | Survived |
| 53 | 29 | M | 54 | 42 | Flame | 24 | 2 | Survived |
| 54 | 37 | F | 61 | 48 | Flame | 27 | 2 | Survived |
| 55 | 28 | M | 53 | 41 | Flame | 21 | 2 | Survived |
| 56 | 41 | M | 87 | 81 | Inhalation injury | 12 | 1 | Died |
| 57 | 39 | F | 54 | 48 | Flame | 24 | 2 | Survived |
| 58 | 21 | F | 62 | 41 | Flame | 29 | 2 | Survived |
| 59 | 36 | M | 58 | 44 | Flame | 26 | 2 | Survived |
| 60 | 47 | M | 62 | 57 | Flame | 27 | 3 | Survived |
| 61 | 19 | M | 85 | 73 | Inhalation injury | 11 | 1 | Died |
| 62 | 28 | M | 78 | 69 | Electrical | 36 | 3 | Survived |
| 63 | 26 | F | 54 | 41 | Flame | 24 | 2 | Survived |
| 64 | 48 | F | 59 | 49 | chemical | 27 | 2 | Survived |
| 65 | 32 | F | 51 | 40 | chemical | 18 | 2 | Survived |
| 66 | 19 | M | 77 | 62 | Flame | 29 | 2 | Survived |
| 67 | 26 | F | 71 | 64 | Inhalation injury | 33 | 3 | Survived |
F, female; M, male.
% TBSA: burned percent of total body surface area.
Management
Patients with associated inhalation injury were intubated on admission. Initial resuscitation followed the Parkland Formula. Decompression of limb compartments was carried out as necessary. Burn wounds were dressed with Flamazine (1% silver sulphadiazine). Patients were operated on between the third and fifth post‐burn day once they had been stabilised with fluids and electrolytes. The Weck knife and the Humby knife were used to debride until punctuate bleeding occurred and the layer was grossly judged to be viable. When indicated, the entire skin down to the fascia was removed. At each operating session, 15–25% of full‐thickness burns was excised, and the MEEK technique was used to close the wound. When portions of the debrided areas were not able to be covered immediately given the intraoperative stability of the patient, overall length of the operation and location of the burn, Epigard alloplastic material was used to temporarily cover debrided wounds as needed. This procedure was repeated every 2–5 days, providing the patient's condition allowed surgical intervention.
Operative technique
Two teams of surgeons worked simultaneously. One team excised the eschar, while the other harvested the graft and prepared the micrografts. The extent of skin expansion required was determined by the size of the wound and the size of the available skin for grafting. The wounds were excised down to the healthy layers, and haemostasis was secured (Figure 1).
Figure 1.

Tangential excision of full‐thickness burn of the whole back.
As described previously, the harvested autograft skin was placed on 42 × 42 mm dampened cork with the dermis side down and trimmed to the required size (Figure 2). Then, it was placed on the carrier block and passed through a modified MEEK–Wall dermatome, which contains 13 parallel blades, spaced 3 mm apart. These blades cut the graft but not the cork (Figure 3). After the first pass, the cork plate was rotated to 90°, reclaimed and passed through the dermatome once more, thus cutting the graft in to 14 × 14 small square islands measuring 3 × 3 mm.
Figure 2.

After harvesting the split‐thickness skin grafts in a normal fashion, the grafts are manually cut in pieces and stretched over the 42 × 42 mm moistened cork plate with the inner layer facing downward for final cutting.
Figure 3.

Meshing of the graft is achieved using the modified MEEK–Wall dermatome.
The cork, with the cut graft in place, was removed. The epidermal side of the graft was then uniformly sprayed with an adhesive dressing spray (Leukospray, Beiersdorf GmbH, Germany) (Figure 4). After about 2–5 minutes, the sticky surface of the graft was brought in contact with the pre‐folded (pleated) gauze (Figure 5), and the pleats were pulled out on all the four sides to provide uniform expansion of the islands (Figure 6), with ratios varying from 1:3 to 1:9. The gauze was pulled steadily in all directions until it was completely smooth and flattened.
Figure 4.

The epidermal surface of the graft is sprayed with adhesives.
Figure 5.

The cork is removed once the minced grafts are transferred to the pre‐folded polyamide gauze backed with aluminium foil.
Figure 6.

The gauze is pulled steadily and firmly in all directions until it is completely smooth and flattened.
The recipient site was cleaned in the usual manner; the graft was applied to the wound bed, and the gauze was tacked down with surgical staples (Figure 7). The grafted wound was covered with Jelonet gauze impregnated with antimicrobial (Lavasept‐Gel) ointment. The operative sites in the trunk and extremities were additionally dressed with Jelonet and wrapped with elastic bandages. The wound was left undisturbed for 3–5 days. Thereafter, the wound care regimen consisted of dressing changes every 2 days. Staples were left in place for 7–10 days (Figure 8), and 1% silver sulfadiazine cream was used to cover the wound if it showed signs of local infection.
Figure 7.

The skin graft is applied onto the wound bed, and the edges of the gauze are tacked down with surgical staples.
Figure 8.
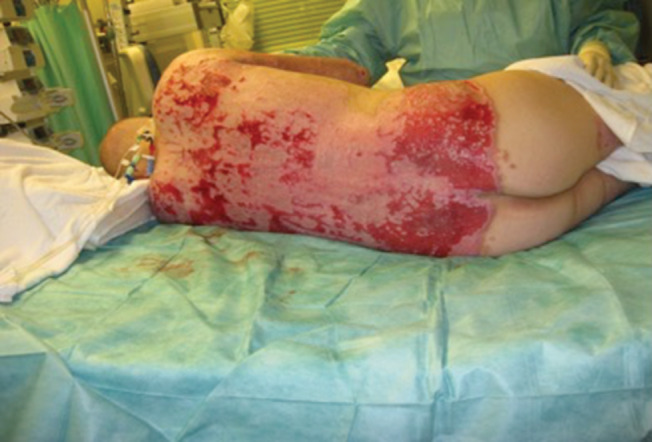
The appearance of the grafted wound at the 10th day after the procedure. The viability of the graft was assessed as satisfactory.
Results
The mean area graft per procedure was 20% (range 15–25%). The viability of the graft as assessed between the 7th and 10th day was generally in the range of 60–90% (Figure 8). The average number of operations required was 2·21. When the initial graft application failed, infection or haematoma was most commonly responsible. Although blood replacement was prescribed as needed in the operating theatre, transfusions seldom exceeded two units per session. Functional and aesthetic outcomes of wounds treated with MEEK grafts were satisfactory in most instances.
The mean length of stay was 27 days (range 4–62 days). Infection was documented in five patients. There were seven deaths among this group of patients. Four patients died from respiratory failure because of severe inhalation injury, and three died from septic shock. The mean follow‐up was 3·2 years (ranging from 3 months to 8 years).
Discussion
Meshed split‐thickness skin grafting has been an accepted method of treatment for severely burned patients at most burn centres 13, 14, 15. However, especially in large area burns, lack of autograft skin may become a limiting factor. In order to prevent wound infection and septicaemia, remaining areas of eschar should be excised even if they can not be covered with autografts immediately 4, 16. Our experience using the MEEK technique in large burn areas suggests that it provides a reliable method to achieve wound healing, with expanded autografts. The advantage is that the MEEK technique allows a greater expansion ratio compared with mesh grafts 16. The small autografts can be easily applied, in contrast to the often challenging handling of higher expansion (1:6 or 1:9) mesh grafts 12, 17.
Infection, as noted in five of our cases, can be a common cause for graft failure. Similar to others, we found that the thickness of skin grafts used for wound coverage does not appear to affect the incidence of infection. Indeed, small postage stamp skin grafts appear to be more resistant to invasion by microorganisms, and we also observed that spacing and distribution of the micrografts allowed for faster and more uniform epithelialisation 16, 17, 18.
In our experience, the cosmetic result following the MEEK graft technique is comparable with that of widely expanded mesh grafts. Other studies, especially concerning long‐term follow‐up, are still rare but underline our experience 12. Some authors speak even from a subjectively superior cosmetic outcome after a 6‐month follow‐up 18 and a faster and more uniform reepithelialisation 19, 20.
A major downside of the micrografting technique is the fact that it is expensive and requires more staff in the operating room in order to be carried out 18, 21. However, like Lumenta et al. have stated, we see the application of the MEEK technique as an integral part of our treatment concept, with which we have achieved good results 21. Furthermore, we have also observed that our patient collective had a comparable amount of operative sessions and length of hospital stay, compared with less severely injured patients who were not treated with the MEEK technique. However, this aspect was also not statistically analysed in this study.
Conclusion
When faced with large surface area burns and limited donor sites, the MEEK technique is a satisfactory method to cover large wounds. Although it is labour‐extensive, paying attention to the outlined principles allows achieving good functional and aesthetic results in this challenging patient population.
References
- 1. Bang RL, Gang RK, Sanyal SC, Mokaddas EM, Ebrahim MK. Burn septicaemia an analysis of 79 patients. Burns 1998;24:354–61. [DOI] [PubMed] [Google Scholar]
- 2. Wurtz R, Karajovic M, Dacumos E, Jovanovic B, Hanumadass M. Nosocomial infections in a burn intensive care unit. Burns 1995;21:181–4. [DOI] [PubMed] [Google Scholar]
- 3. Jackson D, Topley E, Carson JS, Lowbury EJ. Primary excision and grafting of large burns. Ann Surg 1960;152:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macmillan BG. Early excision of more than 25% of body surface in the extensively burned patients. Arch Surg 1958;77:369. [DOI] [PubMed] [Google Scholar]
- 5. Gabarro P. New method of skin grafting. Br Med J 1943;1:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanner JC, Vandeput JF, Olley JF. The Mesh skin graft. Plast Reconstr Surg 1964;34:287–92. [PubMed] [Google Scholar]
- 7. Gang RK, Hamberg H, Arturson G, Hakelius L. Histological studies of intermingled transplantation of autografts and allografts in rabbits. Burns 1980;8:80–5. [Google Scholar]
- 8. Yang CC, Shin TS, Chu TA, Hsu WS, Kuo SY, Kuo SY, Chao YF. The intermingled transplantation of auto and homografts in severe burn. Burns 1980;6:141. [Google Scholar]
- 9. Lin SD, Lai CS, Chou CK, Tsai CW, Wu KF, Chang CW. Microskin autografts with pig skin xenograft overlay: a preliminary report of studies on patients. Burns 1992;18:321–5. [DOI] [PubMed] [Google Scholar]
- 10. Meek CP. Successful microdermagrafting using the Meek wall microdermatome. Am J Surg 1958;96:557–8. [DOI] [PubMed] [Google Scholar]
- 11. Meek CP. Extensive severe burn treated with enzymatic debridement and micro dermagrafting: case report. Am Surg 1963;29:61–4. [PubMed] [Google Scholar]
- 12. Kreis RW, Mackie DP, Vloemans AW, Hermans RP, Hockstra MJ. Widely expanded postage stamp skin grafts using a modified Meek technique in combination with an allograft overlay. Burns 1993;19:142–5. [DOI] [PubMed] [Google Scholar]
- 13. Alexander JW, Macmillan BG, Law E, Kittur DS. Treatment of severe burns with widely meshed skin autografts and meshed allograft overlay. J Trauma 1981;21:433. [PubMed] [Google Scholar]
- 14. Gang RK, Arturson G, Hakelius L. The effect of split skin allografts on wound epithelialization from autologous patch grafts. Scand J Plast Reconstr Surg 1981;15:1–4. [DOI] [PubMed] [Google Scholar]
- 15. Minanov O, Peterson HD. Burn injury. In: Georgiade GS, Riefkohl R, Scott Levin L, editors. Plastic maxillofacial and reconstructive surgery, 3rd edn. Baltimore: Williams & Wilkins, 1997:203. [Google Scholar]
- 16. Raff T, Hartman B, Wagner H, German G. Experience with modified Meek technique. Burns 1996;38:142–6. [PubMed] [Google Scholar]
- 17. Kreis RW, Mackie DP, Hermans RP, Vloemans AR. Expansion technique for skin grafts: comparison between mesh and Meek island (Sandwich) grafts. Burns 1994;20:539–42. [DOI] [PubMed] [Google Scholar]
- 18. Zermani RGC, Zarabini A, Trivisonno A. Micrografting in the treatment of severely burned patients. Burns 1997;23:604–7. [DOI] [PubMed] [Google Scholar]
- 19. Lari AR, Gang RK. Expansion technique for skin grafts (Meek technique) in the treatment of severely burned patients. Burns 2001;27:61–6. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh CS, Schuong JY, Huang WS, Huang TT. Five years' experience of the modified Meek technique in the management of extensive burns. Burns 2008;34:350–4. [DOI] [PubMed] [Google Scholar]
- 21. Lumenta DB, Kamoltz LP, Frey M. Adult burn patients with more than 60% TBSA involved Meek and other techniques to overcome restricted skin harvest availability the Viennese concept. J Burn Care Res 2009;30:231–42. [DOI] [PubMed] [Google Scholar]


