Abstract
Antimicrobial resistance is an ever‐increasing global concern, with the era of untreatable infection becoming a reality. Wound care is no exception, with increasing issues of antibiotic‐resistant infections across different wound types and care settings. Antibiotic resistance and stewardship have been the priority for most strategic interventions so far; however, in wound care, alternative or supplementary strategies using antiseptics should be considered. Antiseptics such as silver can provide effective cidal activity across a broad range of wound pathogens, assuming they are used at the correct level for an appropriate duration. Evidence summarised in this manuscript suggests that effective antiseptics, such as nanocrystalline silver, have an increasing body of evidence in support of their use to minimise transmission of antibiotic‐resistant organisms as part of institutional infection control procedures and, in addition, through appropriate early use and stewardship on local wound infections, in conjunction with local procedures, to minimise the need for systemic antibiotic therapy. Engagement, alignment, and collaboration between wound care professionals and wider related teams and governments on antimicrobial stewardship, and the potential role of antiseptics within this, will help to generate further evidence for such interventions in the fight against antimicrobial‐resistant infections in wound care.
Keywords: antimicrobial resistance, antimicrobial stewardship, antiseptics, efficacy, nanocrystalline silver
1. INTRODUCTION
In response to the development of antimicrobial agents, microorganisms have acquired a resistance to drugs through a variety of mechanisms that have emerged through clinical use and continue to challenge both the clinical and financial resources in the majority of institutions worldwide.1 Global surveillance studies report that resistance to nearly all classes of antibiotics is increasing, as is the emergence of what have been termed pan‐drug‐resistant and extremely drug‐resistant pathogens. Concomitantly, bacterial binding sites have been exploited by available antimicrobials, and there has been a decline in the development of antibiotics using novel mechanisms of action.2 Microorganisms of particular concern include many Gram‐negative bacilli such as those containing extended‐spectrum beta‐lactamases, Enterobacter sp., and the non‐fermenters Pseudomonas aeruginosa and Acinetobacter baumannii.3 Carbapenems are amongst the most powerful antibiotics available and are often used to treat infections because of otherwise multi‐drug‐resistant Gram‐negative organisms. The emergence of carbapenem resistance is disturbing as there are few antibiotics in reserve behind this class of drug.4
However, there has been progress in tackling some organisms; stringent infection control and prudent antibiotic use policies in hospitals have led to a reported reduction in infections of methicillin‐resistant Staphylococcus aureus (MRSA) in some European countries.5 This highlights the potential success of intervention strategies to reduce antimicrobial resistance (AMR) now and focus on areas for the future.
Global attention has focused on how to address the issue of AMR. The UK government strategy to control the development of AMR states three overarching strategy aims:6
Improve the knowledge and understanding of AMR
Conserve and steward the effectiveness of existing treatments (antimicrobial stewardship)
Stimulate the development of new antibiotics, diagnostics, and novel therapies
Antimicrobial stewardship (AMS) is the systematic effort to educate and persuade prescribers of antimicrobials to follow evidence‐based prescribing in order to stem antibiotic overuse, and thus AMR. The Centers for Disease Control and Prevention (CDC) simplify this as “The right antibiotic for the right patient, at the right time, with the right dose, and the right route causing the least harm to the patient and future patients” (https://www/cdc.gov/getsmart/healthcare/inpatient-stewardship). There is a large number of publications relating to the topic, and the content is very focused on the use of antibiotics.7, 8, 9 Furthermore, the importance of infection prevention and AMS is recognised by governments, demonstrated in new measures and targets such as the Quality Premium introduced recently by NHS improvement, UK,10 aiming to reduce Gram‐negative bloodstream infections and inappropriate antibiotic prescribing in at‐risk groups.
2. AMS IN WOUND CARE
Within the field of wound care, infection is one of the most frequent complications of, and in many cases perpetuates, non‐healing wounds.11 The clinical, economical, and patient‐related consequences place major burdens on health care systems.12, 13 For this reason, it is also important to focus on potential solutions specifically with wounds in mind.
There is now rapidly gaining interest in AMS within wound care, and both consensus documents and guidelines have been published to help clinical professionals make appropriate decisions about antibiotic use.14, 15, 16, 17 The wound care sector is unique in that there are also a wide range of topical antiseptics available to help both treat and prevent infections locally. The potential role for non‐antibiotic antimicrobials in wound care and AMS has recently been reviewed, including a detailed overview of the broad choices of different interventions available to clinicians.17 The article concludes that, with the prospect of a post‐antibiotic era looming, ways to maintain and extend our antimicrobial armamentarium must be found. In wound management, it is imperative that all antimicrobial interventions are used wisely. Some have proposed that these antiseptic agents should be included as an integral part of AMS decisions, and the same rules should apply in terms of use on appropriate wounds, at appropriate concentrations to ensure rapid kill, and use for appropriate time periods.12, 16 To date, the emergence of resistance to antiseptics, some of which have been used for centuries, has in no way reached the epidemic proportions seen with antibiotic use over a period totalling less than 100 years. This is largely because of the action of most antiseptics on multiple targets within a bacterial cell, making resistance development more difficult.17, 18, 19
2.1. Determination of antiseptic efficacy within boundaries defined by AMS
For antibiotics, international break point committees have issued agreed‐upon concentration levels of antibiotics that define isolates as susceptible, intermediate, or resistant.20 To date, there are no such guidelines and concentration levels agreed upon for antiseptics. However, information can be found that links laboratory data to clinical performance that will give confidence in ensuring that informed treatment choices are made. When considering infected wounds, not only is the ability of an antiseptic to kill quickly important in order to avoid infection worsening, but there is also a protective barrier function that is maintained.21 Acute and chronic wounds frequently become colonised with a wide variety of organisms, including antibiotic‐resistant strains.22, 23 Preventing the spread of such organisms from the wound into the clinical environment should be a key goal, as well as restricting accessibility of environmental pathogens to the open wound.
2.2. Silver use in wound care
Silver is one example of an antiseptic commonly used in wound care as an important contributor to infection prevention and treatment.24 Silver ions (Ag+) have broad‐spectrum antimicrobial activity (microbiocidal) against bacteria, fungi, and viruses18 and can rapidly kill microorganisms. Silver ions bind to multiple targets on bacterial and fungal cells and have a range of effects, including:18
Cell wall and membrane disruption
Denaturing of proteins and enzymes
Preventing respiration
Inhibiting DNA synthesis
Key requirements of effective silver dressings include broad‐spectrum activity, including activity against antibiotic‐resistant bacteria,14 rapid bactericidal activity,25 ability to act on multiple targets on the bacterial cell14, 17, 18, 19, 25 leading to a low potential for resistance development,17, 18, 25 and the ability to provide a bacterial barrier.14, 21
As discussed previously for antibiotics, silver needs to be potent enough to align with the requirements of antimicrobials within AMS, that is, the correct dose for the correct treatment duration.12 Options of dressings containing silver are many, comprising different types of silver, at different concentrations, with the active agent attached to or incorporated into different formulations. The detailed differences between these options have been reviewed previously26, 27 but not in the context associated with AMS. Published evidence highlights that not all silver dressing formats perform well in vitro across multiple tests and have mixed results in the clinic.28, 29, 30 Provision of bench‐to‐bedside evidence is essential to enable clinicians to make informed choices regarding the most appropriate type of silver dressings for their patients' needs. Nanocrystalline silver (NCS) dressings are one type of silver dressing that provides this broad spectrum of evidence. The next sections intend to present both laboratory and clinical evidence that supports the position of effective antimicrobials such as NCS within an AMR and AMS strategy for wound care.
2.3. Dressings containing NCS in wound care
The architecture of silver within a dressing can make a large difference to the antimicrobial efficacy.31 NCS dressings (commercially available as ACTICOAT, Smith&Nephew, Hull, UK) are formed utilising a specialist manufacturing process of physical vapour deposition involving magnetron sputtering that transforms the architecture of metallic silver.32 By changing the physical properties at the crystal level, the biological activity is also changed because of the increased surface area available, allowing rapid and sustained availability of Ag+.31, 32
Many traditional methods for demonstrating antimicrobial efficacy rely on culture techniques following exposure in fluids to the test material. The nature of the solute, for example, the presence of chloride ions and protein, can affect the killing activity of positively charged silver ions. The structure of NCS described above ensures the release of bactericidal levels of silver but also allows compensation for neutralised Ag+ ions by continuous replenishment, hence maintaining bactericidal levels within the wound environment over extended time periods.33 A reduction in microbial cell numbers of 99.9% (three logs or 1000‐fold reduction) is indicative of bactericidal activity.34, 35 The NCS structure provides enhanced antimicrobial activity and clinical outcomes as expected from an effective wound dressing, as summarised in Table 1.
Table 1.
Key requirements of effective antiseptic dressings and the evidence for NCS
| Key requirements | Features of NCS | NCS evidence and outcomes |
|---|---|---|
| Broad‐spectrum activity including activity against antibiotic‐resistant bacteria14 | Silver is effective against Gram‐positive and Gram‐negative bacteria, yeast, and fungi in vitro18 | NCS is effective in vitro against Gram‐positive, Gram‐negative bacteria, yeast, and fungi31, 36, 37, 38, including antibiotic‐resistant organisms such as MRSA, VRE, enterobacteriaceae strains containing NDM‐1 carbapenemases37, 39, 40 |
| Rapid bactericidal activity25 | Large surface area of nanocrystalline structure allows fast release of Ag+ with other oxidation states also proposed (Ag0, Ag2+, and Ag3+) at a level high enough to kill bacteria rapidly in vitro32, 35 | Rapid speed of kill in vitro31, 36, 37, 38. Clinically proven to resolve the signs and symptoms of infectiona faster than other silver dressings41 |
| Acts on multiple targets on bacterial cell14, 17, 18, 19, 25. Low potential for resistance17, 18, 25, 26 | Silver ions (Ag+) bind to multiple targets on bacterial and fungal cells18. Ag+ interacts with thiol groups in enzymes and proteins, inhibits cell division, and damages the cell envelope and DNA18 | Laboratory data highlights potential to develop silver resistance if sub‐lethal levels used;42 however, further in vitro research suggests NCS still effective against these resistant strains43. Clinically, no failure of silver dressings because of silver resistance27 |
| Bacterial barrier property14, 21 | NCS layer provides antimicrobial barrier39 to microorganisms, which are killed rapidly on contact | In vitro evidence against MRSA44 and EMRA 15 and 1639 and clinical evidence showing reduced transfer of MRSA from wounds44 |
| No evidence of delayed wound healing in clinical use14 | Sustained release over wear time of the dressing31 provides sufficient level to kill microorganisms whilst demonstrating minimal toxicity in patients45, 46, 47 | Peer‐reviewed literature suggests that modern silver products do not delay wound healing29. Excellent antimicrobial barrier performance as graft/dermal replacement resulting in outstanding reepithelialisation48 |
Abbreviations: EMRSA, Epidemic methicillin‐resistant Staphylococcus aureus; MRSA, methicillin‐resistant Staphylococcus aureus; NCS, nanocrystalline silver; VRE, vancomycin‐resistant enterococci.
ACTICOAT is cleared as a bacterial barrier only in the United States.
3. HOW CAN DRESSINGS CONTAINING NCS BE INCORPORATED INTO CLINICAL PROTOCOLS TO TACKLE AMR?
It is essential that cidal activity demonstrated in the laboratory by antiseptic interventions is translated to effective clinical outcomes. Within the field of AMR, key aims have been highlighted in various strategy and review documents to tackle the issue of AMR as discussed earlier.1, 6 Action on the focus areas of infection control and also AMS may be supported by improved wound assessment devices and topical antiseptic dressings.
3.1. Infection prevention and control
One of the crucial focus points with regard to AMR is the ability to prevent infections occurring where possible and, if they occur, to control their spread. Key infection prevention and control interventions and strategies include both surveillance and barrier precautions. These are summarised in Figure 1. The potential use of antiseptics to minimise surgical infections was recognised by Joseph Lister in 1868,49 where carbolic acid was used to reduce surgical wound infections such as gas gangrene. Today, many different antiseptics are available to health practitioners in a plethora of formats, including antiseptic/antimicrobial dressings, such as silver dressings, to help minimise the spread of microorganisms.26 Antimicrobial dressings, if providing a sufficient and sustained level of antimicrobial agent, can provide a barrier to ingress and egress of bacteria from a wound, specifically by killing the organisms before they can transfer through the dressing.21, 53, 54 This is particularly important when culprit organisms are resistant to antibiotics in order to minimise spread from a colonised/infected wound to other health care workers or patients.
Figure 1.
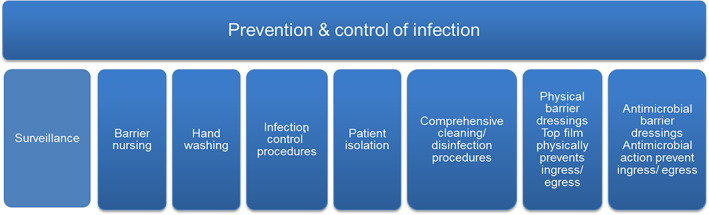
Key factors encompassing infection prevention and control21, 44, 50, 51, 52 [Colour figure can be viewed at wileyonlinelibrary.com]
3.1.1. The benefit of dressings containing NCS against antibiotic‐resistant organisms
The ability of some silver barrier dressings to provide rapid bactericidal activity against antibiotic‐resistant organisms40 indicates that these products may be relevant to managing local wound infections caused by these highly resistant bacteria. In vitro NCS barrier dressings have been shown to be highly effective in killing antibiotic‐resistant bacteria such as MRSA37 (including epidemic MRSA strains39), vancomycin‐resistant enterococci (VRE),37 resistant P. aeruginosa, 37 and carbapenem‐resistant enterobacteriaceae (CRE) bacterial strains carrying NDM‐1 resistance genes.40
This effectiveness of NCS dressings against antibiotic‐resistant organisms in the laboratory is supported by a growing body of clinical evidence. One study by Strohal et al44 on chronic wounds colonised with MRSA showed that the application of the NCS dressing reduced transfer of this organism in 95% cases. Seven patients with a total of 10 MRSA colonised wounds were sampled for MRSA at each dressing change (after 1, 24, 48, and 72 h). Swabs were taken from the upper side of the dressing and the wound bed. None of the dressings showed heavy MRSA load (as denoted by +++) breakthrough on the upper side over the 72‐h observation period. Furthermore, no bacterial penetration through the dressing was shown in seven wounds. Of the remaining three wounds, two dressings had a (++) MRSA colonisation, and one wound had minor colonisation (+) of the upper side. NCS dressings were found to provide a complete, or almost complete, barrier to the penetration/spread of MRSA in 95% of readings. In addition, 67% of all wound observations showed a decrease in the MRSA load with an eradication rate of 11%.
Moreover, NCS dressings have been show to impact MRSA in burn and surgical wounds.55, 56, 57 Huang et al55 performed a randomised controlled trial of NCS dressing treatment compared with silver sulphadiazine cream (SSD) on residual burn wounds, primarily to investigate the effectiveness of each treatment. However, on analysis of bacteria in the wound throughout the 12‐week treatment period, both the NCS dressing and SSD treatment showed that those colonised with MRSA initially became 100% clear of this organism. Interestingly, the clearance rate was found to be faster by 6 days of treatment for the NCS group vs the SSD treatment (33% and 20%, respectively), highlighting the importance of the rapid, effective, and sustained silver provision from the advanced silver dressing.
Wound infection with MRSA and β‐haemolytic streptococci following complex knee surgery was managed using NCS dressings.56 In this case study, antibiotics were used to successfully treat the systemic infection, but they were found to be unable to resolve the persistent superficial infection at the wound site. A subsequent regime of irrigation of the wound and application of NCS resulted in a reduction in exudate and appearance of healthy granulation tissue leading to complete healing with no recurrence in the infection at 3 years post‐surgery follow up. The author also suggested that the continuous bactericidal activity provided by the silver dressing negated the need for frequent hospital admissions for systemic antibiotic treatment. Further cases published in 200857 support this earlier finding; patients were successfully treated with NCS dressings as part of a management protocol without using systemic antimicrobials after developing MRSA infection following wound breakdown after surgical revision procedure on the knee. Successful bacterial clearance helped to reduce the spread of cutaneous infection and subsequent wound necrosis without the use of systemic antibiotics.
3.2. Appropriate use of dressings containing silver within AMS
In 2012, an international consensus group of wound care professionals emphasised that, in order to maintain their efficacy, silver dressings should be used appropriately.24 The panel recommended that silver dressings should not be used in the absence of localised (overt or covert) spreading or systemic infection, unless there are clear indicators that the wound is at high risk of infection or re‐infection. Prophylactic use should be reserved for high‐risk patients/those with multiple comorbidities, and examples of such wounds may include burns; surgical wounds at risk of contamination; pressure ulcers near the anus; or wounds in patients who are immunocompromised, have poor circulation, have unstable diabetes, or have neoplastic disease.
Figure 2 summarises where silver dressings are advocated in a wound on the infection continuum. The guidelines proposed that silver dressings can be used to manage local infection without necessarily the addition of antibiotics, reserving antibiotics for if an infection starts to spread or progress to systemic infection. Furthermore, the group advised that, even in these severe infections, topical antiseptics such as silver play an important role to continue to manage the local infection, particularly where vascular insufficiency may limit the exposure of wounds to systemic antimicrobials locally.
Figure 2.
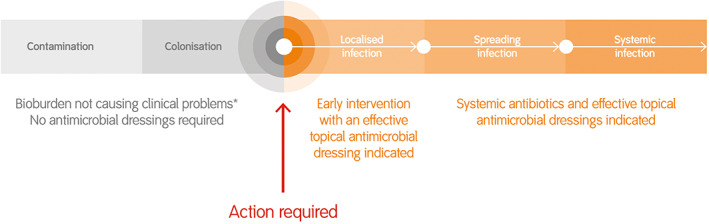
Topical silver dressing and antibiotic treatment recommendations along the wound infection continuum. * With the exception of high‐risk patients, modified from Ayello et al24 [Colour figure can be viewed at wileyonlinelibrary.com]
As discussed, the main focus currently for AMS is around antibiotics; however, it follows that all antimicrobials should be used appropriately to minimise issues of silver resistance in the future. Antibiotics mainly have a narrow spectrum of activity (against specific types of bacteria) and usually act on one target in the cell; however, antiseptics such as silver act on multiple targets as discussed earlier, thus reducing the potential for developing resistance to all targets.17, 18, 25, 26 Currently, silver resistance has been observed in various laboratory studies when bacteria were exposed to repeated low levels (sub‐minimum inhibitory concentrations [MICs]) of silver.42, 58, 59 Moreover, new mechanisms of resistance by interspecies cooperation have been reported in the laboratory.60 Such findings underline the need for increased vigilance of resistant organisms and the understanding of appropriate silver use against bacteria, ensuring the use of an effective level and duration of treatment to minimise this issue across health care.25
Clinically, there is no reliable evidence to date that silver resistance results in treatment failures. Even in cases where wound bacteria have been identified to carry silver‐resistant genes, silver treatment is still effective.61, 62 Alternative explanations for apparent silver resistance may be as a result of inherent tolerance because of structure or phenotypical features of a microorganism (ie, capsules/mucoid strains) or the presence of biofilm known to facilitate antimicrobial tolerance to many antibiotics and antiseptics such as silver, where the concentration required to eradicate biofilm has been found to be 10 to 100 times higher than needed to kill planktonic bacteria.63 Where biofilms are suspected, radical debridement is necessary to open a time‐dependent window enhancing antimicrobial susceptibility of dispersed biofilm bacteria.64 In addition, the use of alternative antimicrobials shown to be effective against biofilms would be more appropriate.65, 66, 67
3.2.1. Practical evidence where appropriate use of an effective dressings containing silver supports the battle against AMR in wounds
Dressings containing NCS have been shown to reduce the systemic spread of MRSA infection in a full‐thickness rat burn wound model,68 with a similar effect in limiting the spread of both fungal infection69 and A. baumannii infections70, 71 into the muscle in these animal models. Furthermore, patients at risk of delayed healing and untreated local infection could progress to systemic sepsis. This was highlighted by Newton,72 who reported the benefit of NCS dressings as part of a strategic early intervention treatment plan to reduce the number of MRSA‐infected wounds progressing to MRSA bacteraemias. Prior to the intervention plan, the wound‐related MRSA bacteraemia rate was found to account for over 20% (n = 8/36) of all MRSA bacteraemia cases. However, following the introduction of a strategic plan addressing the screening and swabbing of patients with wounds; improved access to NCS dressings, education, and training on the treatment pathway; dressing application; and aseptic non‐touch technique, the MRSA bacteraemia rate was reduced to zero, demonstrating a promising role for antiseptics as part of such proactive interventions.
As highlighted by the international consensus group on silver use, the use of effective silver dressings to manage local infections may help to reserve antibiotic treatments for cases where infection is spreading or becomes systemic.24 Case studies highlight the potential for NCS to be used to address local MRSA infections in surgical revision wounds, resulting either in a reduced need for systemic antibiotics56 or successful bacterial clearance without the use of systemic antibiotics in this small number of patients.57 More substantial evidence of this has been demonstrated in burn wounds; a reduction in antibiotic usage was also attributed to the introduction of standard NCS dressings into a paediatric burns unit in Sweden.73 This retrospective study demonstrated a reduction in the number of patients requiring antibiotics from 70% to 25% (P < 0.001) between 2001 and 2007 combined with a significantly reduced length of stay (12.5 to 4.5 days, P < 0.001) following the new protocol introduction. Similar findings were reported by Fong et al 74 and also Tonkin and Wood75 following a clinical audit of burns patients comparing wound dressing regimes using SSD or standard NCS dressing. Tonkin and Wood reported a 50% reduction in antibiotic use (P = 0.016) with NCS dressings compared with SSD, in addition to a significant reduction in hospital length of stay (15.1 and 8.8 days for SSD and ACTICOAT, respectively, P = 0.045). Antibiotic usage was reduced with NCS (5.2%) compared with silvazine (57%) in the audit performed by Fong et al.74 More recently, observations of a substantial increase in antibiotic resistance in a Polish burns unit led to the introduction of a new infection management protocol incorporating antiseptic cleansers and NCS dressings.76 This new intervention resulted in a marked reduction in sepsis cases, particularly those caused by P. aeruginosa. Concurrently, the authors mention increased sensitivity to most antibiotics used to treat P. aeruginosa infections following the protocol modification. In addition, the reported increase in expenditure for antiseptic solutions and dressings (USD 34, 554) was more than offset by the USD 106, 055 decrease in expenditure for antibiotics and antimycotics, resulting in a total reduction in cost of USD 71, 501.76
Furthermore, improved wound assessment has been highlighted in the most recent CQUIN targets.77 Technologies that supplement clinician judgement, particularly from a microbial load perspective, will aid more targeted approach to wound infection. New guidance devices such as Moleculight i:X™, (Moleculight Inc., Toronto, Canada), which not only measure wound surface area but can also detect fluorescent bacteria above a threshold (>104 cfu/g), may aid more targeted sampling and support informed treatment decisions.78, 79, 80 Moreover, this is an important bridge to drive infection diagnostics, highlighted by the UK AMR strategy6 and the O'Neill report1 as one of the key focus areas to tackle AMR.
4. CONCLUSIONS
The current situation with AMR and antibiotics highlights the needs for responsible and appropriate use across all antimicrobials. Clinicians must continue to follow good AMS practice ensuring that antimicrobial treatment is an effective level at the point of infection and used for an appropriate duration. In wound care, clinicians should follow recommendations on appropriate silver dressing use with early intervention, using an effective, clinically proven antiseptic such as NCS dressings.
The clinical evidence summarised in in this paper highlights some of the potential roles for NCS dressings as part of an expanding arsenal to tackle AMS and supporting wider stewardship of antimicrobials; NCS silver dressings are highly effective against antibiotic‐resistant organisms in vitro and clinically, not only providing effective antimicrobial barrier properties but also initial evidence that suggests a role as part of protocols to manage local infections (in locations where these products are cleared for use in this way), which may realise a reduced need for antibiotics and also minimise the progression to more systemic infections when intervening early.
Compared with many clinical infections, wound care is in a fortunate position to be able to use an array of antimicrobial interventions to tackle local infection, including antibiotics and antiseptics. Nevertheless, ongoing surveillance of resistance and greater clarification of AMS strategies is essential across wound care, with wider education around the potential, and limitations, of all antimicrobials. Engagement, alignment, and collaboration between wound care professionals and wider related teams (infection prevention and control, microbiology, and pharmacy departments) and governments on AMS, and the potential role of antiseptics within this, will help to generate further evidence for such interventions in the fight against AMR in wound care.
ACKNOWLEDGEMENTS
Dr Woodmansey is an employee of Smith&Nephew. Dr Roberts provides consultancy support to Smith&Nephew. The authors thank Caroline Williams and Vicky Ward‐Campbell for reviewing early drafts of this article.
Woodmansey EJ, Roberts CD. Appropriate use of dressings containing nanocrystalline silver to support antimicrobial stewardship in wounds. Int Wound J. 2018;15:1025–1032. 10.1111/iwj.12969
REFERENCES
- 1. O'Neill J. Tackling drug‐resistant infections globally: final report and recommendations; 2016, 1‐81.
- 2. Owens RC. Antimicrobial stewardship: concepts and strategies in the 21st century. Diagn Microbiol Infect Dis. 2008;61(1):110‐128. [DOI] [PubMed] [Google Scholar]
- 3. Wilson APR, Livermore DM, Otter JA, et al. Prevention and control of multi‐drug‐resistant Gram‐negative bacteria: recommendations from a Joint Working Party. J Hosp Infect. 2016;92:S1‐S44. 10.1016/j.jhin.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 4. Livermore DM. Has the era of untreatable infections arrived? J Antimicrob Chemother. 2009;64(suppl 1):29‐36. [DOI] [PubMed] [Google Scholar]
- 5. European Centre for Disease Control . Summary of the latest data on antibiotic consumption in the European Union. EARS‐Net Surveillence 2016, 2016.
- 6. Department of Health, Department for Environment Food and Rural Affairs . UK Five Year Antimicrobial Resistance Strategy 2013 to 2018, 43. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf. Accessed November 21, 2017.
- 7. Department of Health ESPAUR SSTF Subcommittee . Start Smart—Then Focus Antimicrobial Stewardship Toolkit for English Hospitals. Public Health England, March 2015, 1‐26. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/417032/Start_Smart_Then_Focus_FINAL.PDF. Accessed November 21, 2017.
- 8. National Institute for Health and Care Excellence . Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use, August 2015.
- 9. Nathwani D, Sneddon J. Practical guide to antimicrobial stewardship in hospitals. BioMérieux Prints. 2013;21‐23. [Google Scholar]
- 10. National Health Service . Technical guidance Annex B information on quality premium, 2017.
- 11. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91‐96. [DOI] [PubMed] [Google Scholar]
- 12. Roberts CD, Leaper DJ, Assadian O. The role of topical antiseptic agents within antimicrobial stewardship strategies for prevention and treatment of surgical site and chronic open wound infection. Adv Wound Care. 2017;6(2):63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13:5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gottrup F, Apelqvist J, Bjarnsholt T, et al. EWMA document: antimicrobials and non‐healing wounds. Evidence, controversies and suggestions. J Wound Care. 2013;22(5):S1‐S89. [DOI] [PubMed] [Google Scholar]
- 15. Swanson T, Angel D, Sussman G, et al. Wound infection in clinical practice. Wounds Int. 2016;5(s3):1‐32. [Google Scholar]
- 16. Lipsky BA, Dryden M, Gottrup F, Nathwani D, Seaton RA, Stryja J. Antimicrobial stewardship in wound care: a position paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J Antimicrob Chemother. 2016;71(11):3026‐3035. [DOI] [PubMed] [Google Scholar]
- 17. Cooper R, Kirketerp‐Møller K. Non‐antibiotic antimicrobial interventions and antimicrobial stewardship in wound care. J Wound Care. 2018;27(6):355‐377. [DOI] [PubMed] [Google Scholar]
- 18. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leaper D. Topical antiseptics in wound care: time for reflection. Int Wound J. 2011;8(6):547‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev. 2007;20(3):391‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutwein LG, Panigrahi M, Schultz GS, Mast BA. Microbial barriers. Clin Plast Surg. 2012;39(3):229‐238. 10.1016/j.cps.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 22. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolcott RD, Hanson JD, Rees EJ, et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2015;24(1):163‐174. [DOI] [PubMed] [Google Scholar]
- 24. Ayello EA, Carville K, Fletcher J, et al. International consensus. Appropriate use of silver dressings in wounds. An expert working group consensus. Wounds Int. 2012;1‐24. [Google Scholar]
- 25. Chopra I. The increasing use of silver‐based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother. 2007;59(4):587‐590. [DOI] [PubMed] [Google Scholar]
- 26. Leaper DJ. Silver dressings: their role in wound management. Int Wound J. 2006;3(4):282‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ousey K, Roberts C, Leaper D. Silver containing dressings. In: Agren MS, ed. Wound Healing Biomaterials, Volume 2: Functional Biomaterials. London, England: Elsevier; 2016:403‐430. [Google Scholar]
- 28. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LG, Martyn‐St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;1(12):CD003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michaels JA, Campbell B, King B, Palfreyman SJ, Shackley P, Stevenson M. Randomized controlled trial and cost‐effectiveness analysis of silver‐donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br J Surg. 2009;96(10):1147‐1156. [DOI] [PubMed] [Google Scholar]
- 30. Leahy‐Gilmartin A, Edwards‐Jones V. Clinical practice challenging silver: a comparison of in vitro testing methods. Wounds Int. 2018;9(2):35‐42. [Google Scholar]
- 31. Wright JB, Hansen DL, Burrell RE. The comparative efficacy of two antimicrobial barrier dressings: in vitro examination of two controlled release silver dressings. Wounds. 1998;10(6):179‐188. [Google Scholar]
- 32. Sant SB, Gill KS, Burrell RE. Nanostructure, dissolution and morphology characteristics of microcidal silver films deposited by magnetron sputtering. Acta Biomater. 2007;3(3):341‐350. [DOI] [PubMed] [Google Scholar]
- 33. Roberts CD. Use of interventional approaches to controlling healthcare acquired infections in wounds. The role of silver. J Wound Technol. 2008;2(October):58‐60. [Google Scholar]
- 34. Stratton CW, Cooksey RC. Susceptibility tests: Special tests (log reduction of 3). In: Balows A, Hausler WJ, Herrmann KL, Isenberg HD, Shadomy H, eds. Manual of Clinical Microbiology. 5th ed. Washinton, DC: American Society for Microbiology; 1991:1153‐1165. [Google Scholar]
- 35. Hall RE, Bender G, Marquis RE. Inhibitory and cidal antimicrobial actions of electrically generated silver ions. J Oral Maxillofac Surg. 1987;45(9):779‐784. [DOI] [PubMed] [Google Scholar]
- 36. Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control. 1999;27:344‐350. [DOI] [PubMed] [Google Scholar]
- 37. Wright JB, Lam K, Burrell RE. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control. 1998;26(6):572‐577. [DOI] [PubMed] [Google Scholar]
- 38. Yin HQ, Langford R, Burrell RE. Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J Burn Care Rehabil. 1999;20(3):195‐200. [DOI] [PubMed] [Google Scholar]
- 39. Edwards‐Jones V. Antimicrobial and barrier effects of silver against methicillin‐resistant Staphylococcus aureus . J Wound Care. 2006;15(7):285‐290. [DOI] [PubMed] [Google Scholar]
- 40. Hope R, Mushtaq S, Vaughan K, Woodmansey E, Roberts C, Livermore D. The in‐vitro antibacterial activity of nanocrystalline silver dressings against bacteria with NDM‐1 carbapenemase. Vienna: European Wound Management Association; 2012.
- 41. Gago M, Garcia F, Gaztelu V, Verdu J, Lopez P, Nolasco A. A comparison of three silver‐containing dressings in the treatment of infected, chronic wounds. Wounds a compend. Clin Res Pract. 2008;20(10):273‐278. [PubMed] [Google Scholar]
- 42. Randall CP, Gupta A, Jackson N, Busse D, O'Neill AJ. Silver resistance in Gram‐negative bacteria: a dissection of endogenous and exogenous mechanisms. J Antimicrob Chemother. 2015;70(4):1037‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nadworny Patricia L.. Biological activity of nanostructured silver. University of Alberta; 2010. https://era.library.ualberta.ca/files/fn106z779/Nadworny_Patricia_Spring2010.pdf. Accessed March 10, 2017.
- 44. Strohal R, Schelling M, Takacs M, Jurecka W, Gruber U, Offner F. Nanocrystalline silver dressings as an efficient anti‐MRSA barrier: a new solution to an increasing problem. J Hosp Infect. 2005;60(3):226‐230. [DOI] [PubMed] [Google Scholar]
- 45. Sibbald RG, Contreras‐Ruiz J, Coutts P, Fierheller M, Rothman A, Woo K. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care. 2007;20(10):549‐558. [DOI] [PubMed] [Google Scholar]
- 46. Vlachou E, Chipp E, Shale E, Wilson YT, Papini R, Moiemen NS. The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns. 2007;33(8):979‐985. [DOI] [PubMed] [Google Scholar]
- 47. Moiemen NS, Shale E, Drysdale KJ, Smith G, Wilson YT, Papini R. Acticoat dressings and major burns: systemic silver absorption. Burns. 2011;37(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 48. Demling RH, Leslie DeSanti MD. The rate of re‐epithelialization across meshed skin grafts is increased with exposure to silver. Burns. 2002;28(3):264‐266. [DOI] [PubMed] [Google Scholar]
- 49. Lister J. An address on the antiseptic system of treatment in surgery. Br Med J. 1868;2(394):53‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Henderson DK. Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms. Am J Infect Control. 2006;34(suppl 5):46‐54. [DOI] [PubMed] [Google Scholar]
- 51. Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug‐resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362‐386. [DOI] [PubMed] [Google Scholar]
- 52. Tanguy M, Kouatchet A, Tanguy B, Pichard É, Fanello S. Management of an Acinetobacter baumannii outbreak in an intensive care unit. Médecine Mal Infect. 2017;47(6):409‐414. [DOI] [PubMed] [Google Scholar]
- 53. Thomas S, McCubbin P. A comparison of the antimicrobial effects of four silver‐containing dressings on three organisms. J Wound Care. 2003;12(3):101‐107. [DOI] [PubMed] [Google Scholar]
- 54. Thomas S, McCubbin P. An in vitro analysis of the antimicrobial properties of 10 silver‐containing dressings. J Wound Care. 2003;12(8):305‐308. [DOI] [PubMed] [Google Scholar]
- 55. Huang Y, Li X, Liao Z, et al. A randomized comparative trial between Acticoat and SD‐Ag in the treatment of residual burn wounds, including safety analysis. Burns. 2007;33(2):161‐166. [DOI] [PubMed] [Google Scholar]
- 56. Bhattacharyya M, Bradley H. Management of a difficult‐to‐heal chronic wound infected with methycillin‐resistant Staphylococcus aureus in a patient with psoriasis following a complex knee surgery. Int J Low Extrem Wounds. 2006;5(2):105‐108. [DOI] [PubMed] [Google Scholar]
- 57. Bhattacharyya M, Bradley H. A case report of the use of nanocrystalline silver dressing in the management of acute surgical site wound infected with MRSA to prevent cutaneous necrosis following revision surgery. Int J Low Extrem Wounds. 2008;7(1):45‐48. [DOI] [PubMed] [Google Scholar]
- 58. Silver S, Phung LT, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 2006;33(7):627‐634. [DOI] [PubMed] [Google Scholar]
- 59. Gupta A, Phung LT, Taylor DE, Silver S. Diversity of silver resistance genes in IncH incompatibility group plasmids. Microbiology. 2001;147(pt 12):3393‐3402. [DOI] [PubMed] [Google Scholar]
- 60. Muller M. Bacterial silver resistance gained by cooperative interspecies redox behavior. Antimicrob Agents Chemother. 2018;XX:XX‐XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Woods EJ, Cochrane CA, Percival SL. Prevalence of silver resistance genes in bacteria isolated from human and horse wounds. Vet Microbiol. 2009;138(3–4):325‐329. [DOI] [PubMed] [Google Scholar]
- 62. Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J. 2009;6(1):32‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bjarnsholt T, Kirketerp‐Møller K, Kristiansen S, et al. Silver against Pseudomonas aeruginosa biofilms. APMIS. 2007;115(8):921‐928. [DOI] [PubMed] [Google Scholar]
- 64. Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time‐ dependent therapeutic window. J Wound Care. 2010, 8;19:320‐328. [DOI] [PubMed] [Google Scholar]
- 65. Phillips PL, Yang Q, Davis S, et al. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J. 2013;12(4):469‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fitzgerald DJ, Renick PJ, Forrest EC, et al. Cadexomer iodine provides superior efficacy against bacterial wound biofilms in vitro and in vivo. Wound Repair Regen. 2017;25(1):13‐24. [DOI] [PubMed] [Google Scholar]
- 67. Malone M, Johani K, Jensen SO, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non‐healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother. 2017;72(7):2093‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ulkür E, Oncul O, Karagoz H, Yeniz E, Celiköz B. Comparison of silver‐coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and fusidic acid 2% (Fucidin) for topical antibacterial effect in methicillin‐resistant Staphylococci‐contaminated, full‐skin thickness rat burn wounds. Burns. 2005;31(7):874‐877. [DOI] [PubMed] [Google Scholar]
- 69. Acar A, Uygur F, Diktaş H, et al. Comparison of silver‐coated dressing (Acticoat®), chlorhexidine acetate 0.5% (Bactigrass®) and nystatin for topical antifungal effect in Candida albicans‐contaminated, full‐skin‐thickness rat burn wounds. Burns. 2011;37(5):882‐885. [DOI] [PubMed] [Google Scholar]
- 70. Uygur F, Oncül O, Evinç R, Diktas H, Acar A, Ulkür E. Effects of three different topical antibacterial dressings on Acinetobacter baumannii‐contaminated full‐thickness burns in rats. Burns. 2009;35(2):270‐273. [DOI] [PubMed] [Google Scholar]
- 71. Selçuk CT, Durgun M, Ozalp B, et al. Comparison of the antibacterial effect of silver sulfadiazine 1%, mupirocin 2%, Acticoat and octenidine dihydrochloride in a full‐thickness rat burn model contaminated with multi drug resistant Acinetobacter baumannii . Burns. 2012;38(8):1204‐1209. [DOI] [PubMed] [Google Scholar]
- 72. Newton H. Reducing MRSA bacteraemias associated with wounds. Wounds. 2010;6(1):56‐65. [Google Scholar]
- 73. Strand O, San Migue L, Rowan S, Sahlqvist A. Retrospective comparison of two years in a paediatric burns unit, with and without acticoat as a standard dressing. Ann Burns Fire Disasters. 2010;23(4):182‐185. [PMC free article] [PubMed] [Google Scholar]
- 74. Fong J, Wood F, Fowler B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: comparative patient care audits. Burns. 2005;31(5):562‐567. [DOI] [PubMed] [Google Scholar]
- 75. Tonkin C, Wood F. Nanocrystalline silver reduces the need for antibiotic therapy in burn wounds. Prim Intent. 2005;13(4):163‐168. [Google Scholar]
- 76. Glik J, Łabuś W, Kitala D, et al. A 2000 patient retrospective assessment of a new strategy for burn wound management in view of infection prevention and treatment. Int Wound J. 2017;15(3):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. National Health Service . Commissioning for quality and innovation: guidance for 2017/2019; 2016.
- 78. Rennie MY, Lindvere‐Teene L, Tapang K, Linden R. Point‐of‐care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: a clinical study. J Wound Care. 2017;26(8):452‐460. [DOI] [PubMed] [Google Scholar]
- 79. DaCosta RS, Kulbatski I, Lindvere‐Teene L, et al. Point‐of‐care autofluorescence imaging for real‐time sampling and treatment guidance of bioburden in chronic wounds: first‐in‐human results. PLoS One. 2015;10(3):e0116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ottolino‐Perry K, Chamma E, Blackmore KM, et al. Improved detection of clinically relevant wound bacteria using autofluorescence image‐guided sampling in diabetic foot ulcers. Int Wound J. 2017;14(5):833‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]


