Abstract
The aim of this study is to determine the predictors for reulceration, reamputation and mortality in patients with diabetes following toe amputation, and the impact of activities of daily living on clinical outcomes.
This prospective cohort study included 245 patients who had undergone toe amputation (202 healing and 43 non‐healing) and was followed for a 5‐year period. Data regarding new foot ulceration, reamputation and mortality were recorded, and the patients' activities of daily living were evaluated.
The rate of wound healing was 82·4%. The rate of follow‐up in the healed group was 91·6%. In years 1, 3 and 5, the cumulative incidence of patients who developed a new foot ulcer was 27·3%, 57·2% and 76·4%, respectively, leading to reamputation in 12·5%, 22·3% and 47·1%, respectively. The cumulative mortality was 5·8%, 15·1% and 32·7% at 1, 3 and 5 years, respectively. Multivariate analysis showed that GHbA1c > 9% (75 mmol/mol) was identified as an independent predictor of impaired wound healing, reulceration and reamputation. An age of >70 years was identified as an independent predictor of reamputation, mortality and impairment of activities of daily living.
Despite a satisfactory initial healing rate after the first toe amputation, with the extension course after the toe amputation, the long‐term outcomes are not optimistic. In developing countries like China, taking measures to prevent reulceration and reamputation is very important for patients with diabetic foot minor amputations, especially following toe amputation.
Keywords: Diabetic foot, Mortality, Toe amputation, Ulcer
Key Message.
the aim of our study was to determine the predictors for reulceration, reamputation and mortality in diabetic patients following toe amputation, and the impact of activities of daily living on clinical outcomes
Introduction
A diabetic foot ulcer (DFU) is the most common cause of non‐traumatic lower‐extremity amputations (LEAs) associated with diabetes. It not only causes great physical and mental pain in the patients, but is also a considerable financial burden on the patients' families and society as a whole. Toe amputation has the highest incidence among diabetic LEAs 1. Many epidemiological reports have published data regarding the incidences of amputation and mortality after LEAs 2, 3; however, the relative outcomes and the activities of daily living after amputations in diabetic patients have rarely been studied. In this prospective cohort study, we aimed to observe the outcomes in diabetic patients who had undergone toe amputation over a 5‐year follow‐up period, and determine the related factors influencing these outcomes. In particular, we considered the effect of the activities of daily living on these outcomes.
Materials and methods
Subjects
A total of 245 patients with type 2 diabetes and DFU who had undergone toe amputation in our department during the period from January 2003 to December 2007 were included in our study. The patients underwent comprehensive medical treatment and surgical intervention in our department by a multidisciplinary team, which included physicians, diabetes specialist physicians and nurses and surgeons. All subjects signed a consent form before surgery and gave written informed consent prior to study participation. The surgeries were reported to the medical department and the study was approved by the local ethics committee of Tianjin Medical University.
Methods
A total of 245 who underwent toe amputation were included in this study, Follow‐up data were collected using the pre‐designed patient hospital registration form, and recorded and analysed sequentially based on the order of patient admission. Data collected included demographic information, complications due to the patients' diabetes, treatment and primary wound healing. Of these patients, 202 patients who exhibited healing following toe amputation were followed until March 2011 via a telephone interview or a specifically designed questionnaire every 3 months. The patients were asked about new ulceration, reamputation, the date and cause of mortality and their activities of daily living.
The inclusion criteria were as follows: patients with diagnoses of type 2 diabetes mellitus and diabetic foot, who had consented to receive toe amputation surgery (operation methods shown in Table 1).
Table 1.
Operation method and number of cases
| Operation method | n (%) |
|---|---|
| Single toe ray amputation | 30 (12·2%) |
| Single toe amputation | 22 (9·0%) |
| Multiple toe ray amputations | 51 (20·8%) |
| Multiple toe amputations | 23 (9·4%) |
| Great toe amputation | 27 (11·0%) |
| Great toe ray amputation | 64 (26·1%) |
| Metatarsophalangeal joint toe amputation | 28 (11·4%) |
| Total | 245 |
The exclusion criteria were as follows: patients with type 1 diabetes with diabetic foot, residence outside the study region at the time of toe amputation (according to the hospital population register), a level of amputation proximal to the metatarsophalangeal joint and amputation performed for other reasons, such as trauma or tumour.
Peripheral arterial disease (PAD) was identified based on the following criteria: (i) a non‐palpable foot pulse (dorsalis pedis or posterior tibial arterial pulse); (ii) a reduction in lumen diameter of more than 50%, as determined by GE LOGIQ‐3 Color Ultrasound (General Electric Company, Fairfield, CT); (iii) an ankle/brachial pressure index (ABPI) of <0·9 (model BP‐203RPE; Nihon Colin, Tokyo, Japan). If one of the three criteria was identified, the diagnosis of peripheral vascular insufficiency was made. Critical limb ischaemia (CLI) was diagnosed when the ABPI value was <0·5 4 or there was >75% arterial stenosis
Peripheral neuropathy (PN) was diagnosed when one or more of the following criteria were met: (i) light sensory abnormalities, diagnosed with the 5·07 monofilament/10‐g Semmes‐Weinstein monofilament test 1; (ii) deep paresthesia, diagnosed based on the final measured value of 128‐Hz tuning fork tests. When the results of either of the above examinations were abnormal, PN was considered to be present.
Diabetic nephropathy (DN) was diagnosed when the 24‐hour urine radioimmunoassay method for the determination of urinary albumin excretion rate (UAER) yielded a result of >30 mg/24 h.
Diabetic foot infection was diagnosed in accordance with the PEDIS 1 grading standards recommended by the Infectious Disease Society of America (IDSA).
The definition of amputations 5 were as follows: a minor amputation was defined as any LEA distal to the ankle joint, a major amputation was defined as any LEA up to or proximal to the ankle joint and a toe amputation was defined as the level of amputation lower than the metatarsophalangeal joint including the metatarsophalangeal joint amputation. Death was defined as occurring during the perioperative period and before the end of this study.
Wound healing after amputation was defined as a continuous, viable epithelial covering over the entire previously open wound, subsequently within 2 months with no new ulcerations. Non‐healing was defined as no significant reduction in the wound size, or no significant decrease or worsening of secretions within 2 months postoperatively, with no need for major amputation.
Evaluation of activities of daily living
The evaluation of the activities of daily living was performed using Barthel Index classification 6; the grade was determined by scoring ten independent daily activities, including eating, bathing, grooming, dressing, stool control, urine control, toilet transfer, chair transfer, walking on level ground and walking up/down the stairs. The activities were scored from 0 to 100 points, a score of >60 points was classified as ‘good’ or ‘mild dysfunction’, 41 to 60 points was classified as ‘moderate dysfunction’ and <40 was classified as ‘poor, with severe dysfunction’ Based on their Barthel Index classification, patients were divided into two groups: the moderate or severe dysfunction group (≤60 points) and the no or mild dysfunction group (>60 points).
Statistical analysis
The SPSS for Windows 17.0 (SPSS, Chicago, IL) software package was used for statistical analysis. We explored the role of continuous variables using the Student's t‐test and analysis of variance (ANOVA), and the categorical variables were analysed using the Chi‐square test. Multivariate logistic regression was used to predict the factors affecting wound healing after amputation. Cox proportional hazards regression model was used to conduct a unique analysis on the factors affecting the expected outcomes (excluding the healing of patients), and covariates with P < 0·1 from the univariate analyses were used to perform multivariate analysis using the Cox model. The proportion of surviving patients was plotted on a Kaplan–Meier curve. The results of the statistical tests were considered significant at a level of P < 0·05.
Results
A total of 245 patients who had undergone toe amputation were included in this study. Of these 145 patients were male, with an average age of 69·27 ± 9·39 years (range; 38–86 years), duration of DM was from 2 weeks to 30 years, the average duration of foot ulcers before admission was 10·1 ± 15·1 weeks (3 days–15 months), in which the course was <1 week in 13% of patients, 1 week–3 months in 55%, and >3 months in 32%. Of these, 202 (82·4%) patients had healed wounds, whereas 43 (17·6%) had non‐healing wounds. The mean wound healing time for patients in the healing group was 11 ± 6 weeks (range; 3–35 weeks). Comparisons between the healing and the non‐healing patient groups are displayed in Table 2.
Table 2.
Comparison between healing group and non‐healing group after toe amputation
| Variable | Non‐healing group [n = 43 (%)] | Healing group [n = 202 (%)] | P | |
|---|---|---|---|---|
| Completed the follow‐up [n = 185 (%)] | Lost to follow‐up [n = 17 (%)] | |||
| Male | 23 | 119 | 13 | 0·143 |
| Age (years) | 67·9 ± 10·14 | 69·39 ± 9·22 | 71·24 ± 9·31 | 0·314 |
| Duration of DM (years) | 14·14 ± 6·61 | 12·49 ± 7·45 | 12·82 ± 7·40 | 0·188 |
| Duration of diabetic foot ulcer before hospitalisation (weeks) | 8·35 ± 1·19 | 7·51 ± 1·62 | 7·46 ± 1·69 | 0·137 |
| Body mass index | 24·93 ± 3·23 | 23·82 ± 2·45 | 23·72 ± 2·74 | 0·012 |
| Family history of diabetes | 20 (46·5) | 63 (34·1) | 6 (35·3) | 0·126 |
| Smoking history | 23 (53·5) | 74 (40·0) | 9 (52·9) | 0·136 |
| Drinking history | 28 (65·1) | 93 (50·3) | 10 (58·8) | 0·092 |
| Retinopathy | 26 (60·5) | 105 (56·8) | 11 (64·7) | 0·714 |
| Cerebrovascular disease | 15 (34·9) | 69 (37·3) | 6 (35·3) | 0·728 |
| Hyperlipidaemia | 20 (46·5) | 87 (47·0) | 8 (47·1) | 0·951 |
| Hypertension | 25 (58·1) | 104 (56·2) | 9 (52·9) | 0·792 |
| Chronic ischaemic heart diseases | 30 (69·8) | 125 (67·6) | 11 (64·7) | 0·756 |
| Diabetic nephropathy | 34 (79·1) | 88 (47·6) | 3 (17·6) | 0·000 |
| Peripheral neuropathy | 26 (60·5) | 136 (73·5) | 11 (64·7) | 0·108 |
| HbA1c (%) | 12·24 ± 2·12 | 9·91 ± 2·78 | 10·95 ± 2·98 | 0·000 |
| Peripheral arterial disease | 39 (90·7) | 158 (85·4) | 14 (82·4) | 0·339 |
| Critical limb ischaemia | 33 (76·7) | 78 (42·2) | 5 (29·4) | 0·000 |
| Infection | 35 (81·4) | 116 (62·7) | 13 (76·5) | 0·026 |
| Insulin therapy | 26 (60·5) | 121 (65·4) | 12 (70·6) | 0·621 |
Results for the non‐healing group
Of the 43 patients in the non‐healing group, 23 (53·5%) underwent a subsequent major amputation, 11 (25·6%) died and 9 (20·9%) ended treatment because of financial burden or individual cognition. Based on the results of univariate analysis, body mass index (BMI), DN, GHbA1c, CLI and infection were identified as factors affecting wound healing (P < 0·05; Table 2). Based on the results of multivariate logistic regression, DN (OR, 3·86; 95% CI, 1·65–9·03; χ2 = 9·71; P < 0·01), CLI (OR, 5·60; 95% CI, 2·41–12·98; χ2 = 16·10; P < 0·01) and GHbA1c > 9% (>75 mmol/mol; χ2 = 11·95; P < 0·01) were identified as independent risk factors for impaired wound healing.
Results for the healing group
In the 202 patients who had healed after toe amputation, 185 (91·6%) were assessed to the end of the follow‐up period, whereas 17 (8·4%) were lost to follow‐up. The mean duration of follow‐up was 5·4 ± 0·9 years (range, 3·2–7·7 years). The baseline characteristics of our study population are reported in Table 2.
New ulceration
Among the 185 healed subjects who completed the follow‐up, there were 143 (77·3%; 143/185) episodes of new ulceration and 81 subjects (43·8%; 81/185) developed new ulcerations two or more times. It was noted that ipsilateral ulcerations (67·8%; 97/143) occurred more often than contralateral ulcerations (32·2%; 46/143) in Table 3. The cumulative incidence of new ulceration was 27·3%, 57·2% and 76·4% at 1, 3 and 5 years, respectively (Figure 1). Patients in the healed group remained free of new ulceration for an average of 2·7 years. The following predictable risk factors for reulceration were identified: PN (HR, 1·69; 95% CI, 1·18–2·42; χ2 = 8·27; P < 0·01), DN (HR, 1·53; 95% CI, 1·10–2·13; χ2 = 6·31; P < 0·05), longer duration of DM (HR, 1·03; 95% CI, 1·01–1·06; χ2 = 7·18; P < 0·01), amputation of the great toe (HR, 1·67; 95% CI, 1·19–2·36; χ2 = 8·57; P < 0·01), BMI (HR, 1·09; 95% CI, 1·01–1·17; χ2 = 5·44; P < 0·05) and GHbA1c > 9% (>75 mmol/mol; HR, 1·12; 95% CI, 1·06–1·18; χ2 = 15·01; P < 0·01). Based on the results of multivariate logistic regression, ischaemia (HR, 1·68; 95% CI, 1·17–2·43; χ2 = 7·75; P < 0·01), amputation of the great toe (HR, 1·44; 95% CI, 1·01–2·06; χ2 = 4·13; P < 0·05) and GHbA1c > 9% (>75 mmol/mol; HR, 1·08; 95% CI, 1·02–1·15; χ2 = 6·47; P < 0·05) were identified as independent risk factors for reulceration.
Table 3.
Characteristics of cases that were followed up
| Cases (%) | |
|---|---|
| n | 185 |
| Reulceration | 143 (77·3) |
| Ipsilateral ulceration | 97 (67·8) |
| Contralateral ulceration | 46 (32·2) |
| Amputation | 90 (48·6) |
| Ipsilateral minor amputation | 38 (20·5) |
| Contralateral minor amputation | 16 (8·6) |
| Ipsilateral major amputation AK | 7 (3·8) |
| Contralateral major amputation AK | 3 (1·6) |
| Ipsilateral major amputation BK | 17 (9·2) |
| Contralateral major amputation BK | 9 (4·9) |
| Death | 70 (37·8) |
| Barthel Index classification score ≤ 60 points) | 59 (31·9) |
| No ulcer or amputation, and Barthel Index classification score > 60 points | 37 (20·0) |
AK, above the knee; BK, below the knee.
Figure 1.
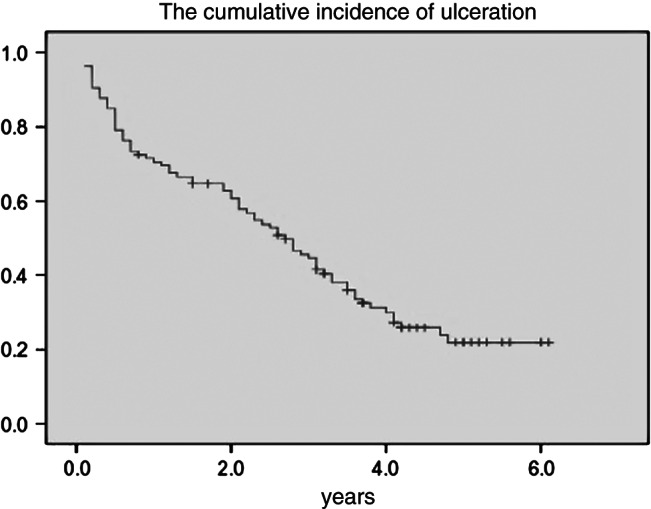
Kaplan–Meier curve. The y‐axis represents the cumulative incidence of ulceration.
Incidence of reamputation
Of the 185 healed subjects who completed the follow‐up, 54 (29·2%) had subsequent minor amputations and 36 (19·5%) had major amputations. The overall incidence of amputation was 48·6%. Of the 90 amputations (Table 3), 77 (85·5%; 77/90) were due to new ulceration. Ipsilateral reamputation (33·5%; 62/185) occurred more often than contralateral (15·1%; 28/185). Among patients who had minor amputations, 25 subjects (13·5%) had minor amputations two or more times. Twenty‐five subjects (13·5%) underwent major amputation, which was performed because of a non‐healing wound following a minor amputation. The cumulative incidence of reamputation was 17·5%, 22·3% and 47·1% at 1, 3 and 5 years, respectively (Figure 2). The median duration for which the subjects remained free of subsequent amputation was 3·2 years. Based on the results of univariate analysis, the male gender (HR, 1·79; 95% CI, 1·10–2·89; χ 2 = 5·57; P < 0·01), ischaemia (HR, 1·65; 95% CI, 1·05–2·57; χ2 = 4·79; P < 0·05), an age of >70 years (HR, 1·07; 95% CI, 1·04–1·10; χ2 = 18·72; P < 0·01) and GHbA1c > 9% (>75 mmol/mol; HR, 1·20; 95% CI, 1·11–1·29; χ2 = 22·35; P < 0·01) were predictors for reamputation. Based on the results of multivariate analysis, the male gender (HR, 1·83; 95% CI, 1·11–3·03; χ2 = 5·54; P < 0·05), age of >70 years (HR, 1·03; 95% CI, 1·00–1·06; χ2 = 3·93; P < 0·05) and GHbA1c > 9% (>75 mmol/mol; HR, 1·23; 95% CI, 1·15–1·33; χ2 = 30·64; P < 0·01) were identified as independent risk factors for reamputation.
Figure 2.
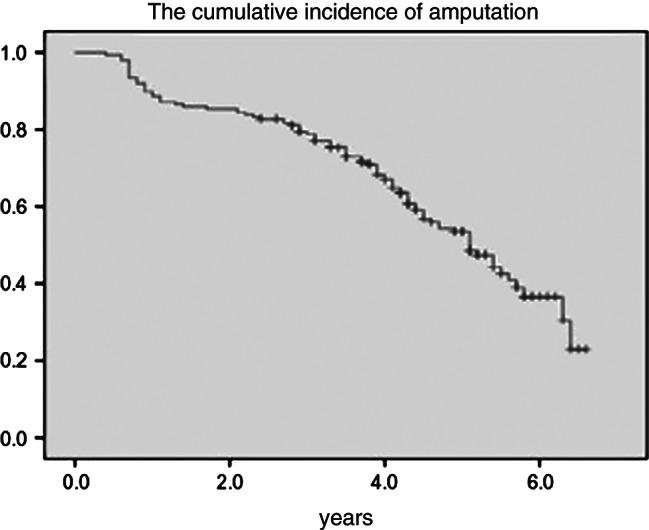
Kaplan–Meier curve. The y‐axis represents the cumulative incidence of amputation.
Evaluation of the activities of daily living
Of the 185 subjects who completed the follow‐up, 59 (31·9%) were classified as having moderate or severe dysfunction and 126 (68·1%) were classified as having no or mild dysfunction of the activities of daily living. At the end of the follow‐up period, 46 (77·9%) subjects in the moderate or severe dysfunction group had undergone reamputation (24 major and 22 minor amputations) and 32 patients (54·2%; 32/59) died. In the group with no or mild dysfunction, 44 (34·9%; 44/126) subjects had undergone reamputation (12 major and 32 minor amputation) and 38 patients (30·2%; 38/126) died. The incidence of reamputation in the moderate or severe dysfunction group was greater than the incidence in the group with no or mild dysfunction (χ2 = 29·8; P < 0·01; χ2 = 24·9; P < 0·01). Based on the results of univariate analysis, DN (HR, 1·74; 95% CI, 1·03–2·94; χ2 = 4·30; P < 0·05), coronary heart disease (CHD; HR, 1·93; 95% CI, 0·39–0·99; χ2 = 4·01; P < 0·05) and age of >70 years (HR, 1·08; 95% CI, 1·03–1·13; χ2 = 11·97; P < 0·01) were identified as factors that affected the activities of daily living. Based on the results of multivariate analysis, an age of >70 years (HR, 1·06; 95% CI, 1·01–1·11; χ2 = 5·95; P < 0·05) was identified as an independent factor that affected the activities of daily living.
Causes of death
Of the 185 subjects who completed the follow‐up, a total of 70 (37·8%) subjects died. The causes of death included the following: 18 (25·7%) foot‐related deaths, 16 (22·9%) deaths due to renal failure, 13 (18·6%) deaths due to heart failure, 12 (17·1%) cancer‐related deaths, 5 (7·1%) deaths due to cerebrovascular disease (CVD), 3 (4·3%) deaths due to lung infection and 3 (4·3%) deaths with unknown causes. The average survival time of the deceased patients was 3·8 years (range, 3·4–4·1 years). The mean survival time of male patients was 3·3 years (range, 2·7–3·9 years), whereas the mean survival time of female patients was 4·1 years (range, 3·6–4·6 years). At the end of follow‐up period, the mean survival time of the 115 patients who had not died was 5·3 years (range, 5·1–5·4 years). The cumulative mortality of the subjects was 5·8%, 15·1% and 32·7% at 1, 3 and 5 years, respectively (Figure 3). Based on the results of univariate analysis, an age of >70 years (HR, 1·15; 95% CI, 1·10–1·20; χ2 = 43·42; P < 0·01), hypertension (HR, 2·23; 95% CI, 1·34–3·71; χ2 = 9·53; P < 0·01), DN (HR, 2·57; 95% CI, 1·47–4·51; χ2 = 10·92; P < 0·01) and CVD (HR, 1·85; 95% CI, 1·15–2·96; χ2 = 6·54; P < 0·05) were identified as predictors for death. Based on the results of multivariate analysis, an age of >70 years (HR, 1·12; 95% CI, 1·07–1·17; χ2 = 22·10; P < 0·01) and DN (HR, 2·31; 95% CI, 1·24–4·30; χ2 = 6·92; P < 0·01) were identified as independent predictors for death.
Figure 3.
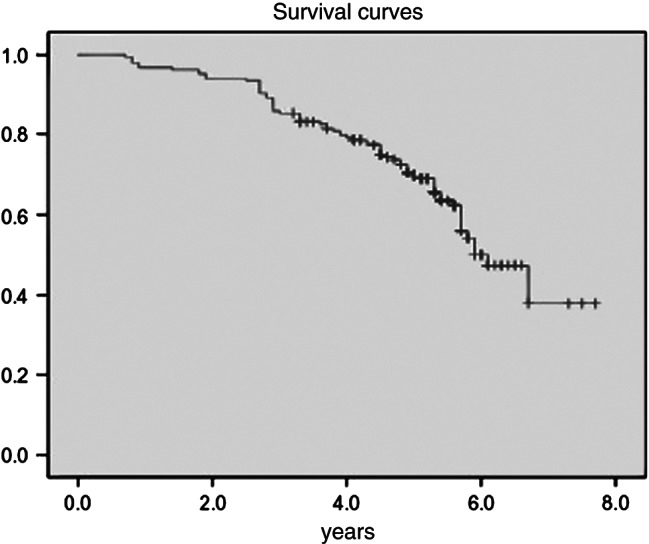
Kaplan–Meier curve. The y‐axis represents the cumulative incidence of mortality.
Discussion
Annually, approximately 2–6% of all diabetic patients exhibit poor healing of wounds and frequently develops chronic lesions on the feet that are associated with a high risk of minor or major amputation, especially if treated inadequately 7. Patients with amputations have a high incidence of reulceration, reamputation and mortality 8. There are relatively numerous studies discussing the outcomes of major amputations in patients with diabetic foot; however, studies that report the outcomes after toe amputation are rare. In this study, toe amputations accounted for 64·1% of amputations in diabetic foot patients. The diabetic foot patients who had undergone toe amputation were compared with patients who had undergone other amputations, and it was noted that the toe amputation patients had a less affected quality of life and higher survival rates. Through follow‐up of diabetic foot patients after toe amputation, we came to know about the predictors for reulceration, reamputation and mortality, and the factors that affected the activities of daily living. Activities of daily living were found to have a significant effect on the prevention of reulceration and reamputation, thus improving quality of life and prolonging survival time, while also guiding preventative measures and treatment.
This study showed that the healing rate of toe amputations in Tianjin, China was 82·4%, which was noted to be higher when compared with related foreign studies 9. In factors affecting outcomes compared with other studies, some differences may be related to the severity of patients enrolled, the number of samples and the regional differences etc. In this study, DN, CLI and GHbA1c > 9% (>75 mmol/mol) were identified as independent risk factors for impaired wound healing. In this study, the incidences of ischaemia and ischaemia associated with neuropathy were 85·4% and 42·2%, respectively. As reported in the literature, obesity, atherosclerotic disease, high smoking rates and old age were risk factors for ischaemic lesions 10. With the exception of old age, the other risk factors varied between the studies. Early intervention with lifestyle changes, smoking cessation, a reasonable diet and the maintenance of a normal body weight in patients with diabetes may prevent the occurrence of atherosclerosis. Reducing the occurrence of atherosclerosis can decrease the incidence of peripheral arterial lesions and the degree of ischaemia, which will facilitate the healing of ulcers.
Reulceration rates of 77·3% were noted in this study. This was most likely due to the fact that, while patients were still able to walk following toe amputation, the bone structure and the distribution of the pressure on the plantar region had been altered. The reulceration rates showed in this study were higher than those reported in the literature 11. Ischaemia, amputation of the great toe and GHbA1c > 9% (>75 mmol/mol) were identified as independent risk factors for reulcerations. When analysing the causes of reulcerations, it should be noted that in developing countries, medical resources are relatively limited and investment funds for postoperative rehabilitation are not sufficient. Furthermore, China currently has almost no specialised factories that produce therapeutic footwear and most patients in need of therapeutic shoes need to import them from abroad. In this study, almost no patients were willing to undergo treatment with therapeutic footwear (which is relatively expensive and not covered by medical insurance), thus leading to poor wound healing after toe amputation and the recurrence of ulcers. As reported in the literature, therapeutic footwear can evenly distribute plantar pressure in patients who have undergone toe amputation, reducing the rates of reulceration 12.
A diabetic foot can lead to a lower limb amputation. A comparison of amputation rates among different studies showed great variability, which might be associated with the definition of amputation and the selection of a study population that may not be entirely representative of the general population. Distinctions should be made between major and minor amputations, as the causes, related costs and functional impairment differ between the two types. In this study, the overall reamputation rate was 48·6% and the 5‐year cumulative amputation rate was 47·1%. The amputation rate noted in this study was lower than those reported in some studies 13, 14, but higher than those in certain other studies 2, 15. Overall, approximately 20% of patients with toe amputation undergo a major amputation after 5 years 1, 3. The rate of major amputation in this study was 19·5%, which is comparable to the overall rate. There are many factors influencing the need for amputation. In addition to the severity of disease, complications and patient factors, the social health care system is also involved, including availability of primary care, the quality of primary care, the number of experts and quality and current medical concepts. The high amputation rate reflects a high incidence of DFU, limited resources and treatment that is not administered by an expert team.
Toe amputation is a process and not a disease. Assessment of the patients' activities of daily living after toe amputation is important, but has been rarely reported in the literature 16. The results of this study showed that in patients with good scores for activities of daily living, the reamputation and major amputation rates were lower. The Taylor study pointed out 17 that not only should wound healing and survival rates be monitored after amputation, but that it was also important to observe toe function. Performing postoperative exercises offered a 20% protective effect. In elderly patients, who cannot move well and often have a variety of complications, the amputations serve as treatment for the symptoms, and not the disease. However, this does not improve the quality of their life, and reamputation and mortality rates remain high.
Studies reporting mortality rates following amputations are rare 13, 18. This study noted that complications and comorbidities accounted for a large proportion of the causes of death. In this study, old age and DN were identified as independent risk factors for death.
In this study, GHbA1c > 9% (>75 mmol/mol) was identified as a factor affecting wound healing and was an independent risk factor for predicting reulceration and reamputation. The results of the Wisconsin epidemiological studies 19 and the UKPDS studies 20 support our findings. As the level of GHbA1c decreases, the risk of developing diabetes complications also decreases. The Steno‐2 study 21 further proposed that a strict multifactorial intervention could decrease the microvascular risk by half and significantly reduce the risk of non‐fatal CHD. Therefore, good blood glucose control is essential.
China's population is ageing. Old age (>70 years) was identified as an independent risk factor affecting postoperative activities of daily living and was found to be a predictor of mortality after toe amputation. A German study suggested that the risk of amputation of patients who were >80 years was five times that of patients aged 40–59 years. Two‐thirds of all amputees were older than 60 years. In this study, the mean age of patients was 69·4 ± 9·2 years. Elderly patients often experience macro‐ and microvascular complications, as well as many cardiovascular and cerebrovascular complications. This study showed that the age and the preoperative function were very important for postoperative results 22. The mortality rate of old patients (age > 70 years) was three times higher than that of younger patients (age < 50 years).
In conclusion, despite a satisfactory initial healing rate after toe amputation, the bone structure and the distribution of the pressure on the plantar region had been altered and other risks were presented, with the extension of the duration after toe amputation, the long‐term outcomes are not optimistic. The cumulative incidence of postoperative reulceration and reamputation and the risk of death increased year by year. In developing countries like China, taking measures to prevent reulceration and reamputation is very important for patients with diabetic foot minor amputations, especially following toe amputation.
Author contribution
P‐HW initiated the study, developed the study protocol, wrote the manuscript and is the guarantor for the article. Y‐JC performed data analysis and provided clinical expertise. X‐WL and JX performed the statistical analysis and developed the study protocol. H‐JS performed data analysis. MD and SF provided statistical expertise. JJ and X‐YJ developed the study protocol and performed data analysis. All the authors commented on the manuscript drafts.
Acknowledgements
This research was supported by Tianjin Natural Science Foundation of China (10JCZDJC19800), Tianjin Medical University Science Foundation (2008KY17) and Tianjin Health Bureau Technology Fund (09KZ87). No potential conflicts of interest relevant to this article were reported.
References
- 1. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV. Diabetic foot disorders: a clinical practice guideline. J Foot Ankle Surg 2006;45(5 Suppl):S1–66. [DOI] [PubMed] [Google Scholar]
- 2. Uzzaman MM, Jukaku S, Kambal A, Hussain ST. Assessing the long‐term outcomes of minor lower limb amputations: a 5‐year study. Angiology 2011;62:365–71. [DOI] [PubMed] [Google Scholar]
- 3. Wong YS, Lee JC, Yu CS, Low BY. Results of minor foot amputations in diabetic mellitus. Singapore Med J 1996;37:604–6. [PubMed] [Google Scholar]
- 4. Coutinho T, Rooke TW, Kullo IJ. Arterial dysfunction and functional performance in patients with peripheral artery disease: a review. Vasc Med 2011;16:203–11. [DOI] [PubMed] [Google Scholar]
- 5. Houtum WV, Rauwerda JA, Ruwaard D, Schaper NC, Bakker K. Reduction in diabetes‐related lower‐extremity amputations in the Netherlands 1991–2000. Diabetes Care 2004;27:1042–6. [DOI] [PubMed] [Google Scholar]
- 6. Mahoey FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 7. Wolf G, Müller N, Busch M, Eidner G, Kloos C, Hunger‐Battefeld W. Diabetic foot syndrome and renal function in type 1 and type 2 diabetes mellitus show close association. Nephrol Dial Transplant 2009;24:1896–901. [DOI] [PubMed] [Google Scholar]
- 8. Schofield CJ, Libby G, Brennan GM, MacAlpine RR, Morris AD, Leese GP. Mortality and hospitalization inpatients after amputation: a comparison between patients with and without diabetes. Diabetes Care 2006;29:2252–6. [DOI] [PubMed] [Google Scholar]
- 9. Svensson H, Apelqvist J, Larsson J, Lindholm E, Eneroth M. Minor amputation inpatients with diabetes mellitus and severe foot ulcers achieves good outcomes. J Wound Care 2011;20 261–262, 264, 266 passim. [DOI] [PubMed] [Google Scholar]
- 10. Rezende KF, Nunes MA, Melo NH, Malerbi D, Chacra AR, Ferraz MB. In hospital care for diabetic foot: a comparison between the estimated cost and the SUS reimbursement. Arq Bras Endocrinol Metabol 2008;52:523–30. [DOI] [PubMed] [Google Scholar]
- 11. Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from1990 to 1993: a 6.5‐year follow‐up. Diabetes Care 2001;24:78–83. [DOI] [PubMed] [Google Scholar]
- 12. Viswanathan V, Madhavavan S, Gnanasundaram S, Gopalakrishna G, Das BN, Rajasekar S, Ramachandran A. Effectiveness of different types of footwear in soles for the diabetic neuropathic foot. Diabetes Care 2004;27:474–7. [DOI] [PubMed] [Google Scholar]
- 13. Izumi Y, Satterfield K, Lee S, Harkless LB. Risk of reamputation in diabetic patients stratified by limb and level of amputation: a 10‐year observation. Diabetes Care 2006;29:566–70. [DOI] [PubMed] [Google Scholar]
- 14. Jones RN, Marshall WP. Does the proximity of an amputation, length of time between foot ulcer development and amputation, or glycemic control at the time of amputation affect the mortality rate of people with diabetes who undergo an amputation? Adv Skin Wound Care 2008;21:118–23. [DOI] [PubMed] [Google Scholar]
- 15. Pscherer S, Dippel FW, Lauterbach S, Kostev K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real‐life conditions in Germany. Prim Care Diabetes 2012;6:241–6. [DOI] [PubMed] [Google Scholar]
- 16. Van Damme H, Rorive M, Martens De Noorthout BM, Quaniers J, Scheen A, Limet R. Amputations in diabetic patients: a plea for foot sparing surgery. Acta Chir Belg 2001;101:123–9. [PubMed] [Google Scholar]
- 17. Ghanassia E, Villon L, Thuan Dit Dieudonné JF, Boegner C, Avignon A, Sultan A. Longterm outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5‐year follow‐up study. Diabetes Care 2008;31:1288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, Robertson RT, Langan EM 3rd, York JW, Carsten CG 3rd, Snyder BA, Jackson MR, Youkey JR. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg 2005;42:227–35. [DOI] [PubMed] [Google Scholar]
- 19. Hambleton IR, Jonnalagadda R, Davis CR, Fraser HS, Chaturvedi N, Hennis AJ. All‐cause mortality after diabetes‐related amputation in Barbados: a prospective case–control study. Diabetes Care 2009;32:306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moss SE, Klein R, Klein BE. The 14‐year incidence of lower‐extremity amputations in a diabetic population: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 1999;22:951–99. [DOI] [PubMed] [Google Scholar]
- 21. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91. [DOI] [PubMed] [Google Scholar]


