Abstract
Special support surfaces are key in pressure ulcer prevention. The aim of this study was to measure the effects of 3 different types of mattresses (reactive gel, active alternating air, basic foam) on skin properties of the sacral and heel skin after 2 hours loading. Fifteen healthy females (median age 66 years) were included. Transepidermal water loss, skin surface temperature, erythema, stratum corneum hydration, epidermal hydration, skin extensibility, elastic function, and recovery as well as skin roughness parameters were measured under controlled room conditions before loading, immediately after loading, and 20 minutes post‐loading in the supine position on the different mattresses. The highest increases in transepidermal water loss, skin temperature, and erythema were observed for the foam mattress after loading, indicating higher deformation and occlusion. Cutaneous stiffness decreased in all 3 groups, indicating structural changes during loading. There was a substantial decrease of mean roughness at the heel skin in the foam group, leading to a flattening of the skin surface. Study results indicate that the type of support surface influences skin structure and function during loading. The gel and air mattress appeared to be more protective compared with the foam mattress, but the differences between the gel and air were minor.
Keywords: microclimate, pressure ulcer, stratum corneum hydration, support surfaces, transepidermal water loss
1. INTRODUCTION
Pressure ulcers (PUs) are localised injuries to the skin and/or underlying tissue, usually near bony prominences.1 In adults in the supine position, the lateral areas of the heel and the sacral area are most often affected (PU predilection sites). PUs are considered to be largely avoidable complications and are painful and costly chronic wounds with a significant impact on quality of life.2 The estimated average prevalence in acute care setting worldwide ranges from 7.8%3 to 54%4 and in nursing homes from 2.5%5 to 29.2%.6
The primary cause of PUs is prolonged skin and soft tissue deformation due to mechanical loading.1, 7 One main pathological pathway includes the (partial) occlusion of blood vessels, leading to reduced perfusion and ischaemia and, in the case of reperfusion, to additional damage. Another pathway of PU development is direct deformation injury.7 When soft tissue deformation exceeds the resistance of cells, they die. Because muscle and subcutaneous fat are considered more susceptible to both ischaemia and direct deformation injury, PU development typically starts in deeper soft tissues under intact skin, which is also called bottom‐up or inside‐out pathway.7, 8, 9
In recent years, the concept of “microclimate” also received increasing attention in PU prevention research.10 It is defined as the temperature, humidity, and air flow on and near the skin surface. The parameters skin temperature and humidity are closely related to functional and mechanical skin properties and to the susceptibility to PU development. For instance, higher skin temperature leads to an increase of cutaneous stiffness (under loading) and metabolic demand and a decrease of dermo‐epidermal adhesion, leading to higher susceptibility to deformation injury and cutaneous irritation.11, 12, 13 Increased humidity near and on the skin surface increases the stratum corneum hydration (SCH) and is associated with an increased coefficient of friction between the skin and support surface and, therefore, may increase the risk of shear damage.14, 15
Support surfaces are key interventions for PU prevention. They may be broadly classified into active (eg, alternating mattresses) and reactive (eg, special foams) systems.1 Reactive support surfaces are powered (requiring or using external sources of energy to operate) or non‐powered support surfaces that are able to change their load distribution properties in response to applied load. They allow immersion and envelopment and distribute loads over larger areas, reducing the magnitude of strains. Active support surfaces are powered, which generate alternating high and low interface pressures, leading to the temporary recovery of loaded soft tissues that bear or do not bear load.
PU prevention support surfaces affect the microclimate as well and, thus, the skin.10 The construction and material of the support surfaces (eg, foam vs low‐air‐loss) or the cover type are key predictors for temperature and humidity between the skin support surface interface.10, 16, 17 The interaction between skin function and fabrics (eg, bed sheets, clothing) is also well known.18, 19 It has also been previously demonstrated that prolonged loading on a standard hospital foam mattress leads to a broad range of structural and functional skin changes on loaded heel and sacral skin.20, 21 PU preventive support surfaces in general modify the degree of skin and tissue deformation and microclimate at the same time. Therefore, an effect of the type and working mechanism of a PU support surface on skin function during loading is highly likely. However, direct comparisons of different support surface designs and characteristics of skin responses due to loading have not been performed so far. Therefore, the aim of this exploratory study was to compare the effects of 3 different support surfaces on the structure and function of the most important PU predilection areas, heel and sacral skin, after loading.
2. METHODS
2.1. Trial design
A randomised, controlled, explorative clinical study with cross‐over design was conducted. Each of the n = 15 subjects was invited to consecutively lie, according to a standardised procedure, in supine position on 3 different support surfaces. The study was approved by the Ethics Committee of the Charité‐Universitätsmedizin Berlin (EA1/270/15). The trial was registered prior to study initiation on 26·09·2016 at clinicaltrial.gov (NCT 02930590).
2.2. Participants and settings
The study took place at the Department of Dermatology and Allergy, Charité‐Universitätsmedizin Berlin, Germany, from September 2016 to March 2017. Eligibility criteria were: female gender, age 60 to 80 years, BMI 18.5 to 29.9 kg/m2, skin phototype I to III according to Fitzpatrick classification,22, 23 non‐smoker of at least 1 year, absence of skin diseases or scars in the skin areas of interest, ability to move independently and to maintain supine and prone positions, and no use of cosmetic products or topical drugs at the study areas at least 12 hours before measurement.
2.3. Interventions
Upon receiving written informed consent, subjects were randomly allocated to 1 of 6 study groups. There was a predefined order of the 3 interventions per group. On the day of the examinations, the subjects were acclimatised to standardised room conditions (40%–60% relative humidity and a temperature of 20°C–22°C) for 30 minutes. These room conditions are widely recommended to perform comparable skin measurements.24, 25 The investigational skin areas (sacrum, right lateral heel) were marked using a skin marker and a template and remained uncovered during that time. These areas were chosen because these are the most important PU predilection areas among adults at risk of PU in the supine position. The right lateral heel was selected because empirical evidence suggests symmetry between contralateral body parts. After the acclimatisation period, the baseline values were measured on the right heel and sacral area while the subjects lay in the prone position. Subjects then turned on their back and stayed in the supine position for 2 hours. During this period, the skin of the investigational areas was in direct contact with the cotton bed sheet, which was on top of the mattresses. During the loading period, subjects were requested not to move their legs or body. They were allowed to sleep, to listen to music, or to move their arms for reading. A study assistant was present all the time to ensure compliance and safety. After 2 hours, subjects returned to the prone position, and the heel and sacral skin areas were measured immediately and again 20 minutes later.
All study procedures and measurements were conducted in the morning at the same time to minimise possible circadian influences. The subject's blood pressure, heart rate, and body temperature were recorded at the beginning of each visit and after the 2‐hour loading period. The performance of the study procedures was identical at all 3 visits, and only the support surface changed. There was at least 1 day between the measurements to prevent any possible carry‐over effects.
2.4. Support surfaces
Three different support surfaces were used according to the manufacturer instructions. The Stryker IsoAir System (Stryker Medical, Portage, MI, USA) was an alternating pressure mattress with low‐air‐loss function, where air is circulated underneath the top of the water vapour‐permeable cover. The coverlet material was a breathable Dartex cover (Dartex Coatings Ltd., Slaterville, RJ, USA). The system was composed of a support surface with air cells (14 cm) inside positioned every 10 cm from the head to the foot, which connects to a main control unit and pump. The pump produced a consistent air flow and provided a pressure source for inflating and deflating the air cells in the support surface every 6 minutes. The depth of the surface was 18 cm.
The gel mattress (IsoFlex LAL, Stryker Medical, Portage, MI, USA) was a reactive, non‐powered, support surface. It had a breathable cover and an open gel column design, which allows air to flow freely throughout the surface without obstruction. The support surface thickness was 15 cm.
The Basic Foam (Stryker Medical, Portage, MI, USA) was a standard hospital mattress made of foam material with a mattress depth of 12 cm. All 3 support surfaces were covered by a standard hospital cotton sheet.
2.5. Outcome measures and variables
Because this was an exploratory study, no distinction was made between primary and secondary outcomes. Each skin measurement was performed twice per skin area and time point.
2.5.1. Skin function parameters
Five non‐invasive measurements were conducted. Transepidermal water loss (TEWL) was measured with an open chamber device (Tewemeter TM 300; Courage & Khazaka electronic GmbH Cologne, Germany) and expressed in g/m2/h. According to the manufacturer, the Tewameter TM 300 (Courage & Khazaka electronic GmbH, Cologne, Germany) has an accuracy of ±0·5 g/m2/h under normal room conditions (10°C to 30°C), with TEWL‐values lower than 70 g/m2/h. Higher TEWL is regarded as an indicator for impaired skin barrier function.26
Stratum corneum hydration (SCH) was measured with the Corneometer CM 825 (Courage & Khazaka electronic GmbH) in arbitrary units (AU) (range 0‐120 AU).This parameter expresses the relative humidity in the stratum corneum. This devices measure the SCH from the skin surface to approximately 20 μm. According to the manufacturer, the accuracy is ±3%.
The MoistureMeterEpiD (Delfin Technologies Ltd. Kuopio, Finland) was used to measure the hydration of deeper epidermal and dermal skin layers up to 0·5 mm. The values are expressed in percentage of local tissue water (0‐100%).
The Mexameter MX 18 (Courage & Khazaka electronic GmbH) was used to measure erythema. This was a narrow‐band reflectance spectrophotometer. An erythema index was expressed in arbitrary units (AU) (range 0‐999). The values are the decimal logarithm of the ratio between the intensity of the reflected red (λ = 660 nm) and green (λ = 568 nm) lights (accuracy ±5%).
Skin surface temperature (°C) was measured with a skin thermometer based on the infrared technique (Courage & Khazaka electronic GmbH).
2.5.2. Skin structure parameters
The Cutometer MPA 580 and its corresponding software (Courage & Khazaka Electronic GmbH) were used to measure structural stiffness, deformability, and elasticity. The measurements were conducted with a negative pressure of −450 mbar, with a probe opening of 2 mm diameter for 2 seconds (on‐period) and a relaxation phase of 2 seconds (off‐period). The parameters skin distensibility/total deformation (Uf, mm), relative elastic recovery (Ur/Uf, %), and net elasticity (Ur/Ue, %)27 were calculated for this study. According to the manufacturer, the accuracy of this device is ±3%.
Skin surface images were taken with the Visioscan VC 98 camera (Courage & Khazaka Electronic GmbH) and its software (version 2·5·5). The measurement field was 6 x 8 mm. Estimated values were mean roughness (Rz and Ra) and maximal roughness (Rmax).
2.6. Sample size
Due to the explorative nature of this study, a formal sample size estimation was not performed. Considering the data of a previous study on a standard hospital mattress,21 15 subjects were regarded as sufficient to identify differences between the different support surface groups.
2.7. Randomisation and blinding
The allocation sequence of the participants was performed with a simple computerised random list created by a statistician not involved in the trial. Sequentially numbered, opaque, sealed envelopes containing the group assignment were prepared by the data manager, who has no involvement in any other study preparation or procedures. Envelopes were opened after confirming eligibility and provision of informed consent. Due to the nature of the intervention, the subjects, study assistants and researchers were not blinded.
2.8. Statistical methods
Sample characteristics were described using numbers and median and interquartile ranges (IQR). Structural and functional skin parameters were described using medians and interquartile ranges per group and time point. The skin surface temperature is the strongest predictor for TEWL. Therefore, in addition to the measured values, all TEWL values were adjusted to a standardised skin surface temperature of 30°C according to Mathias et al.28
Median differences measured immediately after off‐loading (after 2 hours) to baseline were calculated. Median differences between groups were compared using the Friedman test. All P‐values were considered descriptive.
3. RESULTS
3.1. Participants
In total, 20 subjects were contacted, and 15 were included between October 2016 and March 2017. All participants completed the study as allocated and planned. Baseline demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics (n = 15)
| Age, median (IQR) in years | 66.0 (63.0‐69.0) |
|---|---|
| BMI, median (IQR) in kg/m2 | 24.5 (23.7‐26.0) |
| Skin phototype | |
| I, n | 3 |
| II, n | 9 |
| III, n | 3 |
| Body temperature, median (IQR) in °C | 36.3 (36.3‐36.6) |
| Heart rate, median (IQR) in beats/min | 78 (66‐80) |
| Blood pressure, median (IQR) in mmHg | |
| Systolic | 125 (115‐140) |
| Diastolic | 75 (70‐85) |
IQR, interquartile range.
3.2. Outcomes
3.2.1. Skin function
The results of the functional parameters at the 2 skin areas, heel and sacrum, per time and mattress type are shown in Table 2. The measured baseline parameters were comparable to previous results at these skin areas.21, 29 There was an increase of median TEWL, temperature, erythema, and SCH on the sacrum as well as on the heel skin for all interventions from baseline to immediately after 2 hours loading followed by a decrease of 20 minutes after off‐loading.
Table 2.
Medians und interquartile ranges of skin function parameters on sacral and right heel skin areas (n = 15)
| Sacral skin area | Right heel | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After 2 h | After 2 h and 20 min | Difference 2 h—baseline | Baseline | After 2 h | After 2 h and 20 min | Difference 2 h—baseline | |
| TEWL in g/m 2 /h | ||||||||
| Air | 7.8 (6.7‐8.8) | 12.4 (11.5‐15.2) | 8.0 (7.0‐8.6) | 5.3 (−0.1‐6.8) | 8.1 (7.6‐10.9) | 19.5 (16.6‐25.3) | 9.6 (9.0‐11.2) | 12.3 (8.0‐17.0) |
| Gel | 7.7 (6.7‐8.2) | 10.4 (8.5‐12.2) | 7.6 (6.4‐8.6) | 3.3 (1.8‐4.3) | 9.4 (8.2‐11.6) | 19.2 (16.3‐23.3) | 10.4 (8.9‐13.3) | 9.3 (7.5‐13.1) |
| Foam | 7.1 (6.8‐8.8) | 14.5 (9.3‐20.5) | 8.0 (6.8‐9.0) | 6.2 (2.6‐12.6) | 8.6 (7.4‐12.1) | 28.4 (15.9‐32.3) | 11.1 (9.9‐14.8) | 17.6 (8.1‐20.4) |
| P‐value | — | — | — | 0.105 | — | — | — | 0.155 |
| TEWL adjusted to 30° in g/m 2 /h | ||||||||
| Air | 8.2 (7.6‐9.6) | 10.8 (10.2‐13.5) | 7.6 (7.0‐8.3) | 3.4 (−0.8‐4.9) | 13.6 (12.1‐16.2) | 29.3 (21.3‐37.6) | 14.6 (12.2‐16.2) | 12.5 (10.1‐19.4) |
| Gel | 8.1 (7.2‐9.2) | 9.6 (8.5‐11.6) | 7.7 (6.7‐8.7) | 1.5 (0.4‐2.8) | 14.5 (9.2‐16.5) | 27.2 (25.1‐33.6) | 15.2 (13.8‐19.4) | 14.3 (10.4‐21.3) |
| Foam | 8.1 (7.3‐8.9) | 12.2 (8.1‐17.6) | 7.7 (6.7‐8.3) | 4.1 (0.4‐8.6) | 16.0 (11.7‐18.1) | 34.2 (22.2‐38.1) | 16.4 (14.1‐19.6) | 16.5 (9.6‐22.7) |
| P‐value | — | — | — | 0.189 | — | — | — | 0.627 |
| Temperature in °C | ||||||||
| Air | 28.8 (28.2‐29.2) | 31.4 (31.3‐32.0) | 30.3 (29.8‐30.6) | 2.8 (2.1‐3.2) | 24.4 (24.1‐25.2) | 26.5 (25.4‐26.9) | 25.4 (24.9‐26.0) | 1.4 (0.8‐2.5) |
| Gel | 28.9 (28.3‐29.2) | 30.7 (30.5‐31.3) | 29.4 (29.2‐30.1) | 2.3 (1.8‐2.7) | 25.2 (24.3‐25.4) | 25.5 (24.7‐26.2) | 25.2 (23.8‐25.4) | 0.4 (−0.40‐0.9) |
| Foam | 29.1 (28.1‐29.8) | 32.0 (31.3‐32.2) | 30.3 (29.8‐31.1) | 3.1 (2.3‐3.8) | 24.3 (23.3‐25.4) | 26.6 (25.5‐27.3) | 25.9 (24.9‐26.6) | 1.7 (1.1‐3.4) |
| P‐value | — | — | — | 0.002 | — | — | — | < 0.001 |
| Erythema index in AU | ||||||||
| Air | 188 (164‐224) | 220 (185‐251) | 201 (178‐234) | 18 (1‐64) | 195 (132‐225) | 247 (147‐279) | 232 (146‐246) | 32 (5‐67) |
| Gel | 177 (143‐211) | 206 (153‐238) | 194 (147‐219) | 21 (4‐48) | 178 (143‐251) | 226 (160‐273) | 189 (143‐236) | 22 (8‐66) |
| Foam | 167 (151‐194) | 236 (191‐275) | 221 (198‐240) | 61 (17‐104) | 186 (117‐235) | 225 (184‐291) | 227 (144‐247) | 46 (22‐94) |
| P‐value | — | — | — | 0.074 | — | — | — | 0.319 |
| SCH in AU | ||||||||
| Air | 27.2 (19.7‐34.7) | 34.6 (25.3‐40.4) | 29.3 (25.6‐35.9) | 5.6 (2.6‐7.4) | 13.3 (8.3‐19.2) | 18.0 (13.9‐23.4) | 14.4 (10.7‐22.1) | 4.5 (1.0‐5.4) |
| Gel | 23.8 (19.5‐35.8) | 29.8 (22.0‐40.0) | 27.4 (22.7‐36.4) | 4.8 (0.0‐6.7) | 13.6 (9.3‐21.1) | 17.9 (12.5‐23.4) | 12.9 (8.3‐19.2) | 4.5 (2.7‐6.4) |
| Foam | 27.5 (25.1‐36.2) | 31.6 (26.1‐42.5) | 30.1 (23.9‐39.6) | 3.0 (−1.6‐5.8) | 12.9 (8.7‐17.8) | 20.2 (14.5‐24.6) | 15.3 (10.8‐17.8) | 5.9 (2.5‐9.3) |
| P‐value | — | — | — | 1.000 | — | — | — | 0.247 |
| Moisture in % | ||||||||
| Air | 41.0 (39.6‐43.6) | 44.5 (42.5‐48.5) | 44.5 (43.0‐46.0) | 3.0 (1.9‐5.1) | 25.5 (21.5‐29.0) | 28.0 (25.0‐32.0) | 25.5 (22.5‐30.0) | 3.0 (1.0‐5.0) |
| Gel | 40.0 (36.5‐42.0) | 41.5 (40.5‐47.5) | 42.5 (39.5‐46.5) | 4.0 (1.0‐6.5) | 25.5 (21.5‐30.0) | 25.5 (25.0‐27.5) | 25.5 (23.5‐28.0) | 2.5 (−3.0‐4.5) |
| Foam | 41.0 (39.0‐47.0) | 43.5 (41.0‐49.5) | 44.0 (41.0‐48.0) | 1.0 (−0.5‐5.0) | 24.0 (21.5‐27.5) | 28.0 (27.0‐31.5) | 25.5 (23.5‐28.5) | 3.5 (0.5‐6.5) |
| P‐value | — | — | — | 0.607 | — | — | — | 0.692 |
AU, arbitrary units; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
At the sacral skin, the highest increases of TEWL, temperature, and erythema were observed in the foam group, but median differences were comparable to the other groups except for erythema. The erythematous response was 3 times higher in the foam group (61 AU), compared with the gel and air mattresses (21 and 18 AU). Increases of SCH and epidermal hydration were slightly higher in the air and gel groups compared with the foam group, but overall differences were minor.
Similar to the sacral skin area, the highest TEWL, temperature, and erythema increases immediately after unloading were measured on the heel skin in the foam group. Temperature‐adjusted TEWL was approximately twice as high compared with baseline. Increases of SCH were also slightly higher in the foam group but comparable to the other groups and to the sacral skin.
3.2.2. Skin structure
Results of the stiffness, elasticity, and topography measurements are shown in Table 3. Baseline values were similar to previous study results,20 and heel skin was stiffer and less elastic compared with sacral skin. Immediately after loading, the maximum extensibility (Uf) was higher compared with baseline, indicating decreased stiffness. Uf decreased to baseline after 20 minutes. In parallel, there was a decrease of the elastic function (Ur/Ue) and minor changes of elastic recovery (Ur/Uf).
Table 3.
Medians and interquartile ranges of skin structure parameters on sacral and right heel skin areas (n = 15)
| Sacral skin area | Right heel | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After 2 h | After 2 h and 20 min | Difference 2 h—baseline | Baseline | After 2 h | After 2 h and 20 min | Difference 2 h—baseline | |
| Extensibility—Uf in mm | ||||||||
| Air | 0.32 (0.31‐0.35) | 0.34 (0.32‐0.40) | 0.33 (0.30‐0.38) | 0.03 (−0.01‐0.07) | 0.08 (0.07‐0.12) | 0.10 (0.08‐0.12) | 0.08 (0.07‐0.12) | 0.01 (−0.01‐0.02) |
| Gel | 0.29 (0.26‐0.33) | 0.32 (0.28‐0.37) | 0.31 (0.27‐0.35) | 0.03 (0.01‐0.05) | 0.08 (0.06‐0.10) | 0.10 (0.10‐0.12) | 0.09 (0.06‐0.11) | 0.03 (−0.01‐0.04) |
| Foam | 0.32 (0.28‐0.34) | 0.35 (0.32‐0.37) | 0.32 (0.30‐0.37) | 0.03 (0.01‐0.05) | 0.09 (0.07‐0.10) | 0.10 (0.08‐0.12) | 0.10 (0.08‐0.12) | 0.02 (−0.01‐0.04) |
| P‐value | — | — | — | 0.819 | — | — | — | 0.819 |
| Elastic function—Ur/Ue in % | ||||||||
| Air | 0.79 (0.54‐0.90) | 0.81 (0.65‐0.87) | 0.75 (0.56‐0.92) | −0.01 (−0.06‐0.04) | 0.67 (0.64‐0.77) | 0.66 (0.58‐0.79) | 0.67 (0.61‐0.79) | −0.02 (−0.10‐0.05) |
| Gel | 0.78 (0.65‐0.96) | 0.70 (0.63‐0.93) | 0.66 (0.57‐0.97) | −0.03 (−0.11‐0.03) | 0.68 (0.63‐0.98) | 0.70 (0.62‐0.83) | 0.67 (0.64‐0.74) | −0.06 (−0.20‐0.02) |
| Foam | 0.80 (0.67‐0.97) | 0.65 (0.56‐0.90) | 0.72 (0.59‐0.80) | −0.10 (−0.15‐0.02) | 0.68 (0.62‐0.85) | 0.70 (0.62‐0.81) | 0.67 (0.61‐0.75) | −0.02 (−0.17‐0.15) |
| P‐value | — | — | — | 0.282 | — | — | — | 0.766 |
| Elastic recovery—Ur/Uf in % | ||||||||
| Air | 0.55 (0.42‐0.64) | 0.55 (0.42‐0.65) | 0.52 (0.41‐0.68) | 0.01 (−0.06‐0.03) | 0.36 (0.32‐0.42) | 0.35 (0.30‐0.37) | 0.34 (0.32‐0.38) | −0.01 (−0.05‐0.01) |
| Gel | 0.52 (0.46‐0.64) | 0.50 (0.42‐0.66) | 0.45 (0.37‐0.66) | 0.01 (−0.04‐0.03) | 0.35 (0.33‐0.39) | 0.36 (0.32‐0.40) | 0.36 (0.33‐0.38) | −0.01 (−0.06‐0.04) |
| Foam | 0.57 (0.43‐0.66) | 0.44 (0.42‐0.64) | 0.51 (0.44‐0.64) | −0.03 (−0.11‐0.01) | 0.35 (0.33‐0.43) | 0.38 (0.32‐0.42) | 0.35 (0.32‐0.37) | −0.03 (−0.04‐0.06) |
| P‐value | — | — | — | 0.127 | — | — | — | 0.627 |
| Skin maximum roughness—Rmax in μm | ||||||||
| Air | 51.0 (42.5‐56.0) | 51.0 (45.5‐59.0) | 57.0 (49.5‐63.5) | 2.0 (−1.5‐8.0) | 63.5 (44.0‐93.0) | 67.5 (59.0‐83.0) | 71.5 (61.5‐94.5) | 1.5 (−10.0‐9.5) |
| Gel | 47.5 (42.8‐57.0) | 50.0 (46.0‐57.5) | 52.5 (45.5‐65.0) | 1.0 (−3.8‐9.1) | 63.8 (46.6‐79.5) | 73.5 (57.0‐100.5) | 81.5 (69.0‐110.5) | 5.3 (−3.8‐13.6) |
| Foam | 46.5 (38.5‐53.5) | 52.0 (43.5‐58.5) | 52.0 (47.5‐61.0) | 2.5 (−1.5‐8.5) | 70.0 (44.5‐91.0) | 67.5 (52.0‐93.0) | 70.0 (54.0‐81.5) | −8.0 (−11.0‐9.5) |
| P‐value | — | — | — | 0.849 | — | — | — | 0.424 |
| Mean roughness depth—Rz in μm | ||||||||
| Air | 39.5 (33.5‐41.0) | 39.0 (36.0‐43.5) | 43.0 (38.5‐50.5) | 2.5 (−2.0‐6.0) | 48.5 (32.0‐68.0) | 50.5 (43.5‐63.5) | 52.5 (46.0‐73.0) | 2.0 (−9.5‐9.5) |
| Gel | 35.5 (33.3‐44.6) | 40.0 (37.0‐43.5) | 40.5 (36.0‐49.0) | 1.8 (−2.0‐6.9) | 48.3 (36.1‐59.4) | 51.5 (45.0‐72.0) | 59.5 (53.0‐88.0) | 4.0 (−4.5‐9.1) |
| Foam | 34.0 (32.0‐41.5) | 39.5 (34.0‐44.0) | 42.5 (37.0‐46.0) | 2.5 (−2.5‐5.0) | 51.5 (34.5‐72.0) | 51.0 (39.5‐70.5) | 55.0 (40.0‐58.5) | −8.5 (−10.5‐5.5) |
| P‐value | — | — | — | 0.708 | — | — | — | 0.223 |
| Arithmetical mean roughness value—Ra in μm | ||||||||
| Air | 9.0 (8.0‐10.0) | 9.0 (8.0‐11.5) | 10.0 (8.5‐12.0) | <0.001 (−1.0‐1.0) | 9.0 (7.5‐13.5) | 10.0 (7.5‐12.0) | 10.5 (8.5‐14.0) | < 0.001 (−1.5‐1.0) |
| Gel | 8.3 (6.9‐10.1) | 9.0 (8.5‐10.0) | 9.5 (8.5‐10.0) | 0.8 (−0.6‐1.5) | 9.8 (7.9‐12.0) | 10.0 (9.0‐12.0) | 11.5 (9.5‐17.0) | −0.3 (−1.0‐1.0) |
| Foam | 8.0 (7.5‐10.0) | 8.5 (7.5‐10.0) | 9.0 (8.0‐11.0) | 1.0 (−1.0‐1.5) | 9.5 (7.0‐15.5) | 8.5 (7.5‐13.0) | 9.0 (8.0‐11.5) | −1.5 (−3.0‐0.5) |
| P‐value | — | — | — | 0.673 | — | — | — | 0.180 |
Selected skin surface images for the sacral and heel skin are shown in Figures 1 and 2, respectively. There were inter‐individual differences in terms of primary and secondary lines, skin patterns, pigmentation, and hair growth visible at the sacral skin, but there were no clear changes of skin surface topography over time (Figure 2). At the heels, there was a reduction of the height of ridges and the number and size of scales after 2 hours loading on the basic foam mattress. No clear changes were observed in the other groups (Figure 1). At the sacral skin, maximum (Rmax) and mean roughness (Rz, Ra) increased slightly and comparably from baseline to immediately after 2 hours loading in all 3 groups. In contrast, there were slight increases of Rmax and Rz at the heel skin in the air and gel groups but a median roughness reduction of 8 μm (Rmax) and 8·5 μm (Rz) in the foam group.
Figure 1.
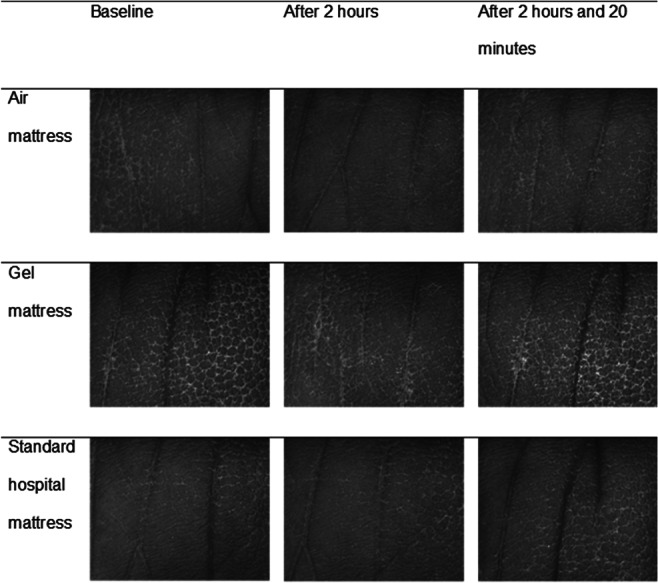
Visioscan images at heel skin per intervention and time point for 1 subject. This figure shows skin surface images of the right lateral heel skin taken with a Visioscan VC 98 camera before loading (baseline), after 2 hours loading, and 20 minutes after off‐loading per 3 different support surfaces (air, gel and standard foam)
Figure 2.
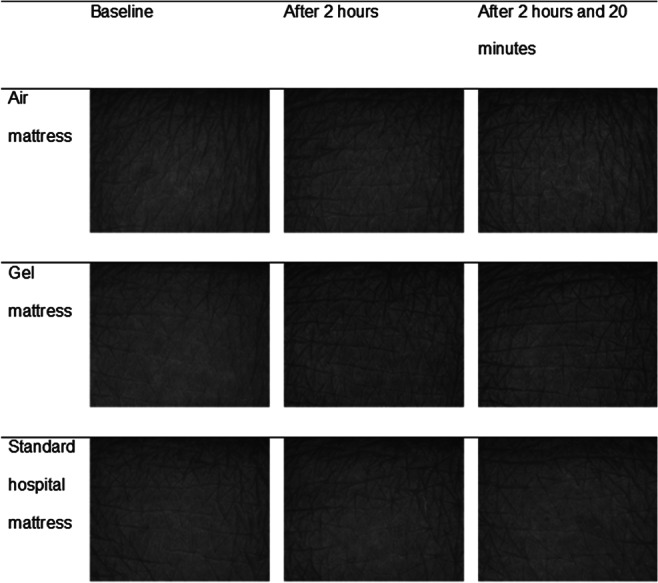
Visioscan images at sacral skin per intervention and time point for 1 subject. This figure shows skin surface images of the sacral skin taken with Visioscan VC 98 camera before loading (baseline), after 2 hours loading, and 20 minutes after off‐loading per 3 different support surfaces (air, gel, and standard foam)
4. DISCUSSION
Results of this cross‐over study indicate that 2 hours loading on support surfaces causes changes in skin function and structure of the 2 most important PU predilections areas, sacrum and heel. These changes appear to be specific for the skin area and are influenced by the type of support surface. Higher TEWL, SCH, and skin temperature immediately after 2 hours loading indicate previous occlusion. Due to the limited air convection and radiation, there was a gradual increase of skin temperature and an accumulation of water molecules in the stratum corneum. After off‐loading, the accumulated water molecules evaporate, and the temperature returns to baseline. Compared with the all 3 groups, the occlusive effect was the highest in the foam group.
Local accumulation of heat during loading is supported by previous studies,21, 30 which may be considered a risk factor for PU development.13, 31 However, results clearly indicate that the type of mattress affects the cutaneous response. Previous study results support the association between support surface characteristics and skin temperature (eg, Ref. 32). The air and gel mattresses appeared to allow better heat convection, leading to reduced skin temperature increase.
There is a direct relationship between skin surface temperature and skin perfusion regardless of the level of loading or deformation.13 The reactive hyperaemia (erythema) after release of sustained loading of the skin is the result of increased compensatory cutaneous blood flow due to previous occlusion of the blood vessels. This physiological response was the strongest for the foam probably due to the highest deformation of the soft tissue structure. Wong et al33 support our findings regarding the hyperaemic response and increased temperature after loading at the heel skin. Erythema indices measured at the sacral skin in our study returned nearly to baseline approximately 20 minutes after loading, whereas this rather fast recovery at the heel skin could be observed for the gel mattress only.
Increases of SCH and epidermal hydration provide further evidence of the occlusive effects of all 3 mattress types. Studies suggest that microstructural changes34 and an increase of the coefficient of friction14 that occur with increased moisture content may increase the risk of skin and soft tissue damage. Slightly higher increases of the hydration of the stratum corneum for the foam mattress at the heel skin indicate that this support surface was the most occlusive, but overall, accumulation of water molecules during loading appeared to be comparable in all 3 groups. Because the MoistureMeterEpiD has a higher penetration depth, results indicate that there is increased hydration of the entire thick heel stratum corneum.
The structural stiffness decreased slightly, indicating higher pliability due to loading. These results are supported by previous research20 and could be associated with the increased hydration.35 For the elastic function and the ability to recover, we observed a slight decrease for the foam mattress compared with the other 2 support surfaces, indicating possible higher structural changes due to higher deformation.
Two hours loading appears to slightly increase sacral skin roughness. Overall changes were minor and are of questionable clinical relevance. There were also minor roughness increases of the heel skin in the air and gel groups but a substantial decrease in the foam group. This finding is supported by previous results.20 The stiffer foam mattress obviously caused higher skin and soft tissue deformation at the heels compared with the much softer gel and air mattresses. Prolonged loading of heel skin on a “hard” surface appears to lead to a flattening of the skin surface, possibly leading to a higher contact area between the skin and the support surface.
It is well known that the water transport and thermo‐mechanical properties of the material of the cover17 and/or textile18 have an impact on the local water accumulation during loading. Layers of linens or underpads on mattresses could reduce the ability to transport heat and evaporate moisture.36 Because we used standard hospital cotton linens on all 3 support surfaces, a possible confounding effect was found to be similar in all 3 groups.
The effects of PU prevention support surfaces to influence microclimate can be measured using standardised testing protocols in laboratories.37 The microclimate properties humidity and temperature have been explicitly proposed as important parameters.38 Here, we provide evidence that the characteristics of support surfaces make a difference in skin responses. The standard mattress caused the highest occlusion (and deformation), whereas the gel and air mattresses showed better heat conductivity and ability to transport moisture, especially at the heel skin.
There is substantial empirical evidence that special PU preventive support surfaces are effective for PU prevention.39 Our study results support this finding. Both the reactive (gel) and active (alternating pressure) mattresses caused less skin functional and structural changes compared with the standard mattress. A major challenge in evidence‐based PU prevention is the lack of high‐quality, robust, direct, support surface comparisons in clinical research.40 Because high‐quality RCTs using PU incidence as a clinical endpoint are time consuming and financially costly, it is unlikely that this research gap will be closed in the near future. Therefore, there is a need for alternative ways of measuring susceptibility to pressure ulceration and effects of preventive measures.39, 41 Our results indicate direct relationships between biophysical skin parameters and mattress performance.
The strength of this study was the cross‐over design, which reduced inter‐individual variability. The simulation of 2 hours lying in bed was also successful to measure differences. A possible limitation was that 2 hours may be much too short to capture longer‐term effects. The subjects’ skin properties and the surface may also not have reached equilibrium within this short period.38 The sample characteristics may not be representative of the population at PU risk who may also suffer from additional systematic disease like diabetes, which is known to be an additional PU risk factor. Furthermore, we included females only to avoid a possible gender bias.
We conclude that the type of support surface has an impact on skin and soft tissue structure and function during and after sustained loading. Compared with the active and reactive support surfaces, the standard foam caused higher tissue deformation at the heels and was most occlusive for the sacral and heel skin. Therefore, patients at PU risk might benefit from specialised support surfaces.
ACKNOWLEDGEMENTS
The authors highly appreciate the support of the CRC team and the healthy volunteers.
This investigator‐initiated study was supported by Stryker European Operations BV, Amsterdam, Netherlands.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Tomova‐Simitchieva T, Lichterfeld‐Kottner A, Blume‐Peytavi U, Kottner J. Comparing the effects of 3 different pressure ulcer prevention support surfaces on the structure and function of heel and sacral skin: An exploratory cross‐over trial. Int Wound J. 2018;15:429–437. 10.1111/iwj.12883
REFERENCES
- 1. National Pressure Ulcer Advisory Panel , European Pressure Ulcer Advisory Panel , Pan Pacific Pressure Injury Alliance . In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Osborne Park: Cambridge Media; 2014. [Google Scholar]
- 2. Gorecki C, Brown JM, Nelson EA, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175‐1183. [DOI] [PubMed] [Google Scholar]
- 3. Mehta C, George JV, Mehta Y, Wangmo N. Pressure ulcer and patient characteristics–a point prevalence study in a tertiary hospital of India based on the European pressure ulcer advisory panel minimum data set. J Tissue Viability. 2015;24(3):123‐130. [DOI] [PubMed] [Google Scholar]
- 4. Moore Z, Johansen E, Etten M, et al. Pressure ulcer prevalence and prevention practices: a cross‐sectional comparative survey in Norway and Ireland. J Wound Care. 2015;24(8):333‐339. [DOI] [PubMed] [Google Scholar]
- 5. Halfens RJ, Meesterberends E, van Nie‐Visser NC, et al. International prevalence measurement of care problems: results. J Adv Nurs. 2013;69(9):e5‐17. [DOI] [PubMed] [Google Scholar]
- 6. Hahnel E, Lichterfeld A, Blume‐Peytavi U, Kottner J. The epidemiology of skin conditions in the aged: a systematic review. J Tissue Viability. 2017;26(1):20‐28. [DOI] [PubMed] [Google Scholar]
- 7. Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Ann Biomed Eng. 2015;43(2):297‐305. [DOI] [PubMed] [Google Scholar]
- 8. Berlowitz DR, Brienza DM. Are all pressure ulcers the result of deep tissue injury? A review of the literature. Ostomy Wound Manage. 2007;53(10):34‐38. [PubMed] [Google Scholar]
- 9. Kottner J, Balzer K, Dassen T, Heinze S. Pressure ulcers: a critical review of definitions and classifications. Ostomy Wound Manage. 2009;55(9):22‐29. [PubMed] [Google Scholar]
- 10. Clark M, Romanelli M, Reger S, Ranganathan V, Black J, Dealey C. Microclimate in Context. International Review Pressure Ulcer Prevention: Pressure, Shear, Friction and Microclimate in Context a Consensus Document. 2010: 19–25.
- 11. Kokate JY, Leland KJ, Held AM, et al. Temperature‐modulated pressure ulcers: a porcine model. Arch Phys Med Rehabil. 1995;76(7):666‐673. [DOI] [PubMed] [Google Scholar]
- 12. Hatje LK, Richter C, Blume‐Peytavi U, Kottner J. Blistering time as a parameter for the strength of dermoepidermal adhesion: a systematic review and meta‐analysis. Br J Dermatol. 2015;172(2):323‐330. [DOI] [PubMed] [Google Scholar]
- 13. Patel S, Knapp CF, Donofrio JC, Salcido R. Temperature effects on surface pressure‐induced changes in rat skin perfusion: implications in pressure ulcer development. J Rehabil Res Dev. 1999;36(3):189‐201. [PubMed] [Google Scholar]
- 14. Gerhardt LC, Strassle V, Lenz A, Spencer ND, Derler S. Influence of epidermal hydration on the friction of human skin against textiles. J R Soc Interface. 2008;5(28):1317‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gefen A. How do microclimate factors affect the risk for superficial pressure ulcers: a mathematical modeling study. J Tissue Viability. 2011;20(3):81‐88. [DOI] [PubMed] [Google Scholar]
- 16. Lachenbruch C. A laboratory study comparing skin temperature and fluid loss on air‐fluidized therapy, low‐air‐loss, and foam support surfaces. Ostomy Wound Manage. 2010;56(8):52‐60. [PubMed] [Google Scholar]
- 17. Posada‐Moreno P, Losa Iglesias ME, Becerro de Bengoa Vallejo R, Soriano IO, Zaragoza‐Garcia I, Martinez‐Rincon C. Influence of different bed support surface covers on skin temperature. Contemp Nurse. 2011;39(2):206‐220. [DOI] [PubMed] [Google Scholar]
- 18. Schario M, Tomova‐Simitchieva T, Lichterfeld A, et al. Effects of two different fabrics on skin barrier function under real pressure conditions. J Tissue Viability. 2017;26(2):150‐155. [DOI] [PubMed] [Google Scholar]
- 19. Hong K, Hollies N, Spivak S. Dynamic moisture vapor transfer through textiles: part I: clothing hygrometry and the influence of fiber type. Text Res J. 1988;58(12):697‐706. [Google Scholar]
- 20. Dobos G, Gefen A, Blume‐Peytavi U, Kottner J. Weight‐bearing–induced changes in the microtopography and structural stiffness of human skin in vivo following immobility periods. Wound Repair Regen. 2015;23(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 21. Kottner J, Dobos G, Andruck A, et al. Skin response to sustained loading: a clinical explorative study. J Tissue Viability. 2015;24(3):114‐122. [DOI] [PubMed] [Google Scholar]
- 22. Magin P, Pond D, Smith W, Goode S, Paterson N. Reliability of skin‐type self‐assessment: agreement of adolescents' repeated Fitzpatrick skin phototype classification ratings during a cohort study. J Eur Acad Dermatol Venereol. 2012;26(11):1396‐1399. [DOI] [PubMed] [Google Scholar]
- 23. Roberts WE. Skin type classification systems old and new. Dermatol Clin. 2009;27(4):529‐533. viii. [DOI] [PubMed] [Google Scholar]
- 24.COURAGE + KHAZAKA electronic GmbH. TM‐ The Tewameter TM 300. TM probe English. Germany, Köln; 2016/ 10 DK. p. 1–16.
- 25. Rogiers V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Ski Physiol. 2001;14(2):117‐128. [DOI] [PubMed] [Google Scholar]
- 26. Kottner J, Lichterfeld A, Blume‐Peytavi U. Transepidermal water loss in young and aged healthy humans: a systematic review and meta‐analysis. Arch Dermatol Res. 2013;305(4):315‐323. [DOI] [PubMed] [Google Scholar]
- 27. Rodrigues L. EEMCO guidance to the in vivo assessment of tensile functional properties of the skin. Part 2: instrumentation and test modes. Skin Pharmacol Appl Ski Physiol. 2001;14(1):52‐67. [DOI] [PubMed] [Google Scholar]
- 28. Mathias CG, Wilson DM, Maibach HI. Transepidermal water loss as a function of skin surface temperature. J Invest Dermatol. 1981;77(2):219‐220. [DOI] [PubMed] [Google Scholar]
- 29. Scheel‐Sailer A, Frotzler A, Mueller G, Annaheim S, Rossi RM, Derler S. Challenges to measure hydration, redness, elasticity and perfusion in the unloaded sacral region of healthy persons after supine position. J Tissue Viability. 2015;24(2):62‐70. [DOI] [PubMed] [Google Scholar]
- 30. Yusuf S, Okuwa M, Shigeta Y, et al. Microclimate and development of pressure ulcers and superficial skin changes. Int Wound J. 2015;12(1):40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sae‐Sia W, Wipke‐Tevis DD, Williams DA. Elevated sacral skin temperature (T(s)): a risk factor for pressure ulcer development in hospitalized neurologically impaired Thai patients. Appl Nurs Res. 2005;18(1):29‐35. [DOI] [PubMed] [Google Scholar]
- 32. Fisher SV, Szymke TE, Apte SY, Kosiak M. Wheelchair cushion effect on skin temperature. Arch Phys Med Rehabil. 1978;59(2):68‐72. [PubMed] [Google Scholar]
- 33. Wong V. Heel response to external pressure: is it the same in healthy community‐dwelling adults and in hip surgery patients? J Wound Ostomy Continence Nurs. 2014;41(6):539‐548. [DOI] [PubMed] [Google Scholar]
- 34. Wu KS, van Osdol WW, Dauskardt RH. Mechanical properties of human stratum corneum: effects of temperature, hydration, and chemical treatment. Biomaterials. 2006;27(5):785‐795. [DOI] [PubMed] [Google Scholar]
- 35. Dobrev H. Use of cutometer to assess epidermal hydration. Skin Res Technol. 2000;6(4):239‐244. [DOI] [PubMed] [Google Scholar]
- 36. Williamson R, Lachenbruch C, VanGilder C. A laboratory study examining the impact of linen use on low‐air‐loss support surface heat and water vapor transmission rates. Ostomy Wound Manage. 2013;59(8):32‐41. [PubMed] [Google Scholar]
- 37. Figliola RS. A proposed method for quantifying low‐air‐loss mattress performance by moisture transport. Ostomy Wound Manage. 2003;49(1):32‐42. [PubMed] [Google Scholar]
- 38. Stone A, Brienza D, Call E, et al. Standardizing support surface testing and reporting: a National Pressure Ulcer Advisory Panel Executive Summary. J Wound Ostomy Continence Nurs. 2015;42(5):445‐449. [DOI] [PubMed] [Google Scholar]
- 39. McInnes E, Jammali‐Blasi A, Bell‐Syer S, Dumville J, Cullum N. Preventing pressure ulcers—are pressure‐redistributing support surfaces effective? A Cochrane systematic review and meta‐analysis. Int J Nurs Stud. 2012;49(3):345‐359. [DOI] [PubMed] [Google Scholar]
- 40. Haesler E, Kottner J, Cuddigan J. The 2014 international pressure ulcer guideline: methods and development. J Adv Nurs. 2017;73(6):1515‐1530. [DOI] [PubMed] [Google Scholar]
- 41. Gefen A, Kottner J, Santamaria N. Clinical and biomechanical perspectives on pressure injury prevention research: the case of prophylactic dressings. Clin Biomech (Bristol, Avon). 2016;38:29‐34. [DOI] [PubMed] [Google Scholar]


