Abstract
Current detection of pressure ulcers relies on visual and tactile changes at the skin surface, but physiological changes below the skin precede surface changes and have a significant impact on tissue health. Inflammatory and apoptotic/necrotic changes in the epidermal and dermal layers of the skin, such as changes in interstitial fluid (also known as subepidermal moisture (SEM)), may precede surface changes by 3–10 days. Those same epidermal and subepidermal changes result in changes in the electrical properties (bioimpedance) of the tissue, thereby presenting an objective, non‐invasive method for assessing tissue damage. Clinical studies of bioimpedance for the detection of pressure ulcers have demonstrated that changes in bioimpedance correlate with increasing severity of pressure ulcer stages. Studies have also demonstrated that at anatomical locations with pressure ulcers, bioimpedance varies with distance from the centre of the pressure ulcers. The SEM Scanner, a handheld medical device, offers an objective and reliable method for the assessment of local bioimpedance, and therefore, assessment of tissue damage before signs become visible to the unaided eye. This literature review summarises pressure ulcer pathophysiology, principles of bioimpedance and clinical research using bioimpedance technology to assess pressure ulcers.
Keywords: Assessment, Bioimpedance, Literature review, Pathophysiology, Pressure ulcer
Introduction
In spite of efforts to prevent pressure ulcers [e.g. Ref. 1], the incidence rate remains high worldwide, especially in hospitals and nursing homes 2, 3, 4, with a mean incidence in the acute‐care setting of 17·6% (range 1·4–49%) and in the long stay setting of 6·63% (range 3·1–8·4%) 4. The cost of treating pressure ulcers increases dramatically once the skin is broken, with the average cost of hospital for a Stage IV pressure ulcer1 acquired either in the hospital or community setting exceeding $120 000 5. Prevention provides significant cost savings compared to treatment 6, 7, for example, prevention is estimated to cost up to 87·57 € per patient per day, but treatment can cost up to 470·49 € per patient per day 6. Therefore, detection of a pressure ulcer at its earliest stage is imperative to afford intervention. In 2008, the World Union of Wound Healing Societies presented a call to action for the development of objective tests to support treatment decisions and aid in the cost‐effective use of limited resources.
Bioimpedance techniques constitute painless and harmless methods for acquiring data from human subjects and have been extensively reviewed 9, 10. Measures of bioimpedance such as total impedance, capacitance, resistive or reactive components and change in impedance can be correlated to physiological events related to changes in volume, orientation and distribution of dermal fluids and tissues 10. Bioimpedance techniques have been used to monitor the respiratory and cardiovascular systems, the brain and the distribution of fluids in the body because of events (e.g. surgery, dialysis) or conditions (e.g. lymphedema, malnutrition) 9. Changes in bioimpedance because of the pathophysiological processes of early pressure‐induced tissue damage may prove to be useful clinical information in the prevention of more advanced stages of pressure ulcers.
The SEM Scanner (Bruin Biometrics, LLC, Los Angeles, CA) is a handheld medical device that offers an objective and reliable method for the assessment of local bioimpedance, and therefore, detection of early tissue damage and pre‐stage I pressure ulcers before the damage becomes visible to the unaided eye. The SEM Scanner was designed for use by health care providers as part of pressure ulcer prevention programmes with the hope of leading to targeted interventional efforts prior to rupture or breakage of the skin. This review of the literature suggest that while the pathophysiology of pressure ulcers is complex at the molecular and cellular level, the consequence of these effects is a change in the bioelectrical properties that can be detected readily in the area of a developing ulcer.
Pathophysiology of pressure ulcer development
Tissue ischaemia, with or without reperfusion injury, and cellular deformation caused by mechanical loading are the commonly accepted aetiological factors for pressure ulcer development 11, 12, 13. Furthermore, lymphatic dysfunction caused by compression or ischaemia 14 likely contributes towards pressure ulcer development. Figure 1 presents a conceptual framework for the physiological events that occur in pressure ulcer aetiology.
Figure 1.

Conceptual framework for physiological processes leading to pressure ulcer development. The manifestation threshold marks the point at which damage is apparent at the skin and is the point of pressure ulcer detection and intervention under today's standard of care. The damage threshold 8 marks the point at which an objective test of physiological changes below the skin could reveal early damage that, with intervention, could prevent a pressure ulcer.
Studies have suggested that tissue ischaemia (i.e. local obstruction of blood vessels) is a primary concern 15 as it reduces the supply of nutrients to cells and increases the accumulation of toxic metabolites, leading to hypoxia, apoptotic or necrotic events and tissue damage 16. Further research has suggested that reperfusion injury following ischaemia may be a significant contributor to the early stages of pressure ulcer development 17. Reperfusion triggers inflammatory processes, increases indicators of oxidative stress and decreases indicators of antioxidant activity, leading to necrosis 18. Inflammatory mediators and oxygen‐free radicals have both been shown to modify microvascular permeability 19, resulting in fluid accumulation in the extracellular spaces 20.
Recent research has demonstrated that cellular deformation likely contributes significantly to the development of pressure ulcers, particularly when muscle tissue is involved 21, 22, and that ischaemia and deformation may be related at greater tissue depths 23. Cellular deformation leads to cell death 24 and may occur more rapidly than cell death caused by ischaemia and its downstream events (e.g. reperfusion injury, oxidative stress, nutrient depletion. See Figure 1) 25. Impaired lymphatic draining, a consequence of both ischaemia and deformation, has been associated with metabolic waste product accumulation and interstitial fluid increases 26, and the inflammatory response itself can lead to cell death [e.g. Ref. 27]. These physiological processes lead to apoptosis, necrosis and an inflammatory response with characteristic heat (calor), redness (rubor), swelling (tumour) and pain.
The extracellular matrix (ECM) contributes to the unique properties of the skin, including its strength, elasticity and compressibility 28. The ECM is composed of proteins and polysaccharides in water occupying the extracellular space and provides a medium through which nutrients and wastes can be transported to and from the cell 29 as well as being a key component in the wound healing process 28. Fluid accumulation in the extracellular space can result from change in hydrostatic or oncotic pressure acting on microvascular walls, alterations to the endothelial walls of cells or changes in the lymphatic outflow; accumulation of interstitial fluid can also be the result of inflammatory mediators 20. Inflammation as a response to tissue injury includes the release of these inflammatory mediators responsible for microvessel permeability, vasodilation and leukocyte recruitment that results in the release of reactive oxygen and nitrogen species that degrade the ECM in an attempt to relieve pressure from the additional fluid 20.
Ultimately, apoptosis, necrosis and the inflammatory process lead to leakage from vascular vessels and other changes that modify the underlying structure of the damaged tissue, including variation in interstitial fluid, which can also be described as subepidermal moisture (SEM). While the biochemical and physiological processes involved with the aetiology of pressure ulcers is complex, an increase in interstitial fluid is an integral part of the process. Correspondingly, changes in SEM become a logical choice for a physiological marker of pressure ulcer development.
Electrical bioimpedance measurements in the clinical setting
Bioimpedance, electrical properties that can be used to study biological tissues 30, is determined by applying a voltage to an object and measuring the current passing through the object, or vice‐versa, by applying a fixed current and measuring the voltage difference at the receiving electrode (Figure 2). Electrical bioimpedance monitoring is considered a diagnostic method based on passive electrical properties of biological tissues 31.
Figure 2.
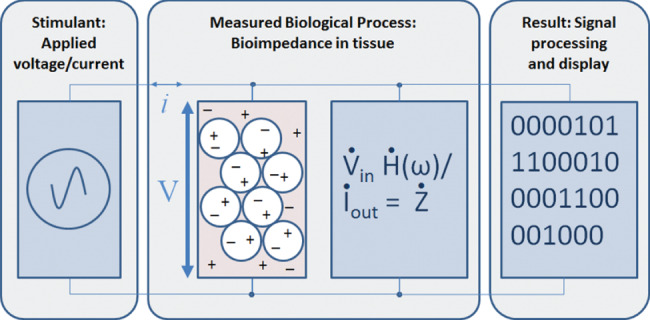
Schematic illustrating the use of a device to determine the bioimpedance of a biological tissue. Application of a current of known frequency, transmission of that current through the extracellular and intracellular spaces and measurement of the resulting voltage.
In a simplified model [Figure 3, adapted from Ref. 32], the extracellular fluid2 and the intracellular fluid are modelled as resistors (Re and Ri, respectively) that transmit current, and the lipid bilayer of the cell membrane is modelled as a capacitor (C m). With direct current, there is negligible conductance of current through the cells; rather, the current travels around the cells through the extracellular space. With alternating current, the level of conductance through the cells increases with increasing frequency of the current. Therefore, at sufficiently high frequencies, above 10 MHz 33, the capacitance of the cell membrane becomes insignificant 34, and the measured bioimpedance represents both the intracellular and extracellular spaces.
Figure 3.
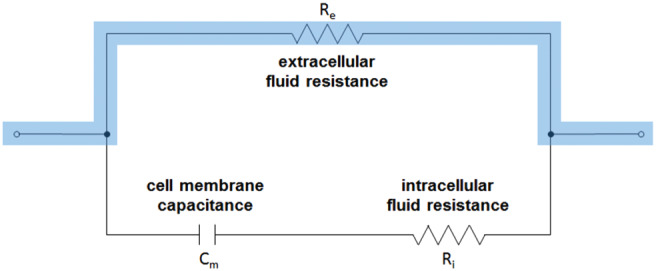
Simplified circuit diagram representing the bioimpedance of a biological tissue. Lower frequencies, highlighted, predominantly interrogate the extracellular space, whereas higher frequencies interrogate both the extra‐ and intracellular spaces because of reduced capacitance of the cell membranes with increasing frequency.
Bioimpedance values vary by cell type and tissue or organ type 35, 36 and, therefore, by anatomical site and almost certainly from person to person. A complete understanding of the reasons for bioimpedance variations associated with physiological activity is probably impossible 10, but changes in bioimpedance measures over time have been used reliably for evaluating numerous medical conditions [reviewed in Refs 9, 31]. In approaching the task of designing a biomedical device for patient monitoring, it becomes important to narrow the problem down to the specific parameters of interest and to control as many of the variables as possible. In the case of bioimpedance, some obvious variables include intracellular versus extracellular fluid, tissue type or anatomical site being assessed and user training or skill. For example, Gonzalez‐Correa et al. 37 demonstrated that bioimpedance readings increased with increasing pressure in both human and rat tissues and hypothesised that the pressure‐related changes were caused by a loss of tissue fluid and extracellular space available to the current flow. This observation that bioimpedance readings change with increasing pressure highlights the need to control for probe application pressure when collecting readings.
The measurement of bioimpedance in tissue reduces to a few principles that have been modelled as an electrical circuit. Tissue bioimpedance had been established as a method for the detection of local oedema in patients with chronic lymphatic obstruction resulting from uterine cancer surgery and has been validated by computed‐tomography (CT) analysis 38. Both methods concur with the model of SEM as a change in the ratio of tissue fluid to subcutaneous tissue area. Furthermore, Swisher 39 recently published an animal model of pressure‐induced injury that clearly shows changes in tissue bioimpedance corresponding with spatial distribution and severity of the induced injury. Their results demonstrated in vivo that an electronic device can be used to non‐invasively detect an ensuing pressure ulcer before it could be visually observed.
Use of a handheld device for the detection of pressure ulcers
The detection of pressure ulcers has been performed through visual and tactile examinations of the skin. Localised erythema that results from pressure can be transient (resolves within 20–30 min) or persistent (does not resolve); it can also be blanchable (visible transition from rubor to pallor to rubor upon application and release of pressure) or non‐blanchable (no transition with pressure) 40, 41. Transient erythema is considered a characteristic of reactive hyperaemia 40, a restorative increase in blood flow following ischaemia. Persistent erythema is considered a pathological response to ischaemia and is distinct from reactive hyperaemia 40. Non‐blanchable erythema is a key component of the NPUAP/EPUAP definition of a Stage I pressure ulcer 8. Clinical studies have demonstrated that not all persistent erythema is non‐blanchable and that not all transient erythema is blanching 40, 41, suggesting that what is observed at the surface is not sufficient to understand the underlying damage. Furthermore, visual identification of tissue colour changes can be difficult in dark skin tone patients, as suggested by the discrepancy in Stage I and II pressure ulcers detected in Caucasians (38% Stage I and 37% Stage II) and African Americans (13% Stage I and 41% Stage II) in a prevalence survey 42. The clinical utility of a reliable, objective medical device to assist in the identification of early stage pressure ulcers is clear.
In an effort to identify objective measures associated with early development of pressure ulcers, bioelectrical impedance readings were collected from three progressively larger zones over the trochanter and coccyx from 10 patients at high risk for pressure ulcers (hospitalised patients) and 10 volunteers from the community in a control group by way of a portable, single‐frequency bioelectrical impedance analyser 43. Local bioelectric impedance was lower in a group at high risk for pressure ulcers as compared with age‐matched controls for each individual site and zone (P < 0·01). Bioelectrical impedance was subsequently investigated as a surrogate measure of SEM in nursing homes 44, 45 and in subjects with spinal cord injuries 46, 47. Measures of SEM from handheld devices were useful for detecting pressure ulcer development in these populations, including in those subjects with dark skin in whom visual assessments can be problematic 48. Ching 49 described an exploratory study in which electrodes were used to investigate the electrical properties of tissue close to and more distant from the pressure ulcer site in patients with Stage I or Stage II sacral pressure ulcers. Similarly, Harrow and colleagues found that tissue impedance varies with distance from the centre of Stage III and IV pressure ulcers in spinal cord injury patients 47.
The spatial distribution of damage in pressure ulcers
Both Ching 49 and Harrow 47 observed differences in bioimpedance measures at and around the wounds as compared with the surrounding, unaffected tissues. These findings are consistent with imaging and biochemical research. Using scanning electron microscopy, Arao et al. 50 observed morphological changes to the dermal papillae and collagen fibres suggestive of impaired micro‐circulation at the border of a Stage II pressure ulcer as compared with healthy and undamaged areas. In healthy tissue, the papillary layer is the site of oxygen and nutrient transfer to the epidermis and is critical for maintaining skin integrity. This suggests that while the visible and tactile signs of pressure‐induced tissue damage suggest a particular region of damage, underneath the observable tissue, cellular damage has occurred across a larger region. This spatial distribution of damage has also been observed histologically in model systems of pressure ulcers 51.
A recent study of Stage III and IV pressure ulcers assessed inflammatory cytokines and growth factors at the centre of the wound and at the margin 52. This study demonstrated that interleukin‐1 beta (IL‐1β), tumour necrosis factor alpha (TNF‐α) and vascular endothelial growth factor (VEGF) mRNA levels were elevated at the centre of the wound compared with normal skin and were even more elevated at the margin of the wound than at the centre or in normal skin. Caspase‐3, an executive mediator of apoptosis, is highest at the centre of these wounds, elevated but slightly lower at the wound margin and lowest in normal skin. This finding suggests that inflammatory and apoptotic processes are underway both within the wound and around the edges of the wound site but to a different extent, depending upon spatial orientation.
Assessment of tissue damage with the SEM Scanner
The SEM Scanner (Bruin Biometrics, LLC, Los Angeles, CA), a low‐frequency, handheld bioimpedance device, uses measures of capacitance to assess changes in the tissue of patients with and without pressure ulcers. The SEM Scanner, a CE‐marked medical device, has demonstrated inter‐device and inter‐operator reliability in healthy volunteers in the sacral region and heel 53, anatomical areas that are at risk for pressure ulcer development.
The SEM Scanner was used to assess pressure‐induced tissue damage in a multi‐site investigational device study in the United States, and results were presented at the 17th Annual European Pressure Ulcer Meeting in Stockholm, Sweden 54. Participants included those from nursing homes and assisted‐living facilities with Stage I pressure ulcers or suspected deep tissue injury (affected subjects, n = 121, representing 63 sacral pressure ulcers and 66 heel pressure ulcers) and a control group of individuals without pressure‐induced tissue damage recruited from an outpatient medical office (unaffected subjects, n = 50). Spatially distributed SEM Scanner readings were collected from the tissue around pressure ulcers for affected subjects and around the sacrum and heel for unaffected subjects.
SEM Scanner readings were lowest at the centre of the sacral pressure ulcers and highest farther away from the centre (Table 1), a pattern also seen in heels with pressure ulcers (Table 2). This V‐shaped pattern (Figure 4) was not as apparent at sacrum or heels of subjects unaffected by pressure ulcers 54. This pattern of increasing SEM Scanner readings with increasing distance from the centre of a pressure ulcer are similar to results observed by others 43, 47, 49 and demonstrate that the SEM Scanner is useful for assessment of tissue viability and wound status, providing data that is considered to be consistent with a model of inflammation and tissue damage following pressure‐induced ischaemia, reperfusion, hypoxia and/or deformation
Table 1.
Summary of SEM Scanner readings for sacrum with pressure‐induced tissue damage
| SEM Scanner placement | |||||
|---|---|---|---|---|---|
| Centre | Ring 1 | Ring 2 | Ring 3 | Ring 4 | |
| Estimates | |||||
| Mean (SE) | 2·18 (0·09) | 2·35 (0·07) | 2·59 (0·07) | 2·79 (0·07) | 2·84 (0·07) |
| 95% CI | (2·00, 2·35) | (2·20, 2·49) | (2·44, 2·74) | (2·64, 2·93) | (2·69, 2·99) |
| Comparisons to centre* | |||||
| Difference (SE) | 0·17 (0·07) | 0·41 (0·08) | 0·61 (0·09) | 0·66 (0·10) | |
| 95% CI | (0·03, 0·30) | (0·24, 0·57) | (0·42, 0·79) | (0·46, 0·87) | |
| Two‐sided P‐value | 0·0133 | <0·0001 | <0·0001 | <0·0001 | |
CI, confidence interval; SE, standard error; SEM, subepidermal moisture.
Comparisons to centre, 95% CIs and multiplicity‐adjusted P‐values estimated using a linear repeated measures model with ring as fixed effect and subject as random effect (SAS 9.2, SAS Institute, Cary, NC).
Table 2.
Summary of SEM Scanner readings for heels with pressure‐induced tissue damage
| SEM Scanner placement | |||||
|---|---|---|---|---|---|
| Centre | Ring 1 | Ring 2 | Ring 3 | Ring 4 | |
| Estimates | |||||
| Mean (SE) | 1·89 (0·09) | 1·97 (0·08) | 2·07 (0·08) | 2·14 (0·08) | 2·19 (0·08) |
| 95% CI | (1·71, 2·07) | (1·82, 2·13) | (1·92, 2·23) | (1·98, 2·29) | (2·04, 2·35) |
| Comparisons to centre* | |||||
| Difference (SE) | 0·08 (0·05) | 0·18 (0·06) | 0·25 (0·07) | 0·31 (0·09) | |
| 95% CI | (−0·02, 0·19) | (0·06, 0·31) | (0·10, 0·39) | (0·14, 0·48) | |
| Two‐sided P‐value | 0·1166 | 0·0043 | 0·0011 | 0·0005 | |
CI, confidence interval; SE, standard error; SEM, subepidermal moisture.
Comparisons to centre, 95% CIs and multiplicity‐adjusted P‐values estimated using a linear repeated measures model with ring as fixed effect and subject as random effect (SAS 9.2, SAS Institute, Cary, NC).
Figure 4.

Subepidermal moisture (SEM) Scanner readings from the sacral area in subjects with Stage I pressure ulcers or suspected deep tissue injuries (n = 63) show a V‐shape pattern.
Together, this research on the spatial distribution of tissue damage and inflammatory activities suggest that (i) local bioimpedance measurement is a useful tool in the detection of pressure‐induced tissue damage, and (ii) a single bioimpedance reading may not provide sufficient information for the detection of pressure‐induced tissue damage. Furthermore, the SEM Scanner, in combination with the traditional standard of visual skin assessment, has demonstrated clinical utility by decreasing hospital‐acquired pressure ulcers to zero over a 45‐day period in an acute‐care hospital 55 and decreasing pressure ulcer incidence by 95% over a 6‐month period in a community hospital [personal communication from Kirsty Thurlby, Virgin Care]. This review of the literature suggests that the problem of early detection of pressure ulcer development may be reduced to collecting a spatial map of bioimpedance measurements using the SEM Scanner at the anatomical areas that are of high risk for pressure ulcer development.
Summary discussion
The majority of the published literature demonstrates the following:
The pathophysiology of pressure ulcers involves ischaemia, reperfusion injury, lymphatic dysfunction and cellular deformation. Resultant inflammation, vascular changes and cell death contribute to changes in electrical properties of the damaged tissue.
The conceptual framework described in this literature review enumerates the complex physiologic processes which may occur as a result of pressure, sheer or friction applied to the skin over time. As described by the 2014 NPUAP/EPUAP/PPPIA guidelines, when these stresses exceed the tissue's ability to resist, the damage threshold has been reached and early pressure damage has occurred. This occurs prior to seeing any visible signs of damage. This paper's authors intend to investigate this model further in the coming years.
Devices that can detect these physiologic processes provide object tests to support treatment decisions prior to skin ulceration. The SEM Scanner, one such device, has demonstrated high inter-device and inter-operator reliability at the sacrum and heel in a study of healthy volunteers.
Localised tissue bioimpedance varies spatially at and around pressure ulcers and can be used to detect pressure-induced tissue damage.
SEM Scanner readings can distinguish tissue affected by Stage I pressure ulcers and suspected deep issue injuries from tissue unaffected by pressure‐induced tissue damage.
Acknowledgements
This research was conducted with support from Bruin Biometrics. SLR was an employee of and has an equity interest in Bruin Biometrics, a company which may benefit from these research results. The terms of this arrangement are in accordance with conflict of interest policies. We gratefully acknowledge Chip Reuben, MS for assistance in manuscript preparation.
Footnotes
Unless otherwise stated, the terms Stage I, Stage II, etc. refer to the 2014 NPUAP/EPUAP/PPPIA classification system 8.
Extracellular fluid, found in the extracellular space, is composed of the interstitial fluid (or tissue fluid), plasma and transcellular fluid.
References
- 1. Moore Z, Cowman S, Conroy RM. A randomised controlled clinical trial of repositioning, using the 30° tilt, for the prevention of pressure ulcers. J Clin Nurs 2011;20:2633–44. [DOI] [PubMed] [Google Scholar]
- 2. Gardiner JC, Reed PL, Bonner JD, Haggerty DK, Hale DG. Incidence of hospital‐acquired pressure ulcers – a population‐based cohort study. Int Wound J 2014. Dec 3. DOI: 10.1111/iwj.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore Z, Cowman S. Pressure ulcer prevalence and prevention practices in care of the older person in the Republic of Ireland. J Clin Nurs 2012;21:362–71. [DOI] [PubMed] [Google Scholar]
- 4. Moore Z, Johanssen E, van Etten M. A review of PU prevalence and incidence across Scandinavia, Iceland and Ireland (Part I). J Wound Care 2013;22:361–2, 364–8. [DOI] [PubMed] [Google Scholar]
- 5. Brem H, Maggi J, Nierman D, Rolnitzky L, Bell D, Rennert R, Golinko M, Yan A, Lyder C, Vladeck B. High cost of stage IV pressure ulcers. Am J Surg 2010;200:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demarre L, Van Lancker A, Verhaeghe S, Van Hecke A, Grypdonck M, Lemey J, Annemans L, Beeckman D. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud 2015;52:1754–74. [DOI] [PubMed] [Google Scholar]
- 7. Mathiesen AS, Nørgaard K, Andersen MF, Møller KM, Ehlers LH. Are labour‐intensive efforts to prevent pressure ulcers cost‐effective? J Med Econ 2013;16:1238–45. [DOI] [PubMed] [Google Scholar]
- 8. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Haesler E, editor. Prevention and treatment of pressure ulcers: clinical practice guideline. Osborne Park: Cambridge Media, 2014. [Google Scholar]
- 9. Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract 2015;30:180–93. [DOI] [PubMed] [Google Scholar]
- 10. Valentinuzzi ME, Morucci JP, Felice CJ. Bioelectrical impedance techniques in medicine. Part II: monitoring of physiological events by impedance. Crit Rev Biomed Eng 1996;24:353–466. [PubMed] [Google Scholar]
- 11. Brienza D, Antokal S, Herbe L, Logan S, Maguire J, Van Ranst J, Siddiqui A. Friction‐induced skin injuries‐are they pressure ulcers? An updated NPUAP white paper. J Wound Ostomy Continence Nurs 2015;42:62–4. [DOI] [PubMed] [Google Scholar]
- 12. Stekelenburg A, Strijkers GJ, Parusel H, Bader DL, Nicolay K, Oomens CW. Role of ischemia and deformation in the onset of compression‐induced deep tissue injury: MRI‐based studies in a rat model. J Appl Physiol 2007;102:2002–11. [DOI] [PubMed] [Google Scholar]
- 13. Ceelen KK, Stekelenburg A, Loerakker S, Strijkers GJ, Bader DL, Nicolay K, Baaijens FP, Oomens CW. Compression‐induced damage and internal tissue strains are related. J Biomech 2008;41:3399–404. [DOI] [PubMed] [Google Scholar]
- 14. Kasuya A, Sakabe J, Tokura Y. Potential application of in vivo imaging of impaired lymphatic duct to evaluate the severity of pressure ulcer in mouse model. Sci Rep 2014;4:4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jan YK, Lee B, Liao F, Foreman RD. Local cooling reduces skin ischemia under surface pressure in rats: an assessment by wavelet analysis of laser Doppler blood flow oscillations. Physiol Meas 2012;33:1733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bader DL, Barnhill RL, Ryan TJ. Effect of externally applied skin surface forces on tissue and vasculature. Arch Phys Med Rehabil 1986;67:807–11. [PubMed] [Google Scholar]
- 17. Jiang LP, Tu Q, Wang Y, Zhang E. Ischemia‐reperfusion injury‐induced histological changes affecting early stage pressure ulcer development in a rat model. Ostomy Wound Manage 2011;57:55–60. [PubMed] [Google Scholar]
- 18. Sener G, Sert G, Ozer Sehirli A, Arbak S, Uslu B, Gedik N, Ayanoglu‐Dulger G. Pressure ulcer‐induced oxidative organ injury is ameliorated by beta‐glucan treatment in rats. Int Immunopharmacol 2006;6:724–32. [DOI] [PubMed] [Google Scholar]
- 19. Carmo‐Araújo EM, Dal‐Pai‐Silva M, Dal‐Pai V, Cecchini R, Anjos Ferreira AL. Ischaemia and reperfusion effects on skeletal muscle tissue: morphological and histochemical studies. Int J Exp Pathol 2007;88:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scallan J, Huxley VH, Korthuis RJ. Capillary fluid exchange: regulation, functions, and pathology. Morgan & Claypool LIfe Sciences: San Rafael, CA, 2010. [PubMed] [Google Scholar]
- 21. Stekelenburg A, Gawlitta D, Bader DL, Oomens CW. Deep tissue injury: how deep is our understanding? Arch Phys Med Rehabil 2008;89:1410–3. [DOI] [PubMed] [Google Scholar]
- 22. Bergstrand S, Källman U, Ek AC, Lindberg LG, Engström M, Sjöberg F, Lindgren M. Pressure‐induced vasodilation and reactive hyperemia at different depths in sacral tissue under clinically relevant conditions. Microcirculation 2014;21:761–71. [DOI] [PubMed] [Google Scholar]
- 23. Loerakker S, Manders E, Strijkers GJ, Nicolay K, Baaijens FP, Bader DL, Oomens CW. The effects of deformation, ischemia, and reperfusion on the development of muscle damage during prolonged loading. J Appl Physiol 2011;111:1168–77. [DOI] [PubMed] [Google Scholar]
- 24. Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Ann Biomed Eng 2015;43:297–305. [DOI] [PubMed] [Google Scholar]
- 25. Loerakker S, Stekelenburg A, Strijkers GJ, Rijpkema JJ, Baaijens FP, Bader DL, Nicolay K, Oomens CW. Temporal effects of mechanical loading on deformation‐induced damage in skeletal muscle tissue. Ann Biomed Eng 2010;38:2577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller GE, Seale J. Lymphatic clearance during compressive loading. Lymphology 1981;14:161–6. [PubMed] [Google Scholar]
- 27. Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–76. [DOI] [PubMed] [Google Scholar]
- 28. Schultz G, Ladwig G, Wysocki A. Extracellular matrix: review of its role in acute and chronic wounds. World Wide Wounds 2005. URL http://www.worldwidewounds.com/2005/august/Schultz/Extrace‐Matric‐Acute‐Chronic‐Wounds.html [accessed on 16 June 2015].
- 29. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Cell junctions, cell adhesion, and the extracellular matrix. In: Molecular biology of the cell. New York, NY: Garland Science, 2002:1065–125. [Google Scholar]
- 30. Schwan HP. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys 1957;5:147–209. [DOI] [PubMed] [Google Scholar]
- 31. Martinsen O, Grimnes S. Bioimpedance and bioelectricity basics. Oxford: Elsevier Academic Press, 2011. [Google Scholar]
- 32. Kanai H, Haeno M, Sakamoto K. Electrical measurement of fluid distribution in legs and arms. Med Prog Technol 1987;12:159–70. [DOI] [PubMed] [Google Scholar]
- 33. Matthie J, Zarowitz B, De Lorenzo A, Andreoli A, Katzarski K, Pan G, Withers P. Analytic assessment of the various bioimpedance methods used to estimate body water. J Appl Physiol 1998;84:1801–16. [DOI] [PubMed] [Google Scholar]
- 34. De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol 1997;82:1542–58. [DOI] [PubMed] [Google Scholar]
- 35. Gabriel C. Compilation of the Dielectric Properties of Body Tissues At Rf and Microwave Frequencies. Report N.AL/OE‐TR‐1996‐0037. Occupational and Environmental Health Directorate, Radiofrequency Radiation Division, Brooks Air Force Base, TX, 1996. URL www.dtic.mil/dtic/tr/fulltext/u2/a305826.pdf [accessed on 9 April 2015]
- 36. Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol 1996;41:2251–69. [DOI] [PubMed] [Google Scholar]
- 37. González‐Correa CA, Brown BH, Smallwood RH, Walker DC, Bardhan KD. Electrical bioimpedance readings increase with higher pressure applied to the measuring probe. Physiol Meas 2005;26:S39–47. [DOI] [PubMed] [Google Scholar]
- 38. Watanabe R, Kotoura H, Morishita Y. CT analysis of the use of the electrical impedance technique to estimate local oedema in the extremities in patients with lymphatic obstruction. Med Biol Eng Comput 1998;36:60–5. [DOI] [PubMed] [Google Scholar]
- 39. Swisher SL, Lin MC, Liao A, Leeflang EJ, Khan Y, Pavinatto FJ, Mann K, Naujokas A, Young D, Roy S, Harrison MR, Arias AC, Subramanian V, Maharbiz MM. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat Commun 2015;6:6575. [DOI] [PubMed] [Google Scholar]
- 40. Sprigle S, Linden M, Riordan B. Analysis of localized erythema using clinical indicators and spectroscopy. Ostomy Wound Manage 2003;49:42–52. [PubMed] [Google Scholar]
- 41. Nixon J, Cranny G, Bond S. Pathology, diagnosis, and classification of pressure ulcers: comparing clinical and imaging techniques. Wound Repair Regen 2005;13:365–72. [DOI] [PubMed] [Google Scholar]
- 42. VanGilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manage 2008;54:40–54. [PubMed] [Google Scholar]
- 43. Wagner DR, Jeter KF, Tintle T, Martin MS, Long JM 3rd.. Bioelectrical impedance as a discriminator of pressure ulcer risk. Adv Wound Care 1996;9:30–7. [PubMed] [Google Scholar]
- 44. Bates‐Jensen BM, McCreath HE, Kono A, Apeles NC, Alessi C. Subepidermal moisture predicts erythema and stage 1 pressure ulcers in nursing home residents: a pilot study. J Am Geriatr Soc 2007;55:1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bates‐Jensen BM, McCreath HE, Pongquan V, Apeles NC. Subepidermal moisture differentiates erythema and stage I pressure ulcers in nursing home residents. Wound Repair Regen 2008;16:189–97. [DOI] [PubMed] [Google Scholar]
- 46. Guihan M, Bates‐Jenson BM, Chun S, Parachuri R, Chin AS, McCreath H. Assessing the feasibility of subepidermal moisture to predict erythema and stage 1 pressure ulcers in persons with spinal cord injury: a pilot study. J Spinal Cord Med 2012;35:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harrow JJ, Mayrovitz HN. Subepidermal moisture surrounding pressure ulcers in persons with a spinal cord injury: a pilot study. J Spinal Cord Med 2014;37:719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bates‐Jensen BM, McCreath HE, Pongquan V. Subepidermal moisture is associated with early pressure ulcer damage in nursing home residents with dark skin tones. Pilot findings. J Wound Ostomy Continence Nurs 2009;36:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ching CT, Chou MY, Jiang SJ, Huang SH, Sun TP, Liu WH, Liu CM. Tissue electrical properties monitoring for the prevention of pressure sore. Prosthet Orthot Int 2011;35:386–94. [DOI] [PubMed] [Google Scholar]
- 50. Arao H, Obata M, Shimada T, Hagisawa S. Morphological characteristics of the dermal papillae in the development of pressure sores. J Tissue Viability 1998;8:17–23. [DOI] [PubMed] [Google Scholar]
- 51. Houwing R, Overgoor M, Kon M, Jansen G, van Asbeck BS, Haalboom JR. Pressure‐induced skin lesions in pigs: reperfusion injury and the effects of vitamin E. J Wound Care 2000;9:36–40. [DOI] [PubMed] [Google Scholar]
- 52. Jiang L, Dai Y, Cui F, Pan Y, Zhang H, Xiao J, Xiaobing FU. Expression of cytokines, growth factors and apoptosis‐related signal molecules in chronic pressure ulcer wounds healing. Spinal Cord 2014;52:145–51. [DOI] [PubMed] [Google Scholar]
- 53. Clendenin M, Jaradeh K, Shamirian A, Rhodes SL. Inter‐operator and inter‐device agreement and reliability of the SEM Scanner. J Tissue Viability 2015;24:17–23. [DOI] [PubMed] [Google Scholar]
- 54. Gershon S, Okonkwo H, Rhodes S, Burns M. SEM Scanner readings to assess pressure induced tissue damage. Proceedings of the 17th Annual European Pressure Ulcer Advisory Panel (EPUAP) Meeting; 2014 Aug 27–29; Stockholm, Sweden.
- 55. Bullough, L. “Chasing Zero” pressure ulcer prevention & root cause analysis with the SEM Scanner. 25th Conference of the European Wound Management Association; 2015 May 12–15; London, England.


