ABSTRACT
Current wound management through the use of a split‐thickness skin graft often requires hospital admission, a period of immobility, attentive donor site wound care and pain management. This study evaluates the feasibility of using a novel epidermal graft‐harvesting device (CelluTome) that allows pain‐free epidermal skin grafting in the outpatient clinic setting. A prospective series of 35 patients was performed in 2 centres, involving 10 acute and 25 chronic wounds. All patients were subjected to epidermal grafting in the outpatient specialist clinic, without the use of anaesthesia, and allowed to return home after the procedure. Completely healed wounds were noted in 22 patients (62·9%). The overall mean time for 50% and 100% reduction in wound size was 3·31 ± 2·33 and 5·91 ± 3·48 weeks, respectively. There was no significant difference in healing times between the acute and chronic wounds (50% reduction in wound size; acute 2·20 ± 0·91 weeks versus chronic 3·73 ± 2·63 weeks, P = 0·171. Hundred percent reduction in wound size; acute 4·80 ± 1·61 weeks versus chronic 6·83 ± 4·47 weeks, P = 0·183). The mean time for donor site healing was 5·49 ± 1·48 days. The mean pain score during graft harvest was 1·42 ± 0·95, and the donor site Vancouver Scar Scale was 0 for all cases at 6 weeks. This automated device offers autologous skin harvesting in the outpatient setting with minimal or no pain and a scar free donor site, equally benefiting both the acute and chronic wounds. It has the potential to save NHS resources by eliminating the need for theatre space and a hospital bed while at the same time benefiting patient care.
Keywords: Epidermal graft, CelluTome, Outpatient care, Wound management, Wound healing
Introduction
The cost of wound management to the national health service (NHS) is considerable, estimated at about £2·3–3·1 billion per year 1. Chronic wounds can present significant challenges to the practitioner with a long healing time and multiple dressing changes. Most of these wounds are managed in the outpatient setting with protocols that vary according to geography, institution and speciality. Wound coverage by dressings or the use of split‐thickness skin grafts (SSGs) aims to achieve complete healing in the shortest time period with minimal patient morbidity.
Current wound management through the use of SSGs often requires hospital admission, even a day case, anaesthesia and a period of immobility for some patients. The donor site becomes a second, often painful wound, which may take more time to heal than the graft site 2, 3. Newer alternatives to SSG, such as tissue‐engineered skin grafts, carry their own challenges, namely the cost and lack of availability, and are often limited to specialised facilities.
Epidermal grafting (EG) was first described in 1964 by two dermatologists, Kiistala and Mustakallio 4. Their technique proposed the use of negative pressure (150–200 mmHg), with minimal intra‐procedure trauma. However, it did not achieve popularity until 1971, when Falabella 5 showed that EG was a valuable tool for providing coverage of granulating areas and repigmentation of achromic wounds. Although it has been described as cumbersome and time‐consuming, there are reports of successful wound healing through the use of EG; however, to date, it has been minimally explored. This study evaluates the feasibility of using a novel epidermal graft‐harvesting device, CelluTome, that allows epidermal skin grafting to be performed in the outpatient clinic setting, with minimal or no pain as an alternative to the current wound management methodology.
Methods
A prospective case series was conducted, from July 2014 to March 2015, at two regional plastic surgery units in the UK: the Royal Free Hospital, London and the University Hospital of Wales, Cardiff. EG was an established technique in the trust; so, a divisional protocol was developed with trust approval for a new device, and all patients registered for a prospective audit.
Patient selection
Adult patients who had been referred to the department of plastic surgery for the consideration of SSG because of difficult or non‐healing wounds were considered. The wounds were between 2 × 2 cm and 12 × 12 cm and had clean and granulating wound beds. Prior to grafting, the wound bed was prepared as per standard clinical practice, either with negative pressure wound therapy or appropriate dressings, until healthy granulation tissue was present. Wound swabs were performed to exclude infection. Details on patient's demographics, comorbidities, wound aetiology, wound type, wound location, wound size, healing time, pain scores during graft harvest, wound measurements at each visit and donor site scar quality were recorded. The wound type was classified into acute (<3 months in duration) and chronic (≥3 months in duration). This study was conducted in the outpatient setting, and patients were allowed to return home on the same day after the intervention.
Epidermal graft harvest and postgrafting wound care
Epidermal grafts were harvested using an automated harvesting system, CelluTome (Acelity) 6. This device harvests epidermal micrografts, without the use of anaesthesia, via the formation of suction blisters, carried out in the outpatient setting. By combining negative pressure (400–500 mmHg) and heat (40°C), this device produces an array of epidermal blisters within 30–50 minutes, providing autologous keratinocytes for grafting (Figure 1). The microdomes are formed at the layer of the lamina lucida of the dermo‐epidermal junction 6. Following the harvest, the epidermal grafts are transferred onto a non‐adherent silicone dressing (Adaptic Touch, Systagenix) and applied onto the wound (Figure 1). The graft is then secured with a secondary dressing, while the donor site is dressed with an occlusive dressing (Tegaderm Film, 3M) (Figure 2). Patients were allowed to return home on the same day after the procedure and were reviewed on day 7 ± 3 postgrafting. The patients were reviewed weekly for a minimum of 6 weeks or until the wound had healed.
Figure 1.
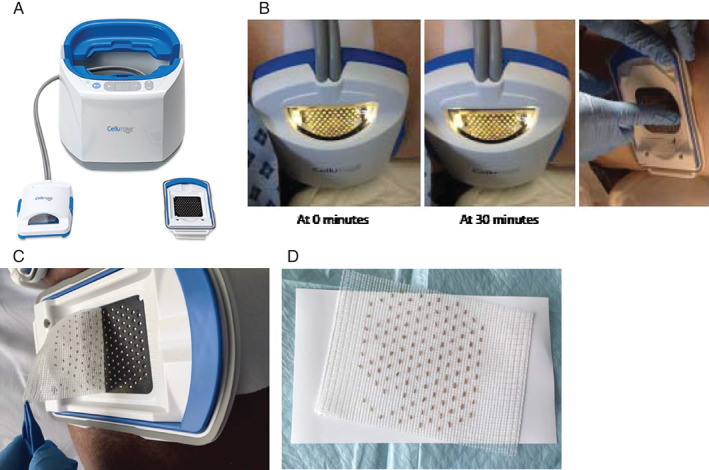
Illustration of the epidermal graft‐harvesting device. (A) The epidermal graft‐harvesting system: (i) the control unit, (ii) the vacuum head, (iii) the harvester. (B) Arrow pointing at the microdomes formed in the vacuum head after 50 minutes of suction. (C) Graft Harvest. (D) Microdomes transferred on to non‐adhesive silicone.
Figure 2.
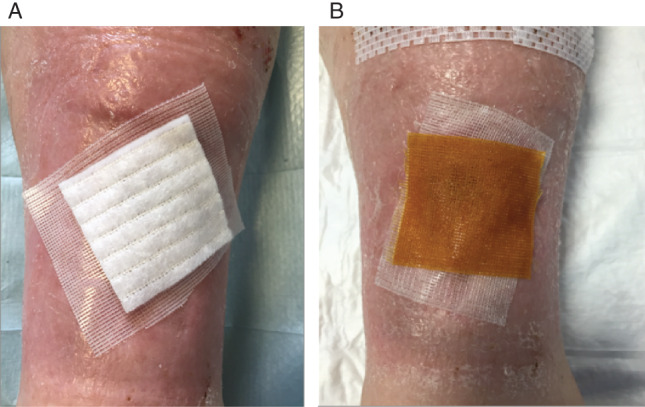
Dressings (A) secondary dressing with Aquacel. (B) Secondary dressing with iNadine. (C) Further occlusive dressing to be applied on top of either (A) or (B).
During the postoperative review, the donor site dressing was removed at week 1, and no further dressing was required. The recipient wound was reviewed by the same clinician for every case to ensure continuity of care and practice as well as reliability in outcome measure assessment. Once the dressing was removed, the bed was not tampered with to allow for the fragile keratinocyte layers to set, and a new dressing was applied, usually in the form of a non‐adherent silicone dressing, Adaptic Touch (Systagenix), followed by a secondary dressing that usually included iNadine (Systagenix) or Aquacel (Figure 2) to deal with the exudate levels. In cases where the exudate level was moderate or high, the secondary dressing was changed twice weekly.
Outcome measures
The wounds were measured (length (cm) × width (cm)) and photographed before and after grafting, and at each wound review. The primary outcomes measured were the time taken for 50% and 100% reduction in wound size as well as the time taken for the donor site to heal. The secondary outcomes measured were pain score during graft harvest and donor site scar quality. The pain score was measured using a Numerical Rating Scale, with 0 being no pain and 10 being the worst pain. The donor site scar quality was evaluated by using the Vancouver Scar Scale (VSS) at 6 weeks postgrafting 7. The VSS assesses four variables: vascularity, height/thickness, pliability and pigmentation. Each variable includes ranked subscales that are summed to obtain a total score ranging from 0 to 13, with 0 representing normal skin and 13 representing maximum alterations of the skin.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 21 software. The P values of <0·05 were considered statistically significant. The results were presented as the mean ± standard deviation. The time for reduction in wound size between the acute and chronic wounds and the size and type of wound were compared using the independent t‐test. The Pearson correlation coefficient was used to determine the association between the age and time for the donor site to heal.
Results
A total of 35 patients were treated with EG, with an average age of 66·1 years (range: 18–93 years). Of these patients, 16 were male (45·7%), and 19 were female (54·3%) (Table 1). The most common aetiology in this patient cohort was wound dehiscence (n = 12). There were 10 acute wounds (mean duration: 1·50 ± 0·81 months) and 25 chronic wounds (mean duration: 26·3 ± 24·3 months), with an average wound duration of 19·4 months (range: 0·5–77 months) (Table 2). The majority of the wounds treated were on the leg (40·0%) followed by the ankle (17·1%) and abdomen (14·3%). The average wound size was 20·5 ± 22·4 cm2. There was no difference between the wound size and type of wound (acute: 11·9 ± 6·65 cm2 versus chronic: 23·9 ± 25·9 cm2, P = 0·079).
Table 1.
Clinical data of patients treated with epidermal graft
| No | Patient | Gender | Age | Comorbidities | Wound aetiology | Location of wound | Duration of wound (month) | Wound size (cm2) | Time for 50% reduction of wound size (weeks) | Time for 100% reduction of wound size (weeks) | Time for donor site healing (days) | Pain score during graft harvest | VSS of donor site scar |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SB | F | 24 | Nil | Pyogenic granuloma | Foot | 4 | 6 | Failed | Failed | 5 | 1 | 0 |
| 2 | HB | M | 18 | Nil | Trauma | Knee | 1 | 13·5 | 2 | 5 | 5 | 3 | 0 |
| 3 | RK | F | 85 | IHD, CABG, HTN, asthma | Venous ulcer | Leg | 4 | 6 | 2 | 5 | 7 | 2 | 0 |
| 4 | BL | F | 93 | Dementia, COPD, HTN, CCF | Trauma | Leg | 1 | 14 | 1 | 3 | 5 | 1 | 0 |
| 5 | JC | M | 54 | Nil | Amputation stump wound dehiscence | Foot | 2 | 28 | 2 | 4 | 5 | 2 | 0 |
| 6 | LH | F | 50 | SLE (oral steroids) | Venous ulcer | Ankle | 5 | 8 | 2 | 5 | 7 | 2 | 0 |
| 7 | JZ | M | 84 | R hemicolectomy, postoperative fistula and hernia | Abdominal wound dehiscence | Abdomen | 60 | 15·8 | 4 | 8 | 5 | 1 | 0 |
| 8 | GM | F | 62 | Bowen's disease, myasthenia gravis (on oral steroids), osteoporosis | Trauma | Leg | 1·5 | 12 | 2 | 6 | 5 | 2 | 0 |
| 9 | RF | M | 78 | Prostate cancer, myelodysplasia, IHD, PVD, multiple BCC/SCC | Wound dehiscence | Leg | 3 | 27 | Failed | Failed | 5 | 1 | 0 |
| 10 | NS | F | 91 | Breast cancer, hypertension, smoker, CKD3 | Trauma | Leg | 3·5 | 3 | 2 | 3 | 5 | 3 | 0 |
| 11 | LR | F | 64 | RA, COPD | Trauma | Ankle | 9 | 40 | Failed | Failed | 5 | 0 | 0 |
| 12 | AL | F | 26 | Anaemia smoker | Burn | Leg | 0·5 | 19 | 1 | 3 | 7 | 1 | 0 |
| 13 | DM | F | 50 | Breast ca and chemo, previous DVT on warfarin | Dehiscence of LD donor site | Back | 4 | 21 | 4 | 8 | 5 | 3 | 0 |
| 14 | JB | F | 52 | Hypertension, gastric banding, abdominoplast, thigh lift | Wound dehiscence | Thigh | 1 | 28 | 4 | 8 | 7 | 2 | 0 |
| 15 | JM | F | 76 | Cerebral palsy, hypothyroid, osteoporosis | SSG donor site | Thigh | 24 | 18 | 2 | 4 | 5 | 2 | 0 |
| 16 | OM | M | 32 | Deaf | Trauma | Foot | 2 | 26 | 3 | 6 | 5 | 2 | 0 |
| 17 | KI | M | 82 | IHD, CABG, HTN, T2DM, RA (on prednisolone + methotrexate), hypercholesterolaemia, CVA, AAA (4·5 cm) | Wound dehiscence | Leg | 2 | 16 | 3 | 6 | 5 | 1 | 0 |
| 18 | BR | M | 78 | Previous sigmoid cancer, colostomy, necrotizing fasciitis abdomen, CVA | SSG donor site | Thigh | 36 | 39 | 2 | 5 | 7 | 2 | 0 |
| 19 | HJ | F | 85 | RA | VLU | Ankle | 24 | 11·25 | 12 | 20 | 7 | 1 | 0 |
| 20 | AL | M | 86 | Arterial leg ulcer | Ankle | 60 | 5·25 | 4 | No change | 9 | 2 | 0 | |
| 21 | IA | M | 65 | PVD diabetes | Diabetic foot ulcer | Ankle | 15 | 0·9 | 1 | 8 | 7 | 0 | 0 |
| 22 | MW 4 applications | M | 70 | Bowel cancer | Wound dehiscence | Abdomen | 42 | 123·5 | 8 | Multiple small areas | 3 | 1 | 0 |
| 23 | EVD 2 applications | F | 80 | PVD | Mixed arterial leg ulcer | Leg | 60 | 15·84 | Failed | failed | 7 | 2 | 0 |
| 24 | EO | F | 72 | RA | Trauma | Leg | 24 | 4·94 | Wound bed more active | Wound bed more active | 7 | 2 | 0 |
| 25 | MM | F | 77 | Diabetes type 1 | Wound dehiscence | Abdomen | 77 | 45 | Failed | Failed | 5 | 1 | 0 |
| 26 | TS 3 applications | M | 72 | Bowel cancer | Wound dehiscence | Abdomen | 72 | 22 | Multiple small areas | Multiple small areas | 3 | 0 | 0 |
| 27 | BH | F | 80 | Nil | Wound dehiscence | Abdomen | 14 | 21·6 | Failed | Failed | 7 | 0 | 0 |
| 28 | PN 2 applications | F | 66 | Systemic sclerosis | VLU | Leg | 72 | 67·2 | Failed | Failed | 3 | 0 | 0 |
| 29 | BG | M | 72 | Nil | VLU | Leg | 18 | 4·05 | 6 | No change | 3 | 0 | 0 |
| 30 | JI | M | 85 | Diabetes | Trauma | Leg | 21 | 2·1 | 4 | No change | 3 | 0 | 0 |
| 31 | JS | M | 88 | IHD, CABG, HTN | Trauma | Forearm | 0·5 | 7 | 3 | 5 | 5 | 2 | 0 |
| 32 | SM | M | 93 | IHD, CABG, HTN, AF | SSG donor site | Leg | 3 | 9 | 2 | 3 | 5 | 2 | 0 |
| 33 | PS | M | 28 | Nil | Wound dehiscence | Ankle | 5 | 6 | 2 | 4 | 5 | 1 | 0 |
| 34 | LM | F | 39 | GORD | Wound dehiscence | Arm | 4 | 18 | 4 | 6 | 7 | 3 | 0 |
| 35 | JK | F | 58 | PE/DVT, PCOS, asthma | Trauma | Pretibial | 5 | 14 | 4 | 5 | 7 | 2 | 0 |
T1DM, type 1 diabetes mellitus; VSS, Vancouver Scar Scale.
Table 2.
Summary of patient demography and wound characteristics
| Characteristics | Number of patients (%) |
|---|---|
| Mean age (years) | 66·1 ± 21·1 |
| Gender | |
| Male | 16 (45·71%) |
| Female | 19 (54·3%) |
| Wound aetiology | |
| Venous ulcer | 5 (14·3%) |
| Arterial ulcer | 2 (5·7%) |
| Burns | 1 (2·9%) |
| Split‐thickness skin graft donor site | 3 (8·6%) |
| Wound dehiscence | 12 (34·3%) |
| Trauma | 10 (28·6%) |
| Pyogenic granuloma | 1 (2·9%) |
| Diabetic foot ulcer | 1 (2·9%) |
| Type of wound | |
| Acute | 10 (28·6%) |
| Chronic | 25 (71·4%) |
| Mean wound duration (months) | 19·4 ± 24·0 |
| Anatomical location | |
| Foot | 3 (8·6%) |
| Ankle | 6 (17·1%) |
| Leg | 14 (40·0%) |
| Knee | 1 (2·9%) |
| Thigh | 3 (8·6%) |
| Abdomen | 5 (14·3%) |
| Back | 1 (2·9%) |
| Arm | 1 (2·9%) |
| Forearm | 1 (2·9%) |
Complete wound healing (100% reduction in wound size) was achieved in 22 patients, 62·9%. Of these 22 patients, 17 patients (77·3%) healed within 6 weeks, 4 patients (18·2%) within 8 weeks and the remaining patient healed within 20 weeks.
The mean time for 50% and 100% reduction in wound size were 3·31 ± 2·33 and 5·91 ± 3·48 weeks, respectively. There was no significant difference in healing times between the acute wounds and the chronic wounds (50% reduction in wound size; acute 2·20 ± 0·91 weeks versus chronic 3·73 ± 2·63 weeks, P = 0·171. Hundred percent reduction in wound size; acute 4·80 ± 1·61 weeks versus chronic 6·83 ± 4·47 weeks, P = 0·183).
The mean time for the donor site to heal was 5·49 ± 1·48 days. There was no correlation between patient's age and donor site healing time (Pearson correlation, P = 0·915). The mean pain score during graft harvest was 1·42 ± 0·95, and the donor site Vancouver Scar Scale was 0 for all cases at 6 weeks, whereby all donor sites looked similar to the surrounding skin.
There were seven graft failures because of infection. No improvement in wound size was seen in two other patients with chronic wounds; however, the wound bed was noted to be more active with granulation tissue. No other complications were experienced by the patients.
Case examples
Case 1: patient 2/HB
A healthy young male sustained a traumatic wound over the left patellar region from a motorbike injury. The wound measured 4·5 × 3·0 cm with exposed infra‐patellar tendon, requiring surgical debridement followed by 4 weeks of negative pressure wound therapy (NPWT). The wound granulated well with the NPWT and subsequently underwent EG (Figure 3). Complete reepithelialisation of the wound was noted at 5 weeks postgrafting, while the donor site healed within the first week without any noticeable scar at week 6.
Figure 3.
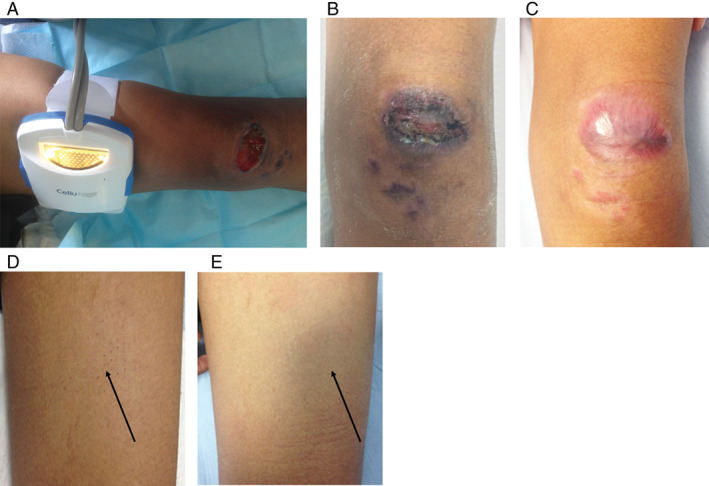
The wound and donor site of Patient 2/HB. (A) Healthy granulation tissue was seen on the wound bed after 4 weeks of negative pressure wound therapy (NPWT). The wound measures 4·5 × 3·0 cm over the left patella region. (B) More than 50% of the wound was reepithelialised at week 3 postgrafting. (C) Complete wound healing was seen at week 5. (D) Minimal scabs were seen at the donor site (black arrow) at week 3. (E) No visible scar was seen at the donor site (black arrow) at week 6. The donor site looks aesthetically similar to the surrounding skin.
Case 2: patient 7/JZ
An 83‐year‐old fit and independent gentleman with a history of appendicectomy and right hemicolectomy complicated by an incisional hernia in 2011 presented with a chronic non‐healing wound over the central abdomen. The wound measured 4·5 × 3·5 cm and was dressed with honey dressings, Inadine (Systagenix) and Silflex (Advancis Medical) prior to EG (Figure 4). Fifty percent reduction in wound size was achieved at week 4, and complete wound healing was achieved at week 8.
Figure 4.
The wound of Patient 7/JZ. (A) 4·5 × 3·5 cm superficial, granulating wound over the central abdomen. (B) At week 4, 50% of the wound was reepithelialised. (C) The wound was completely healed at week 6.
Case 3: patient 8/GM
A 60‐year‐old‐female presented with a 4‐week history of numerous type 1 pretibial lacerations, secondary to a ladder falling on her right leg (Figure 5). The wound had been initially managed with honey dressings, Inadine (Systagenix) and Silflex (Advancis Medical) prior to arrival at our clinic. At presentation, the wound consisted of four small concave granulating wounds all measuring approximately 0·5 × 0·5 cm. There was no growth on microbiology swabs from any of the wounds. The patient had myasthenia gravis for which she was taking oral prednisolone. Epidermal grafts were taken from the right thigh using the CelluTome device and grafted onto the wounds. Adaptic touch (Systagenix) dressings were applied. At 2 weeks, the wounds had reduced in size by 50%, and at 6 weeks postgrafting, all wounds had healed 100%. The donor site healed within 5 days of harvest.
Figure 5.
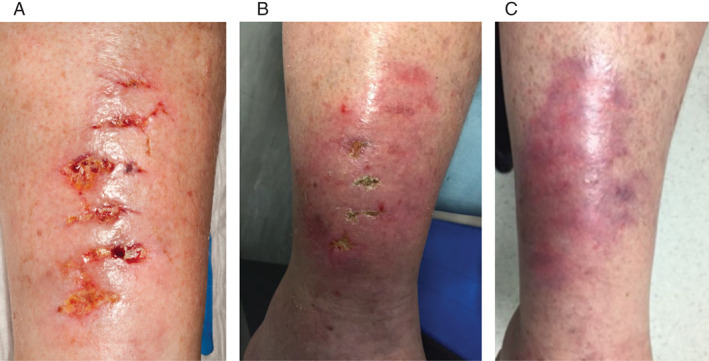
The wound of Patient 8/GM. (A) Multiple 4‐week‐old small type 1 pretibial lacerations on right leg. (B) At 2 weeks after epidermal grafting, the wounds were 50% healed. (C) 100% healing was achieved at 6 weeks postgrafting.
Case 4: patient 12/AL
A 26‐year‐old female presented with a 2‐week history of an acute thermal burn injury to her right leg. The wound measured 9·5 × 2 cm, was granulating and had no growth on microbiology swabs. The patient was a smoker but had no other comorbidities. Epidermal skin grafts were taken from the right thigh using the CelluTome device and grafted onto the wound. Adaptic touch (Systagenix) dressings were applied. Within 1 week, the wound had reduced in size by 50% (Figure 6), and within 3 weeks, the wound had healed 100%. The donor site healed within 7 days of harvest.
Figure 6.
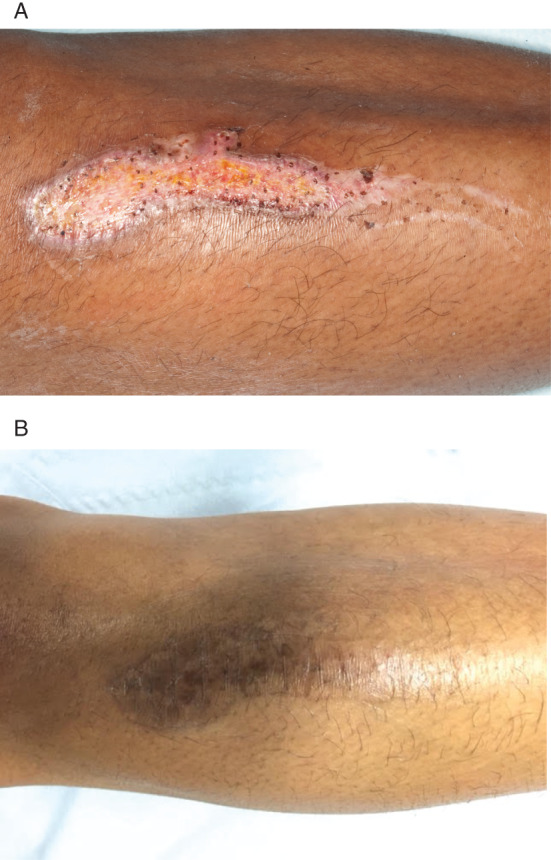
The wound of Patient 12/AL. (A) Burn wound measuring 9·5 × 2 cm over the right leg. (B) Complete healing was achieved at 3 weeks postgrafting.
Case 5: patient 31/JS
An 88‐year‐old male presented with a 2‐week history of a right forearm laceration following trauma. The wound measured 2 × 3·5 cm, was granulating and had no growth on microbiology swabs. The patient had a past medical history of ischaemic heart disease only and was a non‐smoker. Epidermal grafts were taken from the right thigh and applied to the wound. Adaptic touch (Systagenix) dressings were applied. Within 3 weeks, the wound had reduced in size by 50%, and within 5 weeks, the wound was 100% healed (Figure 7). The donor site healed within 6 days after harvest.
Figure 7.
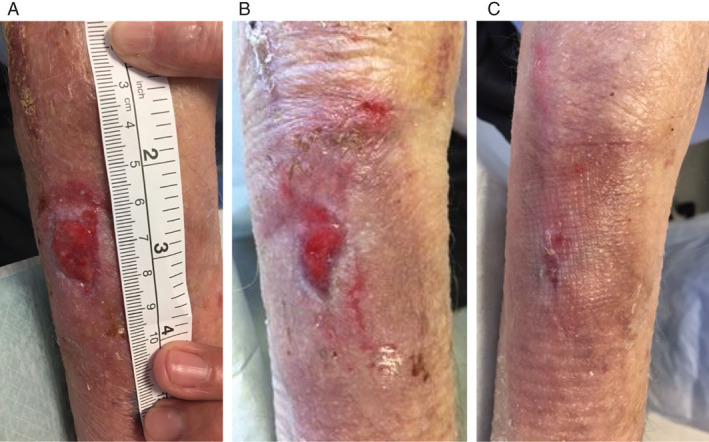
The wound of Patient 31/JS (A) Right forearm 2 × 3·5 cm laceration wound. (B) At 3 weeks after epidermal grafting, the wound was 50% healed; (C) at 5 weeks, the wound was 100% healed.
Case 6: patient 9/RF
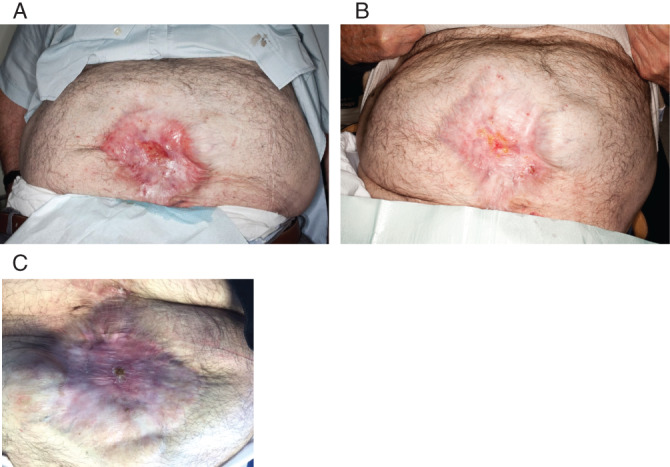
A 78‐year‐old male presented with a 3‐month history of wound dehiscence of the left lateral leg following basal cell carcinoma excision. The wound had broken down shortly after surgery and required debridement in theatre before presentation to our clinic. The wound measured 4·5 × 6 cm, was granulating and had no growth on microbiology swabs. The patient had numerous comorbidities, including prostate cancer (not on active treatment), myelodysplasia, ischaemic heart disease and smoking. Epidermal grafts were taken from the left thigh using the CelluTome device and grafted onto the wound. Adaptic touch dressings were applied. At 2 weeks, the wound was showing signs of over granulation and lack of graft‐take (Figure 8). At 4 weeks, the graft was judged to have failed, and a wound swab confirmed Pseudomonas growth. The donor site healed within 5 days of harvest.
Figure 8.
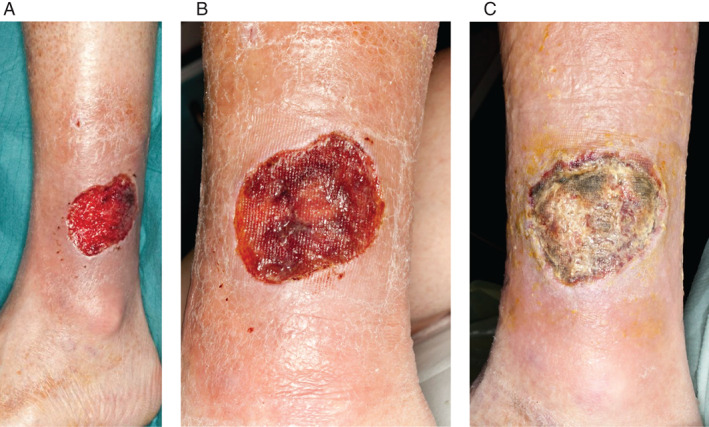
The wound of Patient 9/RF (A) 3‐month‐old left lateral leg wound following basal cell carcinoma (BCC) excision, wound dehiscence and debridement measuring 4·5 × 6 cm. (B) At 2 weeks postgrafting, over granulation can be seen on wound bed. (C) Wound bed was noted to be sloughy at 4 weeks; wound swab confirmed Pseudomonas growth.
Discussion
The use of epidermal grafts or blister grafts for the treatment of vitiligo and chronic wounds has already been widely reported, but its use is limited because of the lack of reproducible and efficient harvesting techniques further limiting its potential to be used in the outpatient setting 8, 9. This study demonstrates the feasibility of using a novel epidermal‐harvesting device to achieve definitive wound coverage in the outpatient setting.
The CelluTome device produces an array of epidermal microdomes, comprising the epidermis down to the basal layer, immediately available for transfer to the recipient site. Epidermal grafts are made of multi‐layered keratinocytes in which a variety of other cell types with specialised functions are embedded, such as the melanin pigment‐producing melanocytes, the immune‐competent Langerhans cells and the neuroendocrine Merkel cell, while its basal layer contains epidermal stem cells 10. During the early stages of wound healing, keratinocytes begin to migrate from wound edges within 24 hours to the wound bed where they proliferate and form new epithelium 11. Migrating keratinocytes synthesise and deposit a variety of extracellular matrix components, such as laminin, fibronectin and type IV collagen 12. In addition, numerous growth factors are also produced, namely, epidermal growth factor (EGF), transforming growth factor alpha (TGF‐a) and heparin‐binding growth factor (HB‐EGF), which acts on the epidermis to drive wound closure 13. Hence, the epidermal grafts act more like a bioengineered skin, stimulating the endogenous process of wound healing.
Another key factor to the success of EG is the ability for basal cell outgrowth from the graft edge, and this occurs for up to a 2 mm distance (REF). This is intrinsic to the design of the harvester, which consists of 128 micrograft pores set at a 2 mm distance apart to allow for the grafts to be raised in this manner (Figure 1).
Wound healing was a key outcome measured, and the results demonstrated that 62·9% of the wounds fully healed with the use of Cellutome. Fifty percent wound healing was achieved within 3·31 ± 2·33 weeks, and complete wound closure was achieved within 5·91 ± 3·48 weeks. Of the wounds that healed, 54·5% were chronic wounds that were not responding to dressings and conservative management, which potentially implies that the epidermal grafts stimulates the healing process in quiescent wounds. The donor site wound healed within 5·49 ± 1·48 days, with excellent aesthetic outcome, requiring neither frequent nursing care nor scar management. This result is encouraging as a donor site from a split‐thickness skin graft can take up to 21 days to reepithelialise with current donor site dressing methods 14. Furthermore, donor site complications such as infection, pain, and hypertrophic scarring can be avoided. Interestingly, there was no significant difference in wound‐healing time between the acute and chronic wounds, making the CelluTome equally useful in both types of wounds. The aetiology of the wound and anatomical site also had no significant impact on the wound‐healing time. Donor site healing was excellent in all patients, with all cases scoring 0 on the Vancouver Scar at 6 weeks. As for the pain scores, these were reported to be were very low for all patients, with a mean pain score during graft harvest of 1·42 ± 0·95, making this a very tolerable technique.
As with all new technologies, the costs of intervention need to be assessed. We did not formally undertake a cost analysis. However, the series included eight acute wounds (1·38 ± 0·54 months) and 22 chronic wounds (29·6 ± 25·2 months); a total of 662 months of dressing care was performed before the intervention of CelluTome. If these wounds had been dressed 2–3 time per week, a total of 5296–7944 dressing changes would have been performed. In total, 30 interventions were performed with 400–600 dressing changes in the 8 weeks of care, with two thirds of patients achieving a dressing‐free (healed) outcome. The intervention and subsequent treatment, therefore, costs less than 10% of the previous management costs.
Our experience shows that this harvesting device can be introduced routinely in the outpatient setting for both acute and chronic wounds. Once a patient has been assessed, they can be invited back to a routine ‘CELLUTOME’ clinic and can undergo the epidermal harvest followed by routine dressing changes by the delivering clinical team. Chronic wounds pose a significant burden on the NHS, representing at least 5·5% of the NHS budget expenditure 15; therefore, such technologies that can introduce lasting improvements to wound management should be welcomed.
Limitations of the study
This is an observational study and therefore prone to selection bias. The data reported includes all cases performed in a sequential manner, and patients were identified from routine referrals. Both units experienced a learning curve, and this was attributed to three main points: the quality of the wound bed preparation, ensuring absence of wound bed infection (responsible for 7 graft failures) and the harvest and postoperative wound care. Following some graft failures because of infection, assessing wounds with a preoperative swab has become the standard approach. The fragility of the epidermal grafts and keratinocyte sheets that develop in the weeks postoperatively was acknowledged, and as such, our practice has changed whereby the wound bed is not touched during the first 3–4 weeks of dressing changes. Furthermore, better management of exudate levels with various secondary dressings was achieved, which also improved results.
The feasibility of treating acute wounds in the outpatient setting reduces the need for patients to be admitted for autologous skin grafting. In addition, the prospect of using this device in the emergency department for the management of acute wounds could potentially reduce the number of hospital visits. The CelluTome is easy to use and well tolerated by patients. Elderly patients with multiple comorbidities would benefit from this technique as it does not require anaesthesia and avoids the complications of bed rest, maintaining a patient's independence and quality of life.
Conclusion
This automated device offers a novel method in autologous skin harvesting, resulting in minimal or no pain and a scar‐free donor site in the outpatient setting. Complete wound coverage is achieved while maintaining patient independence. It has the potential to save health care resources by eliminating the need for theatre space and a hospital bed while at the same time benefiting patient care.
References
- 1. Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–45. [PubMed] [Google Scholar]
- 2. Voineskos SH, Ayeni OA, McKnight L, Thoma A. Systematic review of skin graft donor‐site dressings. Plast Reconstr Surg 2009;124:298–306. [DOI] [PubMed] [Google Scholar]
- 3. Demirtas Y, Yagmur C, Soylemez F, Ozturk N, Demir A. Management of split‐thickness skin graft donor site: a prospective clinical trial for comparison of five different dressing materials. Burns 2010;36:999–1005. [DOI] [PubMed] [Google Scholar]
- 4. Kiistala U, Mustakallio KK. In‐vivo separation of epidermis by production of suction blisters. Lancet 1964;1:1444–1445. [DOI] [PubMed] [Google Scholar]
- 5. Falabella R. Epidermal grafting. An original technique and its application in achromic and granulating areas. Arch Dermatol 1971;104:592–600. [DOI] [PubMed] [Google Scholar]
- 6. Nihr HSC. CelluTome? epidermal harvesting system for autologous skin grafting. New York: NIHR Horizon Scanning Centre, University of Birmingham, 2014. [Google Scholar]
- 7. Duncan JA, Bond JS, Mason T, Ludlow A, Cridland P, O'Kane S, Ferguson MW. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg 2006;118:909–918. [DOI] [PubMed] [Google Scholar]
- 8. Costanzo U, Streit M, Braathen LR. Autologous suction blister grafting for chronic leg ulcers. J Eur Acad Dermatol Venereol 2008;22:7–10. [DOI] [PubMed] [Google Scholar]
- 9. Jung KE, Kim MH, Kim JY, Park BC. Comparison of modified Korean cupping method and conventional respiratory suction unit for epidermal graft. Int J Dermatol 2014;53:e384–e386. [DOI] [PubMed] [Google Scholar]
- 10. Potten CS. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol 1981;69:271–318. [DOI] [PubMed] [Google Scholar]
- 11. Ortonne JP, Loning T, Schmitt D, Thivolet J. Immunomorphological and ultrastructural aspects of keratinocyte migration in epidermal wound healing. Virchows Arch A Pathol Anat Histol 1981;392:217–230. [DOI] [PubMed] [Google Scholar]
- 12. Kirfel G, Herzog V. Migration of epidermal keratinocytes: mechanisms, regulation, and biological significance. Protoplasma 2004;223:67–78. [DOI] [PubMed] [Google Scholar]
- 13. Barrandon Y, Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor‐alpha and epidermal growth factor. Cell 1987;50:1131–1137. [DOI] [PubMed] [Google Scholar]
- 14. Karlsson M, Lindgren M, Jarnhed‐Andersson I, Tarpila E. Dressing the split‐thickness skin graft donor site: a randomized clinical trial. Adv Skin Wound Care 2014;27:20–25. [DOI] [PubMed] [Google Scholar]
- 15. Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J 2015;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]


