Abstract
The aim of this study was to compare changes in wound size and appearance and health complication rates in patients with vasculopathy and lower‐extremity wounds treated with or without low‐frequency contact ultrasound debridement (LFCUD) This study was a randomised controlled trial. The study was conducted in a vascular surgery service, including outpatient wound clinic and inpatient ward, in a tertiary care academic centre. In total, 70 patients with vasculopathy and lower‐extremity wounds of mixed aetiology were enrolled in the trial; 68 completed the study. Patients were randomised to receive LFCUD plus usual care (n = 33) or usual care (n = 37) at 4 weekly visits, and were followed thereafter for up to 12 wk. The main outcome measures included closed wounds, change in wound surface area (WSA), and wound appearance by the revised Photographic Wound Assessment Tool (revPWAT). After 4 weekly LFCUD treatments, patients in the LFCUD group had significantly better wound appearance (total revPWAT score) compared with the control group treated only with usual care (P = <0.05). LFCUD‐treated wounds also had a significant reduction in WSA over 4 wk that was not found in the UC group. LFCUD treatment was also associated with a greater number of healed wounds, odds ratio 5.00 (95% CI 1.24‐20.25), and fewer instances of wound deterioration. Weekly LFCUD applications to patients with significant vasculopathy resulted in superior healing outcomes when compared with current usual wound care practice.
Keywords: leg ulcers, peripheral arterial disease, ultrasound debridement, wound bed preparation, wound healing
1. INTRODUCTION
Wounds complicated by vascular disease are challenging to heal, and patients face serious health risks. Peripheral arterial disease (PAD) is thought to be underappreciated, under‐diagnosed, and under‐treated.1 PAD is a comorbid condition noted in 29% of those over 50 y of age with diabetes·2 Patients with both diabetes and PAD have an increased risk of serious complications, including serious infections, lower limb amputation, and even death.44 Given an increasing elderly population and what has been described as the “economic tsunami” of diabetes,6 the number of people with PAD‐related wounds is expected to increase to up to 10.8% of Canadians by 20207 Unfortunately, around 15% to 25% of people with diabetes can be expected to develop foot wounds, which are the leading cause of non‐traumatic lower limb amputation. To date, there are few active treatments for people with wounds related to PAD, and wound debridement is often avoided due to safety concerns.
A diagnosis of PAD reduces healing expectations as this pathology results in altered delivery and transport of oxygen and other factors essential for fuelling cellular function and healing. Additionally, poor blood supply to the wound mutes local inflammatory response, increasing excess bacterial growth and increasing the risk of infection·8 Tissue ischaemia can result when arterial compromise is severe, and the accumulation of devitalised tissue (necrosis) within the wound can become an excellent culture medium for bacterial proliferation,9 increasing the risk of systemic infection and wound complications. Importantly, impaired blood flow affects the delivery of medications needed to treat underlying illness and infections·10
Sharp debridement involves the removal of necrotic or unhealthy tissue from the wound,11 providing a fresh, bleeding wound base, and is considered gold‐standard preparation for chronic or non‐healing wounds. Debridement is a widely accepted wound treatment that reduces bacterial burden, improves the wound environment, and promotes healing·12, 13, 14 Sharp debridement improves the wound environment by removing senescent cells and necrotic debris, which may recur. Additionally, increased debridement episodes have demonstrated improved healing rates in diabetic foot ulcers, possibly because this procedure stimulates the release of growth factors that are necessary for tissue repair·13 More recently, sharp debridement has been identified as 1 of few wound treatments that can remove biofilm bacteria from wounds.15, 16, 17, 18 These biofilm‐protected bacterial colonies are notoriously hard to remove, destroy, or detect and have been implicated in recurring wound infections·9 Unfortunately, sharp debridement is not readily available for routine wound care and is seldom offered to those with PAD because of concerns about post‐procedure complications.19 Inconsistent levels of wound provider competency, lack of policy, and limited funding models form additional barriers to performing debridement in many settings·20
2. BENEFITS OF ULTRASOUND
Ultrasound is acoustic energy in the form of sound waves above the range of human hearing (greater than 20 000 kHz).21 There are several variations of therapeutic ultrasound used to treat wounds, including indirect and direct contact methods that may be directed either to the wound or peri‐wound area. These applications incorporate a range of frequencies and formats of ultrasound delivery that may be delivered in continuous or pulsed (intermittent) modes.
Higher‐frequency (MHz) applications are applied using a transducer to the peri‐ulcer skin and are coupled via aqueous gel or through a water bath medium. Lower‐frequency therapeutic systems (kHz) are available that deliver ultrasound by probes or through saline vapour. The lower frequencies have been reported to favourably influence early wound healing in venous leg ulcers and diabetic foot wounds·22 Low‐frequency ultrasound produces a longer wavelength, which penetrates tissue more deeply and generates less heat compared with higher (MHz) frequencies·21
Although these various modes are all based on ultrasound energy, it is inappropriate to compare them directly as indications, dosage, and delivery methods are not equivalent. The focus of this study was to explore the effect of LFCUD, which is a direct wound contact application of ultrasound that immediately and visibly removes necrotic debris and causes a light bleeding response.
3. PHYSIOLOGICAL EFFECTS ON HEALING
Ultrasound has been shown to promote cellular response, including fibroblast activity, collagen deposition, and new blood vessel growth to induce tissue repair·23, 24, 25, 26 Ultrasound has also been shown to induce blood vessel dilation27 and improve the quality of granulation tissue·25, 28 More recently, there is emerging evidence that ultrasound promotes migration mechanisms and promotes cell adhesion, which is necessary for tissue repair to occur·29 Interestingly, increase of local blood flow, oxygen uptake, and tissue regeneration in embryo tissues have all been noted with ultrasound application·30 These particular attributes are of interest for a vascular population. In light of these benefits, our study examines the combination of ultrasound and debridement.
LFCUD devices incorporate potentially beneficial ultrasound energy to precisely remove debris.31 Promising results have been reported in previous case series where LFCUD was used to prepare wounds for skin grafts32 and promote closure of wounds in patients with prosthetic vascular graft infection33 and infected sterno‐cutaneous fistulae.34 A similar form of LFCUD is in common use in dentistry to remove plaque biofilm while causing minimal disturbance of viable tissue·35 We recently completed a feasibility study using LFCUD treatments of 10 patients with vasculopathy. We specifically showed that 4 weekly treatments with LFCUD produced 30% wound size reduction and improved the appearance of the wound bed in people with long‐standing vascular pathology. LFCUD was provided by an Enterostomal nurse (ET RN) with advanced training to patients with moderate to severe vascular disease with minimal and manageable complications in an outpatient setting.
While there is promising research to suggest that LFCUD may be an effective and safe treatment to be used in wound care, there are no properly designed and controlled trials that test its ability to promote either better tissue quality or faster wound healing in a vascular population. The purpose of this study is to explore the effect of adding 22·5 kHz LFCUD to usual care on wound‐healing outcomes of patients of a vascular service at a tertiary centre nurse‐led wound clinic. It is hypothesised that treatment with LFCUD will remove necrotic debris, reduce bacterial burden, and improve wound size and appearance in a high‐risk, vasculopathic population. These improved healing outcomes will be associated with a lower occurrence of infections, amputations, and other serious health complications.
4. METHODS
The study is a 2‐arm randomised controlled trial with single assessor blinding. A study flow diagram is outlined in Figure 1. The study was approved by both the Western University and Ottawa Health Science Network Research Ethics Boards and was registered at the U.S National Institutes of Health Registry (ClinicalTrials.gov, at identifier NCT01973361). As per local requirements for the administration of LFCUD, an ET RN with advanced wound care training was approved by the Department of Vascular Surgery to administer all LFCUD treatments via Medical Directive and a Delegated Medical Act. The ET RN also received training and certification on the use of the device from the company representatives (Misonix, Farmingdale, NY).
Figure 1.
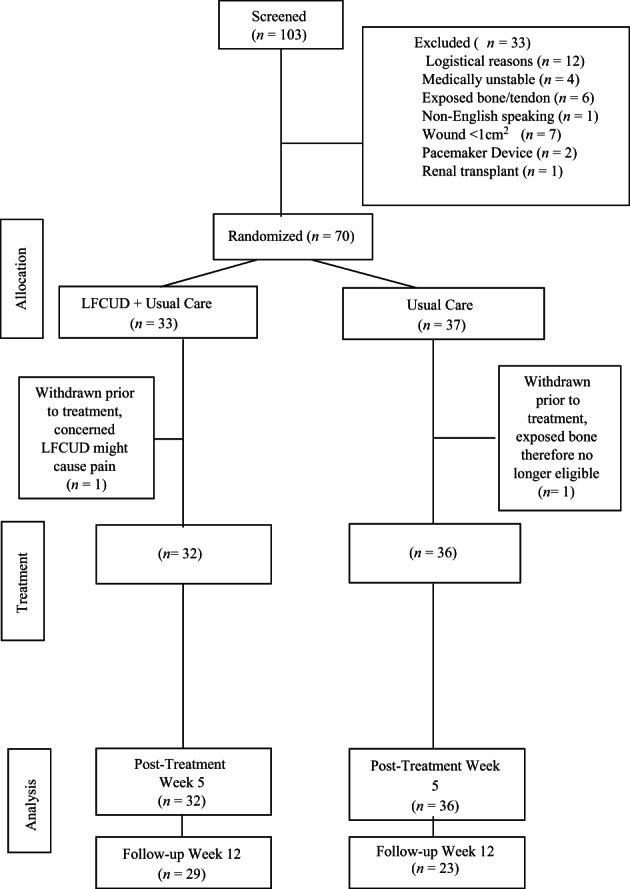
Patient flow diagram
4.1. Study participants
All patients with lower‐extremity wounds who were referred to the vascular service of a tertiary care hospital were approached consecutively by members of the vascular service team and asked if they would participate in the study. Patients were eligible if they were over 18 y of age and had a full‐thickness wound below the knee greater than 1 cm2 in surface area. Patients were excluded if they had conditions that prevented healing or if they had a medical condition that could contraindicate ultrasound treatment or could cause undue pain or post‐procedural bleeding. Patients were also excluded if they were concurrently receiving collagen, extracellular matrix products, or hyperbaric oxygen therapy; had exposed bone or tendon in the wound; were unable to speak English; or were unwilling to complete the 12‐wk study protocol. Patients with more than 1 ulcer were included, and all ulcers were treated. However, only 1 ulcer (the largest area measured at baseline) was followed for study purposes. All patients were screened by a vascular surgeon and the Infectious Diseases physician (ID MD) to rule out the presence of serious or potentially life‐ or limb‐threatening ischaemia or infections.
As part of the screening process, 1 of 6 vascular surgeons confirmed that vascular status was sufficient for healing, and debridement was not contraindicated. An extensive vascular assessment performed by the vascular service includes palpation of pedal pulses and an evaluation of limb perfusion in a clinical vascular laboratory using various methods, including Ankle Brachial Pressure Index, Toe Brachial Index, computerised tomography angiography, or digital subtraction angiography. Throughout the study, the vascular surgeons were not blinded so that patients could discuss any concerns and safety could be monitored.
Provided members of the vascular team felt that patients were able to participate in the study, the same ET RN completed the screening process and provided patients with a letter of information that was approved by the Research Ethics Boards.
4.1.1. Randomisation
All eligible and consenting patients were enrolled and randomly allocated in a 1:1 ratio to either the LFCUD) group plus usual care or usual care alone (UC). Randomisation was performed using web‐based computer software (Empower, Inc., London, Canada) that was off‐site and independent of any of the researchers. We stratified by whether or not the patient was receiving negative pressure wound therapy (NPWT) so that the groups were balanced.
4.1.2. Initial assessment and debridement
The study timeline is outlined in Figure 2. All patients who were enrolled in the study underwent a comprehensive assessment conducted by the ET RN to identify risk factors for delayed healing. A patient history form was used to fully describe patient characteristics and identify all comorbidities known to affect healing (eg, diabetes and associated complications, any recent or serious illness, and/or any recent surgeries). A blood sample was drawn to identify factors that may affect healing (eg, infection, nutritional markers, and anaemia). The ET RN applied the treatments and therefore was not blinded to treatment allocation.
Figure 2.
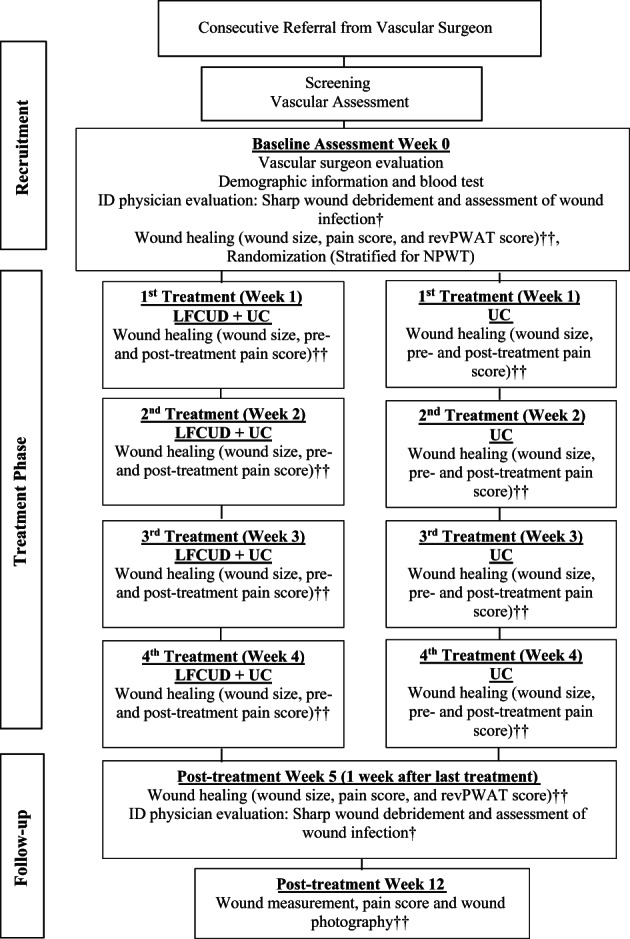
Study visit diagram. Abbreviations: LFCUD, low‐frequency contact ultrasound debridement; UC, usual care; revPWAT, revised photographic wound assessment tool.43 †Wound infection assessed via semi‐quantitative analysis of tissue sample culture and visual wound inspection. ††Wound measurement, photography, and pre‐treatment pain scores obtained and documented by blinded nurse assessor
All participants underwent a comprehensive assessment and extensive debridement by an infection disease physician (ID MD). An extensive sharp debridement procedure was performed at baseline on all enrolled patients by the same ID MD who was blinded to treatment allocation. This sharp debridement procedure involved cleansing with chlorhexidine 0.05% and completely removing all visible necrotic debris on the wound surface with a sterile curette, forcep, and/or scalpel. A tissue sample was taken after cleaning with physiological saline and then analysed for bacteria quantity and sensitivity under both aerobic and anaerobic conditions by an accredited medical laboratory. Results were expressed semi‐quantitatively. The ID MD determined whether the wound was infected or not and prescribed antibiotics according to the Infectious Diseases Society of America's Practice Guidelines for Skin and Soft Tissue Infection36 and Diabetic Foot Infection·37
4.2. Usual care (UC)
All patients regardless of group allocation received routine wound care at the same visit frequency (weekly). UC at our clinic includes removing the dressing; cleansing the wound; performing conservative debridement procedure, which involved removal of any necrotic debris from the wound base; pairing the peri‐wound callus; and replacing with a moist‐interactive dressing. Foot ulcers were evaluated for customised offloading systems as determined by the chiropodist. Compression wraps were applied to patients with tibial wounds and were titrated based on the vascular evaluation at baseline. All study patients received the same topical antimicrobial dressing prophylactically (Silver alginate dressing (Silvercel®, Acelity, San Antonio, TX), which was changed regularly by home care nurses. Typically, patients on vascular service are seen in the outpatient clinic every 4 to 6 wk. Therefore, weekly visits to the clinic for those in the UC group resulted in more frequent access to expertise on the vascular service and more frequent episodes of conservative debridement.
For patients with cavity wounds that extended adjacent to deep structures or with postoperative cavity defects, UC includes the use of NPWT as the wound dressing. For these patients, 1 of 2 NPWT devices was used (VAC®, Acelity, Antonio, TX, or Renasys®, Smith & Nephew, London, UK). NPWT was set at intermittent suction to support granulation response unless wound structural support was needed or the seal was problematic, in which case continuous suction was selected.
4.2.1. Low‐frequency contact ultrasound debridement (LFCUD)
In addition to UC, participants allocated to the LFCUD group received high‐intensity, low‐frequency (22·5 kHz) contact continuous ultrasonic debridement (Sonic One® , Misonix, Farmingdale, NY.). The Sonic One® LFCUD device produces a 22·5 kHz ultrasonic frequency at amplitude settings of 1 to 5 through a piezoelectric crystal in the hand piece, which, in turn, transfers the acoustic energy into the tissue via direct contact with the saline medium. The probe type was selected based on patient pain sensation, wound shape, and tissue adherence. The gold (standard) probe was the usual selection, while the green (gentle) probe was used for patients with any described discomfort or preference; the blue (tunnel) probe was used for wounds with undermined areas, and the magenta (aggressive) probe was used for very adherent necrotic debris. The hand piece and probe were sterilised in the hospital's central processing department by autoclave as per manufacturer's instructions. The treatment was applied by placing the selected and sterilised probe in direct contact with the wound bed. The saline irrigation rate was set at the lowest setting. Treatment continued until light bleeding occurred, and all necrotic tissue was removed.
All treatments were administered by the same qualified ET RN under medically aseptic conditions in the combined vascular surgery inpatient and wound clinic setting. Personal protective equipment, including a face visor, was used for the aerosol‐generating procedure as per local infection control practices. Local anaesthesia (1% lidocaine hydrochloride) was available by local injection prior to the LFCUD procedure and if they appeared uncomfortable at any point during their visit.
4.2.2. Outcomes
Wounds were assessed by a single, trained assessor who was a Registered Practical Nurse (RPN) familiar with wound care. This assessor was trained to use the camera to obtain a good digital image of the wound, use the acetate tracing system (Visitrak, Smith & Nephew, London, UK), and evaluate pain using a visual analogue scale (VAS). This nurse assessor was blinded to treatment allocation and performed weekly assessments prior to any treatments so that visual cues of group allocation were absent.
4.3. Wound‐healing outcomes
4.3.1. Wound appearance
Digital images were taken of the wound after treatment with either LFCUD or UC using a Canon Rebel 300D EOS, 8 megapixel resolution, 60 mm macro lens digital camera with a ring flash. To obtain high‐quality wound images that were comparable over time, patient set up and wound preparation were standardised as outlined previously.38 Briefly, the patient was positioned in a similar fashion in an examination room that had consistent lighting. Wound dressings were removed, and the wound was irrigated with normal saline. A ruler with millimetre graduations was placed against the peri‐ulcer skin and in the same plane as the wound opening. It was labelled with a date and a de‐identified number that was not linked to the patient or the treatment number.
Wound appearance was assessed using the Photographic Wound Assessment Tool (revPWAT),38 which is a pen and paper tool that is used to systematically assess 8 different characteristics of the wound base, edges, and peri‐ulcer skin using a digital image of the wound and peri‐ulcer skin. Each of the 8 domains of the revPWAT is ranked on a 4‐point scale with a total revPWAT score ranging from 0, representing a closed wound, to 32. Previous research has shown that this assessment tool is valid and reliable on different types of open wounds.38 A single assessor rated each wound image using the revPWAT using the instructions provided.38 Wound images were evaluated in a large group so that the assessor did not know who had received LFCUD or how many treatments had been delivered when the photo was taken.
4.3.2. Wound size reduction
Wound surface area was determined by tracing the wound perimeter 3 times onto a multi‐layer acetate designed for single‐patient use (Visitrak, Smith & Nephew, London, UK). Each tracing was then digitised using the previously validated Visitrak planimetry system39 The average WSA was calculated from 3 tracings taken from each wound and then entered into the database. WSA was determined using acetate tracings after cleansing the wound and before debridement from Weeks 1 to 12. Tracings were obtained after the ID physician debridements at Week 0 and Week 5 so that necrotic debris did not obscure the wound edges.
4.3.3. Wound closure
Wound closure was defined as wound edges apposed and the absence of exudate on removal of the in‐place dressing. When confirmed by the ET RN, it was recorded as WSA = 0 cm2 in the database.
4.3.4. Pain and treatment tolerance
Pain intensity was measured using the validated Visual Analogue Scale (VAS).40
This involved asking the patient to identify the level of wound pain experienced on a 100 mm ruler with slide indicator, where 0 mm represents no pain and 100 mm represents the worst pain imaginable. This question was asked by the blinded RPN assessor at the beginning of every visit and was reassessed in an identical manner immediately after treatment by the ET RN. Each assessor recorded their results independently into the database.
4.3.5. Adverse reactions and complications
The number and type of lower limb amputations and any other serious life‐ or limb‐threatening condition were documented. In addition, the frequency of emergency room visits and deaths were recorded.
4.3.6. Statistical analysis
A sample size of 70 participants was calculated based on an effect size determined when a similar treatment protocol was administered in a small pilot study.26 We considered a minimally important difference between healers and non‐healers to be 20% wound area reduction. This estimate was based on available literature of normally perfused patients11 and was supported by the pilot study data, which demonstrated a mean wound area reduction of 39.4% with ultrasound debridement; 19.4% was considered to be an optimistic target for non‐healing PAD wounds, and so, these estimations would yield a conservative sample size calculation. These sample size calculations were reviewed by the Methods Centre statistician during the approval phase.
Data were analysed using SPSS version 23.0 (SPSS, IBM Corporation, Armonk, NY). Baseline characteristics were compared between groups using the x 2 Test for categorical data and Student's t‐Test for continuous variables. The wound‐healing outcome data of change in wound surface area (cm2) and total revPWAT scores were calculated and compared using covariate analysis (ANCOVA) to adjust for baseline score. A 2‐sided P‐value of <0.05 was considered statistically significant. All patients were analysed in the group to which they were allocated. All missing data, which included patients who withdrew once treatment started, were imputed using the last outcome carried forward method.
5. RESULTS
In total, 103 patients were recruited from December 2013 to May 2015; however, 12 declined to participate for multiple reasons (eg, parking costs and frequency of visits), and 21 were screened out during the initial assessment. Of the 21 patients who were screened out, 7 had wounds that were smaller than 1 cm2 in area; 6 had an exposed bone or tendon visible in the wound; 4 were medically unstable; 2 had pacemaker devices in situ; 1 did not speak English; and 1 was taking immunosuppressive medications due to a previous renal transplant, making wound healing unlikely (see Figure 1).
Of the 70 patients who were randomised, 2 patients withdrew after randomisation but before commencing either treatment. One patient chose not to continue in the study (due to concerns about the potential treatment pain), and the other patient originally assigned to the UC group had exposed bone after initial sharp debridement and was therefore no longer eligible.
In total, 68 patients received either LFCUD plus UC (n = 32) or UC alone (n = 36). Of these patient, 11 (34.4%) received NPWT in the LFCUD group and 14 (38.9%) in the UC group. Five patients (3 in UC group and 2 in LFCUD group) withdrew later in the treatment phase. These patients withdrew for: practical reasons such as parking costs and frequency of visits (n = 2, UC group); medical issues, including medical decline to palliative status (n = 1, LFCUD group); infection requiring toe amputation (n = 1, UC group); and change of treatment plan initiated by homecare nurse (n = 1, LFCUD group). In addition to those who withdrew, 10 patients missed 1 treatment visit due to scheduling issues (8 patients receiving UC treatment and 2 patients receiving LFCUD). In total, 16 patients (23.5%) did not return for evaluation at the 12‐wk follow‐up visit, which occurred 7 wk after treatments stopped; 12 of these patients were in the UC group.
5.1. Patient characteristics
Patients are described in Table 1. Of the patients in the study, most were male, and the majority had evidence of significant vascular disease (having undergone either a previous angioplasty or bypass procedure or a major or distal amputation); 47 patients enrolled in the study had diabetes for a mean of 20.5 y and were evenly distributed between the LFCUD group (n = 22), with a mean of 22 y, and the UC group (n = 23), with a mean of 19 y duration. Patients in the LFCUD group had a longer mean duration of diabetes, longer wound duration, lower haemoglobin, and fewer bypass graft procedures, but these differences were not statistically significant. The number of patients receiving antibiotics in the LFCUD group was 62.5% compared with 66.7% UC, which was not a statistically significant difference (P = 0.72). There were more patients in the LFCUD group who had undergone previous trans‐metatarsal or digital amputation procedures, and this was statistically significant (x 2 (1) = 5.88, P = 0.01). Mean ankle brachial pressure index (ABPI) was significantly lower in the LFCUD group (0.83, P = 0.03); however, this assessment was performed on only 25 of 68 included patients.
Table 1.
Demographics of patients in LFCUD and UC groups
| Total sample (n = 68) | LFCUD (n = 32) | UC (n = 36) | P‐value | |
|---|---|---|---|---|
| Age (years) | 65.7 ± 10.5 | 67.2 ± 11.5 | 64.4 ± 9.5 | 0.28 |
| Male | 76.5 (52) | 75.0 (24) | 77.8 (28) | 0.79 |
| BMI | 26.4 ± 5.6 | 25.1 ± 4.8 | 27.6 ± 6.0 | 0.07 |
| Initial wound area (cm2) | 14.6 ± 20.2 | 13.5 ± 23.3 | 15.6 ± 17.3 | 0.68 |
| Wound duration (months) (n = 76) | 14.75 ± 27.32 | 17.06 ± 36.85 | 12.57 ± 13.57 | 0.68 |
| Wound location | ||||
| Toe/toe amputation site | 16.2 (11) | 15.6 (5) | 16.7 (6) | 0.91 |
| Mid‐foot/plantar | 27.9 (19) | 31.3 (10) | 25.0 (9) | 0.57 |
| Heel | 20.6 (14) | 18.8 (6) | 22.2 (8) | 0.72 |
| Malleolar | 7.4 (5) | 9.4 (3) | 5.6 (2) | 0.66 |
| Leg | 27.9 (19) | 25.0 (8) | 30.6 (11) | 0.61 |
| Diabetes | 69.1 (47) | 71.9 (23) | 66.7 (24) | 0.64 |
| Duration diabetes (years) (n = 45) | 20.56 ± 12.30 | 22.23 ± 13.72 | 18.96 ± 10.85 | 0.38 |
| Anticoagulant medication | 64.7 (44) | 65.6 (21) | 63.9 (23) | 0.89 |
| Antibiotic medication | 64.7 (44) | 62.5 (20) | 66.7 (24) | 0.72 |
| Haemoglobin (n = 77) | 114.21 ± 18.99 | 112.2 ± 17.33 | 116.1 ± 20.47 | 0.41 |
| HbA1C | 7.61 ± 1.45 | 7.59 ± 1.24 | 7.63 ± 1.65 | 0.93 |
| Albumin (n = 70) | 31.4 ± 5.68 | 30.9 ± 6.67 | 31.84 ± 4.65 | 0.54 |
| NPWT | 36.8 (25) | 34.4 (11) | 38.9 (14) | 0.70 |
| ABPI (n = 25) | 0.92 ± 2.34 | 0.83 ± 0.19 | 1.03 ± 0.25 | 0.03* |
| Arterial insufficiency | n = 60 | n = 29 | n = 31 | |
| Pedal pulse present a | 18.3 (11) | 13.8 (4) | 22.6 (7) | 0.42 |
| Angioplasty | 55.0 (33) | 55.2 (16) | 54.8 (17) | 0.98 |
| Bypass graft | 33.3 (20) | 27.6 (8) | 38.7 (12) | 0.36 |
| Prior amputation: | ||||
| Major: (transtibial/transfemoral) | 11.7 (7) | 6.9 (2) | 16.1 (5) | 0.43 |
| Distal: (pedal/digital) | 30.0 (18) | 44.8 (13) | 16.1 (5) | 0.02** |
Abbreviations: BMI, body mass index; HbA1C, glycated haemoglobin; LFCUD, low‐frequency (22.5 kHz) contact ultrasound debridement; NPWT, receiving negative pressure wound therapy to wound; UC, usual care.
Unless otherwise stated, values are expressed as mean ± Standard Deviation, with range in parentheses, or percentage (n).
Pedal Pulse Palpable = Dorsalis Pedis and/or Posterior Tibial pedal pulse palpable in affected limb.
Statistically significant difference but partial sample only: t (23)=−2.270, P = 0.033, n = 25.
Statistically significant difference: x 2 (1) = 5.88, P = 0.015.
5.1.1. Ultrasound debridement treatments
LFCUD was applied in continuous mode at amplitude 5 with physiological saline flow at 20% until surface debris was removed and light bleeding response was obtained. The average length of LFCUD treatment was 2 min and 59 s (with a range from 19 s to 6 min). Most patients were treated with the green (gentle) probe (44.0% or the gold (regular) probe (40.8%). Two patients received a total of 6 treatment episodes with the blue (tunnel shape) probe due to wound shape (4.8%), and 1 patient received the magenta (aggressive) probe at 2 visits (1.6%) to treat adherent slough.
5.2. Wound‐healing outcomes
5.2.1. Wound appearance (revPWAT score)
We found a greater improvement in wound appearance for the LFCUD group (adjusted mean = 7.34 (95% CI 5.81‐8.88) compared with the UC group (adjusted mean = 2.98 (95% CI 1.36‐4.60), and this difference was statistically significant (adjusted mean difference = 4.36 (95% CI 2.07‐6.66), P < 0.01) (Figure 3). After LFCUD treatments, revPWAT scores assigned by the blinded assessor for the 4 revPWAT domains that evaluated necrotic and granulation tissue amount and type were consistently lower (better).
Figure 3.
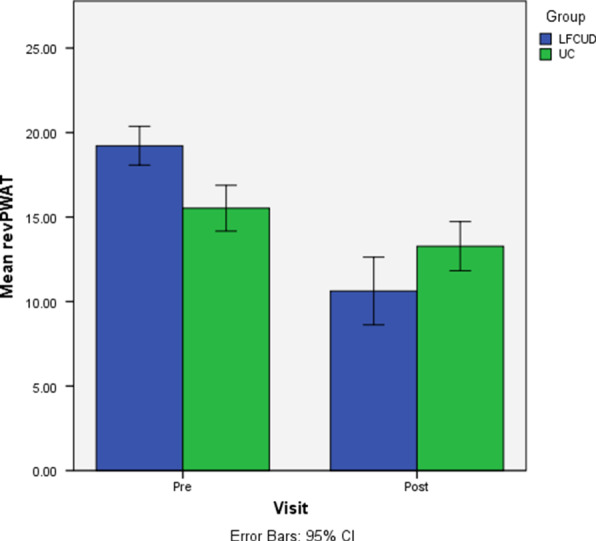
Change in wound appearance (Week 0 to Week 5). Abbreviations: LFCUD, low‐frequency contact ultrasound debridement; UC, usual care; revPWAT, revised photographic wound assessment tool. *Change in wound appearance was significantly greater in the LFCUD group post‐treatment (Week 5) after controlling for baseline revPWAT score (P = <0.01)
5.2.2. Wound size reduction
The LFCUD group showed progression and significant decline in WSA measurements before and after over the 4 weekly LFCUD treatments (P = <0.01, see Figure 4). Changes in WSA in the UC group were variable over the 4 wk of treatment, and no significant change over time was detected (P = 0.93). We found a greater reduction in WSA after 4 wk of treatment (Week 5) in the LFCUD group (adjusted mean = 31.63% (95% CI 3.54‐59.70) than in the UC group (adjusted mean = 18.06%, 95% CI −8.42 to 44.54), but this difference was not statistically significant (P = 0.48). Wounds measured at the 12‐wk follow‐up visit were smaller in the LFCUD group (adjusted mean = 4.83 cm2 (95% CI −10.673 to 1.85) than in the UC group (adjusted mean = 9.25 cm2 (95% CI −10.56 to 1.74); however, because of the large variability in initial wound size, these differences were not statistically different (t (46) = −1.42, P = 0.16).
Figure 4.
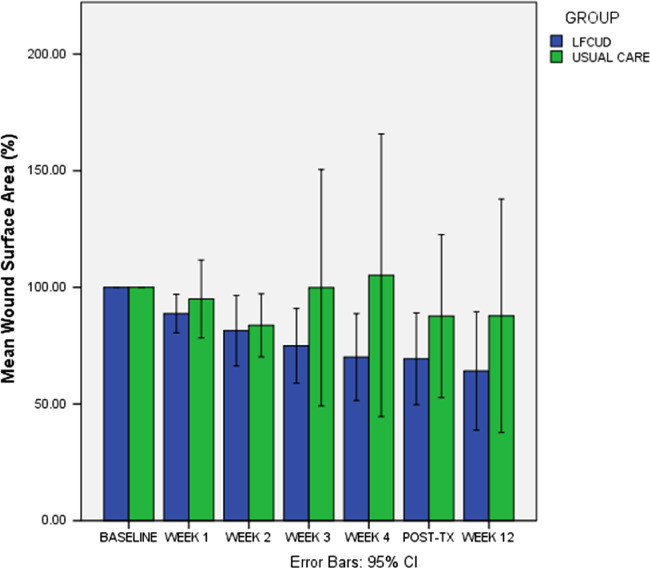
Percentage change in wound size from baseline. Abbreviations: LFCUD, low‐frequency contact ultrasound debridement; UC, usual care. (Baseline wound surface area (WSA) = 100%). Linear trend in WSA reduction was significant (P = <0.01) for LFCUD group during treatment period (Week 0 to Week 5) but not significant for UC; P = (0.935) as adjusted not assuming equal variances
5.2.3. Wound closure
The only 2 patients to achieve wound closure by Week 5 were in the LFCUD group. Of the 47 patients who attended the 12‐wk follow‐up visit, there were significantly more healed wounds in the treatment group (33.3%) compared with the control group (8.3%), x 2 (1) = 5.75, P = 0.03. Therefore, the odds ratio for healing with LFCUD was 5.00 (95% CI 1.24 to 20.25) compared with the control group. The only 2 patients who produced healthy granulation tissue deemed eligible for wound closure by skin graft were in the LFCUD treatment group.
5.2.4. Pain and treatment tolerance
Pain intensity decreased after each of the 4 LFCUD treatment sessions (n = 68). The average reduction in pain intensity was 9·3 mm (95% CI 3.5 to 15.1, P = 0.003) at Week 3 and 16·6 mm (95% CI 9.0 to 24.2, P = <0.001) at Week 2. Pain scores were also lower after the first UC treatment (Week 1), 6.11 mm (95% CI 0.152‐12.1, P = 0.04), but were similar before and after subsequent UC treatments. Patients receiving LFCUD commonly reported a sensation of vibration rather than pain and frequently noted that they were surprised as they had anticipated pain at the first application. All patients were informed that local anaesthesia would be available should the LFCUD or UC procedure be uncomfortable. Two patients requested local anaesthesia prior to initial LFCUD treatments but not thereafter. Only 1 patient requested a local anaesthetic for the initial 2 LFCUD treatments and found no need to continue by the third treatment. Many patients in the LFCUD group reported they believed the treatment was improving their wound, and 1 patient reported restored sensation in his forefoot that had been absent for an extended time. There was minimal bleeding with ultrasound debridement despite most patients receiving anticoagulant therapy.
5.2.5. Adverse reactions and complications
Adverse events in this high‐risk population were surprisingly rare, and none were related to treatment. In total, there were 12 adverse events that were equally distributed between groups. In the LFCUD group, there were: 2 new infections; 1 arterial occlusion requiring admission to hospital for angioplasty; 1 dressing reaction; 1 burn injury from a house fire; and 1 death, which occurred several weeks after treatment had concluded. In the UC group, 4 patients developed new wound infections; 1 patient developed an infection requiring toe amputation; and 1 patient developed a medication‐related rash. There were no episodes of excessive bleeding. A similar proportion of patients in each treatment group had wounds that increased in size between initial visit and the following 12 wk (21.9% LFCUD: 27.8% UC group).
As per the REB requirements, all complications were reported to the Data Safety Monitoring Board and were later deemed unrelated to the device by both the infectious disease specialist and vascular surgeons.
6. DISCUSSION
The results of this study demonstrate that 4 weekly LFCUD treatments produced better‐quality granulation tissue and a lower proportion of necrotic tissue present in the wound base of vasculopathic wounds. These changes in wound appearance were reflected in a significantly lower total revPWAT score in LFCUD compared with UC‐treated wounds. In addition, we found that wound surface area decreased significantly after LFCUD but not UC treatment. A significantly greater number of wounds closed in patients who received LFCUD, with an odds ratio of 5.00 (95% CI 1.24‐20.25), compared with UC. These improved healing outcomes were achieved in a very challenging group of patients with significant vascular disease. Complication rates over the 12‐wk observation period were similar between groups (in 6 of 68 patients). LFCUD was well tolerated, did not induce additional pain, and was not associated with any treatment‐related adverse events.
Our findings show that applying LFCUD results in a significant improvement of wound appearance. Specifically, there was less necrotic tissue and improved granulation tissue appearance after LFCUD treatment. We used the PWAT to detect these improvements in wound appearance. PWAT was designed to detect changes in wound base and edges that occur when wounds heal. It has been validated on all types of wounds and has shown to have excellent intra‐ and inter‐rater reliability and concurrent validity.38 This is the first time such a systematic approach was undertaken to describe changes in wound appearance after LFCUD. Herberger and colleagues reported that LFCUD produced a subjective improvement in wound appearance that was similar to that produced by surgical wound debridement·41
This study also represents the first rigorous evaluation of the use of LFCUD to treat wounds occurring in patients with significant vasculopathy. LFCUD has been used previously in patients with diabetic foot ulcers with osteomyelitis and other types of wounds·32, 41, 42 While other reports have used similar technology to hasten wound healing, this study is the first to complete a well‐controlled clinical trial with calculated sample size that had the power to detect a difference in healing outcomes between groups. Given the nature of the study design, we feel confident suggesting that weekly LFCUD treatments produced better healing outcomes in the challenging patients serviced by this tertiary care service. Having treatment options for patients with long‐standing arterial and/or venous disease is important clinically as this group of patients is often excluded from most clinical trials, and debridement is often considered contraindicated or reserved for only highly skilled clinicians or surgeons.
The mean percentage wound surface area reduction at 12 wk was 47.23% (SD = 51.85) of the initial size when wounds received 4 weekly treatments of LFCUD. This healing rate is impressive considering the extensive vascular pathology present in the subjects included in this study. The average change in wound size was similar to that reported previously in a small pilot study group, 39.4%. Although the mean percentage wound surface area reduction in the UC group was lower, at 19.51% (SD = 137.16), it did not achieve statistical significance. Our inability to detect a statistical difference between LFCUD and the control group was likely due to the large variability in healing response seen in the group that received traditional wound care.
It is also possible that greater differences between groups would have been revealed if LFCUD treatments continued for a few more weeks. The LFCUD treatment schedule used in the present study was selected after review of existing research and considering what would be feasible for this patient group. A previous study showed that LFCUD treatments (25 kHz) applied in weekly intervals, as necessary, to diabetic foot ulcers with osteomyelitis accelerated wound healing at 2‐ and 3‐mo time points·43 Another uncontrolled study involving 10 patients with chronic leg ulcers of mixed aetiologies with an average 20‐mo duration reported that LFCUD produced wound closure in over 33% of patients within 5 treatments and was especially useful for skin graft preparation.42 The ideal timing between LFCUD treatments has yet to be determined. Even though it is known that an increase of conventional sharp debridement sessions promotes wound contraction,13 there is no consensus on the best schedule with that method to achieve the greatest effect. A more frequent or prolonged treatment schedule would not likely be feasible for patients in this vascular service who are typically reviewed in clinic on a monthly basis. As it was, 16 patients, the majority in the UC group, did not attend the 12‐wk follow‐up visit.
Procedural‐related pain after debridement is a common side effect of sharp debridement, which often necessitates the use of local anaesthesia. However, we found that pain was significantly reduced in the LFCUD group after each debridement experience. This is encouraging as anxiety and stress are known to interfere with healing·44 Our findings reflect those of previous researchers, who have found that patients with chronic leg ulcers treated with LFCUD reported little pain42 and that LFCUD was less painful than surgical debridement·41 This finding confirms our previous results from the pilot study and suggests that the LFCUD is well tolerated in a vasculopathic population and may be less painful than current usual practice·
Our data did not allow us to confirm if LFCUD treatments were associated with lower occurrence or recurrence of wound infection or biofilms. New infections during the treatment period were extremely rare. This is likely because the ID physician who assessed patients at the initial assessment prescribed antibiotics to those he felt had or were at risk of infection. Furthermore, all patients underwent an extensive sharp debridement procedure that removed all necrotic or devitalised tissue in the wound base when they entered the study. This judicious approach used to manage wound bioburden that is UC in this facility was likely responsible for the low incidence of new infections and other complications observed in this group of high‐risk patients.
Experimental research suggests that ultrasound may have direct bactericidal effects on common wound pathogens. Schoenbach and Song found that 5 min of low‐frequency ultrasound (20 kHz) applied via a water bath to septic burn wounds of rats decreased Pseudomonas Aeruginosa bacteria and resulted in greater graft survival and wound reepithelialisation.45 These positive outcomes were in contrast to those in the control group that had a 25% death rate due to complications associated with sepsis. Furthermore, bacterial biofilms have been found to be more susceptible to the antibiotic gentamicin when low‐frequency ultrasound was applied46 Low‐frequency ultrasound dismantles the protective blocking effect of Pseudomonas aeruginosa and more than doubles the transport of gentamicin through Escherichia coli biofilms·47 Thus, ultrasound administered along with systemic antibiotics48 may have a synergistic effect on wound bioburden by making biofilm bacteria vulnerable to antibiotic penetration and speeding up bacterial metabolism.49
In addition to removing a potential medium for bacterial proliferation, ultrasound has been shown to stimulate several cells involved in wound‐healing processes, including angiogenesis, inflammation, and collagen synthesis. 23, 24, 25, 26 In addition, sonication of injured tissue is known to increase vasodilation, local blood flow, oxygenation,27 and improve the quality of granulation tissue.25, 28 More recently, ultrasound has been cited to promote cell migration mechanisms and cell adhesion, which are critically necessary in wound healing·29
6.1. Limitations
Patients recruited to this study had a variety of wound aetiologies, including venous leg ulcers, arterial wounds, and diabetic foot wounds. While it may have been preferable to restrict the sample to a particular wound aetiology, it was considered unlikely that sufficient participants could be recruited within the catchment area to permit analysis. We were pleased that our sample was representative of a typical vascular surgery department population, which was clinically relevant.
The number of patients recruited to this study was based on a sample size calculation derived from expected healing rates obtained in a small pilot study. While this approach determines whether studies have ample power to detect differences between study outcomes, it did not result in significant differences in all wound‐healing outcomes. Recruiting additional patients could have resulted in more conclusive and consistent findings.
We were unable to blind the participants to treatment allocation. As patients knew when they received LFCUD treatment, their perceived pain after treatment may have been influenced by knowing they were receiving a “newer therapy”. We did see a proportionally higher rate of missed appointments in those patients assigned to the UC group, an unfortunate result likely due to unblinded treatment groups.
Additionally, the ET RN who provided the LFCUD treatments could not be blinded. Rather, we made sure all assessments were carried out by a single blinded assessor, and we incorporated objective, standardised study outcomes that are free of bias. In addition, changes in wound appearance were assessed on wound photographs that were de‐identified so that the ET RN could not tell the treatment group or the timing of the assessment.
Concurrent wound treatments, including the use of silver alginate dressings or NPWT, may have affected healing outcomes. All patients also received regular dressing changes administered using a publically funded home care system. Any 1 of these interventions could affect healing outcomes. We tried to reduce this confounding variable by having similar UC administered to both treatment groups.
7. CONCLUSION
We found that 4 weekly LFCUD treatments added to UC significantly improved wound appearance and produced reductions in wound size that were not detected in a similar group of patients receiving only UC. This improved wound‐healing outcomes in a complex patient group with significant vascular compromise without increasing their complication rate and with few and minor adverse events. .
7.1. Clinical implications and future research
Our study suggests that LFCUD is a safe and efficient method of wound preparation, which is well‐tolerated and feasible to apply by the ET RN in a tertiary care vascular wound clinic. We also found that LFCUD is a feasible and well‐tolerated method of debridement for a vulnerable population with vascular disease who do not have a plethora of treatment options. Producing improvements in the quality of tissue in the wound bed of these patients may also make these individuals more eligible for wound closure by skin graft. Additionally, LFCUD may improve access to debridement procedures as it was found to be well‐tolerated, without the need of local anaesthesia, and was feasible for application by non‐surgical providers within an outpatient setting.
A major benefit of LFCUD is the ease of application by a non‐physician. Debridement requires specific knowledge and skills and carries inherent risks, which prohibit the availability of the procedure in many areas of practice. Furthermore, there are gaps in education delivery and policy, with few definitive protocols available.20 In a supported environment, the availability of nurse‐applied LFCUD allowed for improved access to care. This benefit was also described for the nurse‐applied LFCUD treatment of a peri‐stomal wound, which allowed for an earlier skin graft and decreased hospital stay.50
Importantly, we believe that high‐risk populations with vascular disease should be included in future LFCUD trials. Future research is needed to determine if better healing outcomes or reduced infection may be attained with increased applications over an extended time.
Murphy CA, Houghton P, Brandys T, Rose G, Bryant D. The effect of 22.5 kHz low‐frequency contact ultrasound debridement (LFCUD) on lower extremity wound healing for a vascular surgery population: A randomised controlled trial. Int Wound J. 2018;15:460–472. 10.1111/iwj.12887
REFERENCES
- 1. Hirsch AT, Haskal ZJ, Hertzer NR, et al. The management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. Catheter Cardiovasc Interv. 2012;79(4):501‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirsch AT, Criqui MH, Treat‐Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317‐1324. [DOI] [PubMed] [Google Scholar]
- 3. Bozkurt AK, Tasci I, Tabak O, Gumus M, Kaplan Y. Peripheral artery disease assessed by ankle‐brachial index in patients with established cardiovascular disease or at least one risk factor for atherothrombosis—CAREFUL study: a national, multi‐center, cross‐sectional observational study. BMC Cardiovasc Disord. 2011;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570‐2581. [DOI] [PubMed] [Google Scholar]
- 5. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16(Suppl 1):S75‐S83. [DOI] [PubMed] [Google Scholar]
- 6. Canadian Diabetes Association . An economic tsunami: the cost of diabetes in Canada. 2009.
- 7. Canadian Diabetes Association and Diabete Quebec . Diabetes: Canada at the tipping point. Charting a new path. 2011:1–58.
- 8. Attinger CE, Evans KK. In: Sidawy AN, ed. Operative Management of Diabetic Foot Wounds and Infections: Maximizing Length and Optimizing Biomechanics. Vol First. Philadelphia: Lippincott, Williams and Wilkins; 2006:50. [Google Scholar]
- 9. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zammit MC, Fiorentino L, Cassar K, Azzopardi LM, LaFerla G. Factors affecting gentamicin penetration in lower extremity ischemic tissues with ulcers. Int J Low Extrem Wounds. 2011;10(3):130‐137. [DOI] [PubMed] [Google Scholar]
- 11. Cardinal M, Eisenbud DE, Armstrong DG, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009;17(3):306‐311. [DOI] [PubMed] [Google Scholar]
- 12. Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous leg ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen. 2005;13(2):131‐137. [DOI] [PubMed] [Google Scholar]
- 13. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic ulcer study group. J Am Coll Surg. 1996;183(1):61‐64. [PubMed] [Google Scholar]
- 14. Steed DL. Debridement. Am J Surg. 2004;187(5):71S‐74S. [DOI] [PubMed] [Google Scholar]
- 15. Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time‐dependant therapeutic window. J Wound Care. 2010;19(8):320‐328. [DOI] [PubMed] [Google Scholar]
- 16. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 17. Kirshen C, Woo K, Ayello EA, Sibbald RG. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care. 2006;19(9):506‐517. [DOI] [PubMed] [Google Scholar]
- 18. Sibbald R, Goodman L, Woo KY, et al. Special considerations in wound bed preparation 2011: an update. Adv Skin Wound Care. 2011;24(9):415‐436. [DOI] [PubMed] [Google Scholar]
- 19. Shannon RJ. A cost‐utility evaluation of best practice implementation of leg and foot ulcer care in the Ontario community. Wound Care Canada. 2007;5:S53‐S56. [Google Scholar]
- 20. Rodd‐Nielsen E, Harris CL. Conservative sharp wound debridement: an overview of Canadian education, practice, risk, and policy. J Wound Ostomy Continence Nurs. 2013;40(6):594‐601. [DOI] [PubMed] [Google Scholar]
- 21. Kloth LC, Niezgoda JA. In: Muccullogh JM, Kloth LC, eds. Ultrasound for Wound Debridement and Healing. Vol Fourth. Philadelphia, PA: F.A. Davis; 2010:545‐575. [Google Scholar]
- 22. Voigt J, Wendelken M, Driver V, Alvarez OM. Low‐frequency ultrasound (20–40 kHz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta‐analysis of eight randomized controlled trials. Int J Low Extrem Wounds. 2011;10(4):190‐199. [DOI] [PubMed] [Google Scholar]
- 23. Webster DF, Pond JB, Dyson M, Harvey W. The role of cavitation in the in vitro stimulation of protein synthesis in human fibroblasts by ultrasound. Ultrasound Med Biol. 1978;4(4):343‐351. [DOI] [PubMed] [Google Scholar]
- 24. Dyson M, Luke DA. Induction of mast cell degranulation in skin by ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 1986;33(2):194‐201. [DOI] [PubMed] [Google Scholar]
- 25. Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol. 1990;16(3):261‐269. [DOI] [PubMed] [Google Scholar]
- 26. Harvey W, Dyson M, Pond JB, Grahame R. The 'in vitro' stimulation of protein synthesis in human fibroblasts by therapeutic levels of ultrasound. EXCERPTA MEDICA, ICS NO 363. 1975:10–21. [DOI] [PubMed]
- 27. Sugita Y, Mizuno S, Nakayama N, et al. Nitric oxide generation directly responds to ultrasound exposure. Ultrasound Med Biol. 2008;34(3):487‐493. [DOI] [PubMed] [Google Scholar]
- 28. Thawer HA, Houghton PE. Effects of ultrasound delivered through a mist of saline to wounds in mice with diabetes mellitus. J Wound Care. 2004;13(5):171‐176. [DOI] [PubMed] [Google Scholar]
- 29. Roper J, Harrison A, Bass MD. Induction of adhesion‐dependent signals using low‐intensity ultrasound. J Vis Exp. 2012;(63):e4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dyson M, Pond JB. The effects of ultrasound on circulation. Physiotherapy. 1973;59(9):284‐287. [PubMed] [Google Scholar]
- 31. Cheng LH, Stewart J, Thompson M, Adlam DM. Ultrasonic debridement of contaminated facial wounds. Br J Oral Maxillofac Surg. 2002;40(2):149‐150. [DOI] [PubMed] [Google Scholar]
- 32. Breuing KH, Bayer L, Neuwalder J, Orgill DP. Early experience using low‐frequency ultrasound in chronic wounds. Ann Plast Surg. 2005;55(2):183‐187. [DOI] [PubMed] [Google Scholar]
- 33. Carmo M, Mazzaccaro D, Barbetta I, et al. Use of ultrasound debridement as an adjunctive tool for treating infected prosthetic vascular grafts in the lower extremities. Ann Vasc Surg. 2015;29(3):607‐615. [DOI] [PubMed] [Google Scholar]
- 34. Tewarie L, Moza AK, Zayat R, Autschbach R, Goetzenich A, Menon AK. Ultrasound‐assisted treatment of sternocutaneous fistula in post‐sternotomy cardiac surgery patients. Eur J Cardiothorac Surg. 2015;47(5):e180‐e187. discussion e187. [DOI] [PubMed] [Google Scholar]
- 35. Drisko CH. Root instrumentation. Power‐driven versus manual scalers, which one? Dent Clin N Am. 1998;42(2):229‐244. [PubMed] [Google Scholar]
- 36. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41:S395‐S519. [DOI] [PubMed] [Google Scholar]
- 37. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):132‐173. [DOI] [PubMed] [Google Scholar]
- 38. Thompson N, Gordey L, Bowles H, Parslow N, Houghton P. Reliability and validity of the revised photographic wound assessment tool on digital images taken of various types of chronic wounds. Adv Skin Wound Care. 2013;26(8):360‐373. [DOI] [PubMed] [Google Scholar]
- 39. Sugama J, Matsui Y, Sanada H, Konya C, Okuwa M, Kitagawa A. A study of the efficiency and convenience of an advanced portable wound measurement system (VISITRAK). J Clin Nurs. 2007;16(7):1265‐1269. [DOI] [PubMed] [Google Scholar]
- 40. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 41. Herberger K, Franzke N, Blome C, Kirsten N, Augustin M. Efficacy, tolerability and patient benefit of ultrasound‐assisted wound treatment versus surgical debridement: a randomized clinical study. Dermatology. 2011;222(3):244‐249. [DOI] [PubMed] [Google Scholar]
- 42. Tan J, Abisi S, Smith A, Burnand KG. A painless method of ultrasonically assisted debridement of chronic leg ulcers: a pilot study. Eur J Vasc Endovasc Surg. 2007;33(2):234‐238. [DOI] [PubMed] [Google Scholar]
- 43. Amini S, ShojaeeFard A, Annabestani Z, et al. Low‐frequency ultrasound debridement in patients with diabetic foot ulcers and osteomyelitis. Wounds. 2013;25(7):193‐198. [PubMed] [Google Scholar]
- 44. Kielcot‐Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. The Lancet. 1995;346:1194‐1196. [DOI] [PubMed] [Google Scholar]
- 45. Schoenbach SF, Song IC. Ultrasonic debridement: a new approach in the treatment of burn wounds. Plast Reconstr Surg. 1980;66(1):34‐37. [PubMed] [Google Scholar]
- 46. Qian Z, Sagers RD, Pitt WG. The effect of ultrasonic frequency upon enhanced killing of P. aeruginosa biofilms. Ann Biomed Eng. 1997;25(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 47. Carmen JC, Nelson JL, Beckstead BL, et al. Ultrasonic‐enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J Infect Chemother. 2004;10(4):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rediske AM, Hymas WC, Wilkinson R, Pitt WG. Ultrasonic enhancement of antibiotic action on several species of bacteria. J Gen Appl Microbiol. 1998;44(4):283‐288. [DOI] [PubMed] [Google Scholar]
- 49. Phillips PL, Yang Q, Sampson E, Schultz G. Effects of antimicrobial agents on an in vitro biofilm model of skin wounds. Adv Skin Wound Care. 2010;1:299‐304. [Google Scholar]
- 50. Shannon MK, Williams A, Bloomer M. Low‐frequency ultrasound debridement (sonaca‐185) in acute wound management: a case study. Wound Pract Res. 2012;20(4):200‐205. [Google Scholar]


