Abstract
Critical lower limb ischaemia is a diffuse pathology that could cause claudication, severe ischaemic pain and tissue loss. The common treatment includes modification of risk factors, pharmacological therapy and endovascular or surgical revascularisation of the lower limb to restore a pulsatile flow distally. Spinal cord stimulator is seen as a valid alternative in patients unsuitable for revascularisation after endovascular or surgical revascularisation failure and as adjuvant therapy in the presence of a functioning bypass in patients with extensive tissue loss and gangrene presenting a slow and difficult wound healing. We report our experience on spinal cord stimulation (SCS) indication and implantation in patients with critical lower limb ischaemia, at a high‐volume centre for the treatment of peripheral arterial disease.
Keywords: Critical lower limb ischaemia, Pain relief, Spinal cord stimulation, Surgical revascularisation, Wound healing
Introduction
Critical lower limb ischaemia (CLI) is a common disease that usually results from progressive narrowing of the arteries caused by atherosclerosis 1. Severe ischaemic pain, claudication, tissue loss and finally limb amputation are common complications of peripheral arterial disease (PAD), especially among patients affected by diabetes mellitus and end‐stage renal disease and on haemodialysis 2, 3, 4. The common treatment of PAD includes correction of risk factors, medical therapy and revascularisation through endovascular treatment or distal bypass, in order to restore a pulsatile flow as distal as possible 2, 3, 4, 5.
However, some patients are unfit for revascularisation or a complete resolution of rest pain is not obtained, so they need alternative treatments to improve their quality of life and achieve pain relief and wound healing 6.
Spinal cord stimulation (SCS) was introduced by Shealy in 1969 7 for the treatment of intractable pain and, more recently, SCS indications were extended to patients with gangrene presenting severe comorbidities that render them unsuitable for surgery or in cases of persistent pain or slow and difficult wound healing after successful revascularisation 6. Complications from SCS include lead migration, lead connection failure, lead break, local pain, wound seroma, haematoma and infection. Hardware‐related complications are the most common and can occur in 11–36% of patients, with infection being the next most common, occurring in 3–6·3% of patients 8, 9, 10, 11.
In this study, we report our experience about SCS implantation in patients affected by critical lower limb ischaemia on Rutherford stages 4, 5 and 6 after revascularisation, in a high volume centre for the treatment of PAD.
Material and methods
From January 2009 to June 2013, 564 patients affected by PAD were found suitable for lower limbs surgical revascularisation in our institution. Prior approval was obtained from the Institutional Review Board (IRB) for this study.
Preoperative clinical and instrumental criteria for the temporary SCS implantation were met.
Clinical criteria were: persistent ischaemic area >2 cm after 15 days of surgical revascularisation and/or absence of granulation tissue. If the clinical criteria were present, instrumental criteria based on transcutaneous oxygen pressure (TcPO2) testing were performed and the cut‐off level was baseline TcPO2 <30 mm/Hg.
We divided our patients into four groups based on TcPO2: initial baseline TcPO2 value was 21·1 ± 3·3 mm/Hg for patients of group A, 14·4 ± 3·6 mm/Hg in group B, 11·3 ± 4·5 mm/Hg in group C and 26·1 ± 4·7 mm/Hg for patients of group D. One patient with steal syndrome presented an initial baseline TcPO2 value of 12·4 mm/Hg.
Of 564 patients affected by PAD, 34 patients (13 females and 21 males, mean age 63·3 ± 6·7 years) were found suitable for SCS implantation: two temporary spinal cord stimulators were explanted 3 weeks after implantation for local infection (one patient in Rutherford class 5 and one in Rutherford class 6) and in other two patients temporary spinal cord stimulators were explanted for malfunction and persistence of symptoms 2 weeks later (Rutherford class 6).
Among the other 30 patients, the indication for SCS implantation was represented by severe ischaemic pain in three cases (Rutherford 4) and by ischaemic lesions in two cases (Rutherford 5), with a very compromised distal arterial bed not responding to medical treatment (group A). The SCS was implanted after late bypass failure in nine patients presenting with persistent ischaemic distal lesions (seven patients in Rutherford class 5 and two in Rutherford class 6; group B) and in seven cases (group C) after early failure of multiple extreme attempts of endovascular and surgical revascularisation (four patients in Rutherford class 5 and three in Rutherford class 6). In eight cases (group D), the SCS was used as adjuvant therapy in patients with functioning bypass, presenting extensive tissue loss and slow wound healing (Rutherford class 6; Figures 1, 2, 3).
Figure 1.
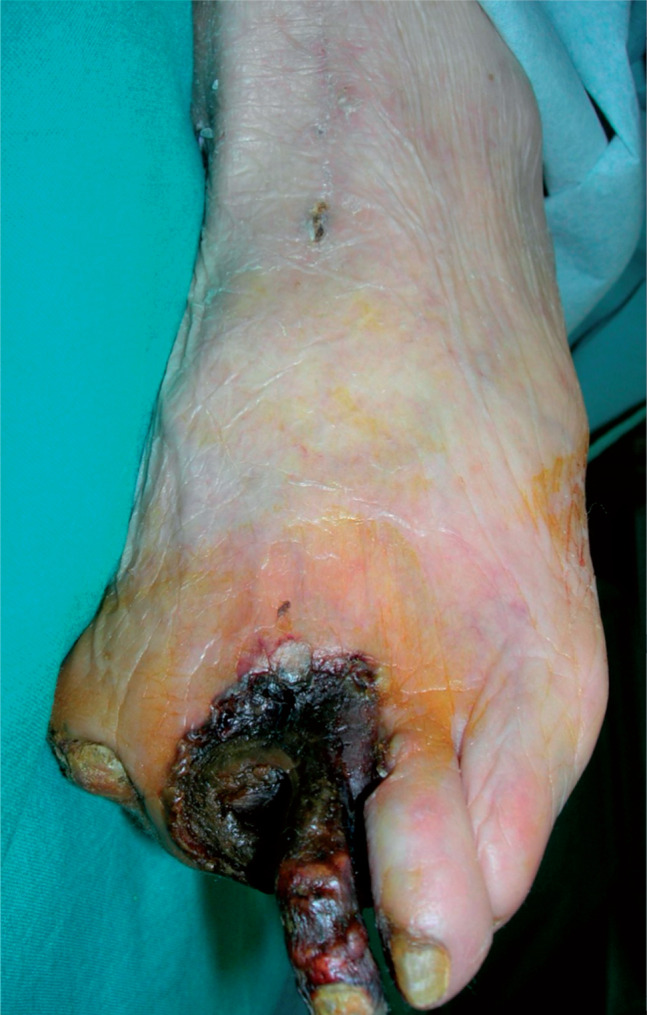
Extensive tissue loss and a slow wound healing.
Figure 2.
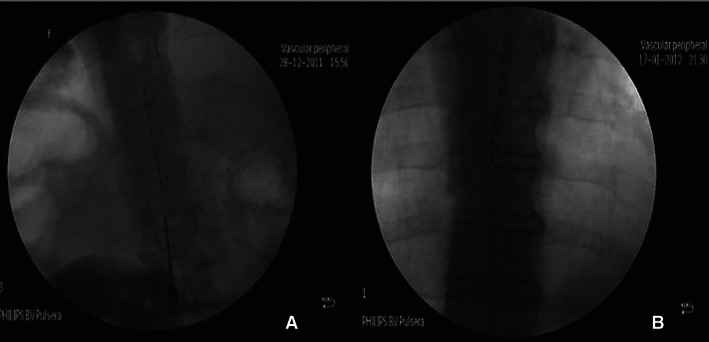
(A) Single octopolar lead; (B) double octopolar lead.
Figure 3.
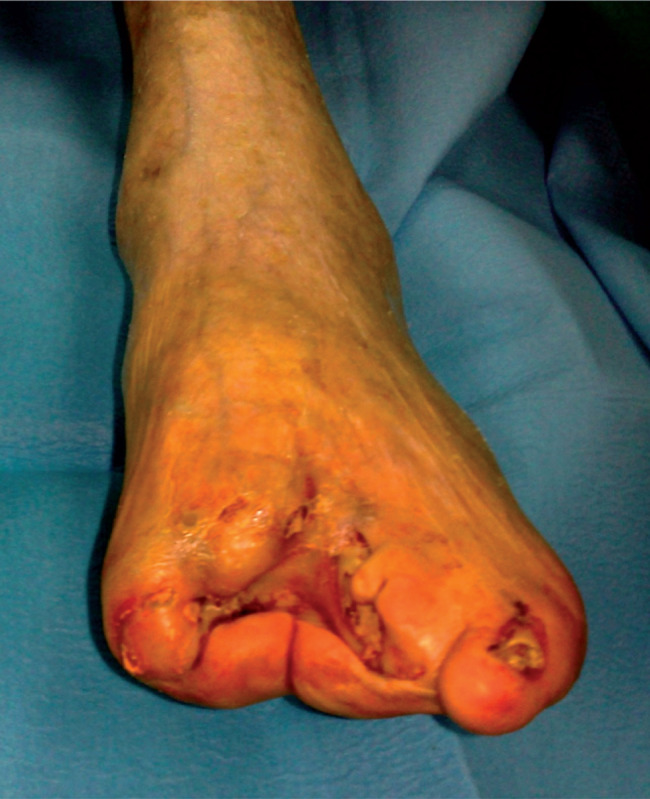
Wound healing after spinal cord stimulation (SCS).
One haemodialysis patient affected by steal syndrome, with left hand pain and cyanosis of the distal phalanx of the fourth finger, underwent upper limb surgical bypass and adjuvant cervical SCS, in order to obtain pain relief and limitation of gangrene in the cervical C3‐C6 peridural space.
The demographic characteristics of the patients, comorbidities and risk factors, degree of ischaemia, previous treatments, indications for SCS implantation procedures and TcPO2 values are reported in Table 1.
Table 1.
Demographic characteristics of patients, comorbidities and risk factors, degree of ischaemia, previous vascular treatments, TcPO2 values, trial SCS therapy failure, indications for permanent SCS implantation, outcomes
|
Demographic characteristics of patients (pts 34) |
|
|
Risk factors and comorbidities (pts 34) |
|
|
Degree of ischaemia (Rutherford class) (pts 34) |
|
|
Previous treatment (pts 34) |
|
| Initial baseline TcPO2 values (supine position) |
|
Pts, patients; TcPO2, transcutaneous oxygen pressure; SCS, spinal cord stimulation.
All implantations were carried out by a single experienced surgeon. Antiplatelet agents were stopped 7 days before the planned intervention and subcutaneous heparin was discontinued 12 hours preoperatively. Electrodes were always implanted under local anaesthesia and intravenous broad‐spectrum antibiotic therapy was administered.
As previously described 8, 9, SCS requires the insertion of single or double leads of four or eight electrodes into the epidural space, between the thoracic 8 and 11 levels, with the electrodes connected to an impulse generator (The Nevro® Senza® SCS system, Menlo Park, CA; Medtronic® Inc., Minneapolis, MN; St. Jude Medical®, St. Paul, MN).
The electrodes are usually centred in the spinal canal or directed off‐centre, depending on the limb with the most significant symptoms. Patients generally may undergo a trial use of SCS to determine the optimal location of lead placement in order to obtain pain relief as expected. As previously shown 11, 12, 13, patients who respond well during the trial period become candidates for permanent lead and generator placement.
An increase of TcPO2 values >10 mm/Hg with trial SCS therapy, represented the indication for definitive device implantation in all cases, except in two patients in whom an increase <10 mm/Hg was recorded. Other two patients received SCS temporary explantation for local infection (Table 1).
After SCS implantation, the patients were monitored for postoperative controls in the outpatient vascular unit after 2 weeks, 1, 3, 6 months, 1 year, and then yearly. These controls include a physical examination and technical controls of the stimulation system.
Results
During follow‐up, we observed the failure of four temporary devices (11·8%): two for local infection 3 weeks after implantation and two for persistence of pain 2 weeks later. Three patients underwent lower limb amputation during follow‐up and one patient was submitted to another extreme surgical revascularisation and he died after 12 days because of myocardial infarction.
Among the remaining 30 cases, patients of group A achieved complete pain relief, improvement of quality of life and complete wound healing in an average period of 1·2 ± 0·5 months after starting therapy.
In six patients (Rutherford class 5) of group B, pain relief and complete wound healing were observed in an average period of 2·7 ± 1·4 months. However, in one of these six patients presenting complete healing of his foot lesion, the SCS was removed following infection, 6 months after implantation. Another Rutherford class 5 patient obtained improvement of ischaemic lesions, but without definitive wound healing in the follow‐up of 15 months. One patient with extensive tissue loss and gangrene (Rutherford 6) required a longer interval time from device implantation (7 months), to achieve a complete healing. The other patient in Rutherford class 6 did not obtain improvement of his clinical status and the definitive SCS was removed 2 months later and he underwent lower limb amputation during follow‐up.
In group C, the clinical status of two patients (Rutherford class 6) did not show improvement; the definitive SCS was removed 5 and 8 months later and they underwent lower limb amputation during follow‐up. Little improvement of trophic lesions was seen in one patient but he died 5 months later with functioning SCS and with a residual gangrene at the calcanear site. The other four patients achieved wound healing in a mean period of 3·4 ± 1·5 months.
Patients of group D presented a complete healing in a mean period of 4·6 ± 2·7 months, except one patient who obtained improvement of ischaemic lesions without definitive complete healing during 17 months of follow‐up. One device was explanted 2 years later for surgical wound dehiscence at the level of impulse generator implantation and the other one at 3 years for surgical wound dehiscence at the site of leads placement for a wound on the back. The patient with upper limb steal syndrome obtained pain relief and complete wound healing of the hand after 7 weeks.
Among the other 30 cases, three permanent SCS were removed after complete remission of symptoms and three were explanted for malfunction, with lower limb amputation during follow‐up. In three cases, the wound healing was incomplete in the follow‐up period. In the remaining 21 cases, a complete healing was achieved, requiring a longer period especially in the presence of extensive gangrene and tissue loss (Rutherford 6). The haemodialysis patient obtained pain relief and hand salvage during follow‐up.
Results are explained in Table 2.
Table 2.
Results
| TcPO2 values after trial SCS therapy (supine position) |
|
| Trial SCS therapy failure |
|
|
Indications for permanent SCS implantation (pts 30) |
|
|
Outcomes (pts 30) |
|
Pts, patients; TcPO2, transcutaneous oxygen pressure; SCS, spinal cord stimulation.
Wound healing
Wound healing was more in those patients who presented a higher baseline value of TcPO2. The following significant increase of TcPO2 obtained with the definitive SCS implantation significantly influenced wound healing: from 21·1 ± 3·3 mmHg to 32·6 ± 3·7 mmHg in group A patients with rest pain (100% wound healing); from 14·4 ± 3·6 mmHg to 34·8 ± 3·9 mmHg in group B patients with late failed revascularisation (78% wound healing); from 11·3 ± 4·5 mmHg to 27·3 ± 4·4 mmHg in group C patients with early failed revascularisation (57% wound healing); in those patients with bypass patent in group D, from 26·1 ± 4·7 to 39·8 ± 4·9 mmHg (88% wound healing).
Discussion
The mechanism of action of SCS is not yet completely clear, and many theories have been reported. In general, the electrodes in the epidural space stimulate sensory unmyelinated c‐fibres and myelinated Aδ‐fibres 14, 15, 16 with activation of cell‐signalling molecules such as extracellular signal‐regulated kinase (ERK) and protein kinase B (AKT) that stimulate the transient receptor potential vanilloid receptor 1 (TRPV1). Activation of TRPV1 and depolarisation of the nerve terminals cause the release of vasodilators such as calcitonin gene‐related peptide (CGRP), which has powerful microvascular vasodilatory effects. The release of CGRP is responsible for endothelial nitric oxide (NO) release and stimulates smooth muscle cell relaxation. These effects lead to a decrease in vascular resistance and an increase in local blood flow 10, 14, 17, 18. In addition, SCS suppresses sympathetic vasoconstriction through inhibition of sympathetic nicotine transmission at the ganglionar level 12, 14, 19. Pain relief is mediated by the suppression of pain or nociceptive transmission and the release of opioid peptides such as met‐enkephalin 17.
SCS is indicated in many conditions such as failed back surgery syndrome, degenerative low back or leg pain, spinal stenosis, nerve root avulsion, traumatic nerve injury, chronic regional pain syndromes, postherpetic neuralgia, neuropathic perineal pain, interstitial cystitis, urge incontinence, refractory angina and peripheral vascular disease 19.
In patients with critical lower limb ischaemia, SCS implantation was introduced in case of severe refractory ischaemic pain. Even if the endovascular approach reduced the number of patients unsuitable for revascularisation, however, some patients cannot be treated by angioplasty or open surgery; moreover, some are unfit for surgery, and others have persistent distal ischaemia and pain with a functioning revascularisation. In these cases, SCS either alone or associated with prostanoids could be indicated and a scrupulous and careful selection of patients on the basis of TcPO2 value measurements and clinical status is essential to for successful treatment of ischaemic pain relief, wound healing, lower limb amputation rate reduction and improvement of quality of life.
Brummer et al. reported their experience on SCS application in eight haemodialysis patients. Intensity of pain and quality of life significantly improved during follow‐up, reporting a limb salvage of 75% 10.
Petrakis and Sciacca described their experience about 150 patients with Fontaine stage III and IV disease: limb salvage was achieved in patients who experienced a significant increase in TcPO2 within the first 2 weeks of the testing period 20.
A Dutch multicentre randomised controlled trial compared 55 patients assigned to standard medical therapy to 56 patients treated with SCS and standard medical therapy. The results of this study suggest that patient selection on the basis of the initial microcirculatory skin perfusion can identify those in whom SCS can improve local skin perfusion and limb survival 21.
Spincemaille et al. reported a limb salvage of 77% at 18 months with an increase of TcPO2 value of at least 15% after a trial of SCS. The outcome in patients with an initial TcPO2 ≤ 10 mmHg was significantly poor compared with those with higher TcPO2 22.
Horsch et al. showed improved limb survival and a delay in time‐to‐amputation in patients with an initial baseline TcPO2 of 10–30 mmHg compared with those with a baseline <10 mmHg 23.
Also, Ubbink and Vermeulen recommended SCS in patients with CLI, particularly if their foot TcPO2 was 10–30 mmHg 24.
The European Peripheral Vascular Disease Outcome Study (SCS‐EPOS) concluded that patient selection based on TcPO2 trial screening further increases the probability of limb preservation with SCS therapy 25.
In a meta‐analysis of 444 patients that compared SCS to any form of conservative treatment for inoperable CLI, patients receiving the device required significantly less analgesia and experienced improvement in their Fontaine stage classification 9.
Pedrini and Magnoni reviewed the efficacy of SCS in patients with untreatable CLI and found that pain relief, ulcer healing and limb salvage were greater in non‐diabetic patients, in diabetic patients without autonomic neuropathy, and in patients with rest pain or ulcer more often than in patients with gangrene 14.
The Cochrane review of SCS for non‐reconstructable CLI found that limb salvage after 12 months was significantly higher in patients with the device. Pain relief was also more prominent in the SCS‐treated patients, and they required significantly less analgesics.
In our experience, of the 33 SCS implanted in patients affected by PAD in Rutherford classes 4, 5 and 6, the majority was implanted after surgical revascularisation as adjuvant therapy in association with functioning bypass or after early or late bypass failure in order to improve wound healing and promote pain relief. Only in five cases the SCS was implanted in patients previously revascularised, presenting a much compromised distal arterial bed and one SCS was positioned in the cervical peridural space for the treatment of steal syndrome in a haemodialysis patient. Application of SCS in the cervical space has been previously reported in literature for the treatment of different diseases 26, 27. Results of our study were very encouraging: group A showed a healing rate of 100%, group B of 78%, group C of 57% and group D of 88%.
SCS could be considered a valid alternative for patients affected by PAD with refractory ischaemic pain and tissue loss in the presence of a compromised distal arterial bed for patients with failed revascularisation and persistent ischaemic symptoms and in association with a functioning bypass in patients with slow and difficult wound healing.
We believe that a correct and scrupulous selection of patients for SCS therapy by including those with a baseline TcPO2 of 10–30 mmHg and who demonstrate ≥10 mmHg increase with an initial SCS trial period is necessary to achieve a good results, thereby improving the quality of life and reducing the amputation rate inpatients.
Acknowledgements
The authors received no funding. The authors declare no conflict of interest.
References
- 1. Blecha MJ. Critical limb ischemia. Surg Clin North Am 2013;93:789–812. [DOI] [PubMed] [Google Scholar]
- 2. Serra R, Grande R, Scarcello E, Buffone G, de Franciscis S. Angiosome‐targeted revascularisation in diabetic foot ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12162[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J 2013;10:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, Buffone G, Serra R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J 2013. DOI: 10.1111/iwj.12085[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Caridi G, Massara M, Villari S, Martelli E, Spinelli F, Grande R, Butrico L, de Franciscis S, Serra R. Extreme distal bypass to improve healing in Buerger's disease. Int Wound J 2014. DOI: 10.1111/iwj.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Caridi G, Massara M, Benedetto F, Tripodi P, Spinelli F, David A, Butrico L, Serra R, de Franciscis S. Adjuvant spinal cord stimulation improves wound healing of peripheral tissue loss due to a steal syndrome of the hand: clinical challenge treating a difficult case. Int Wound J 2014. DOI: 10.1111/iwj.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shealy CN. Dorsal column electrohypalgesia. Headache 1969;9:99–102. [DOI] [PubMed] [Google Scholar]
- 8. Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Physician 2002;5:156–66. [PubMed] [Google Scholar]
- 9. Ubbink DT, Vermeulen H, Spincemaille GH, Gersbach PA, Berg P, Amann W. Systematic review and meta‐analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischemia. Br J Surg 2004;91:948–55. [DOI] [PubMed] [Google Scholar]
- 10. Brümmer U, Condini V, Cappelli P, Di Liberato L, Scesi M, Bonomini M, Costantini A. Spinal cord stimulation in hemodialysis patients with critical lower‐limb ischemia. Am J Kidney Dis 2006;47:842–7. [DOI] [PubMed] [Google Scholar]
- 11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract 2011;11:148–53. [DOI] [PubMed] [Google Scholar]
- 12. Reig E, Abejón D, del Pozo C, Wojcikiewicz R. Spinal cord stimulation in peripheral vascular disease: a retrospective analysis of 95 cases. Pain Pract 2001;1:324–31. [DOI] [PubMed] [Google Scholar]
- 13. Epstein LJ, Palmieri M. Managing chronic pain with spinal cord stimulation. Mt Sinai J Med 2012;79:123–32. [DOI] [PubMed] [Google Scholar]
- 14. Pedrini L, Magnoni F. Spinal cord stimulation for lower limb ischemic pain treatment. Interact Cardiovasc Thorac Surg 2007;6:495–500. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Role of primary afferents in spinal cord stimulation‐induced vasodilation: characterization of fiber types. Brain Res 2003;959:191–8. [DOI] [PubMed] [Google Scholar]
- 16. Provenzano DA, Jarzabek G, Georgevich P. The utilization of transcutaneous oxygen pressures to guide decision‐making for spinal cord stimulation implantation for inoperable peripheral vascular disease: a report of two cases. Pain Physician 2008;11:909–16. [PubMed] [Google Scholar]
- 17. Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 2008;138:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka S, Komori N, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci 2004;114:55–60. [DOI] [PubMed] [Google Scholar]
- 19. Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician 2006;9:347–52. [PubMed] [Google Scholar]
- 20. Petrakis IE, Sciacca V. Spinal cord stimulation in critical limb ischemia of the lower extremities: our experience. J Neurosurg Sci 1999;43:285–93. [PubMed] [Google Scholar]
- 21. Ubbink DT, Spincemaille GH, Prins MH, Reneman RS, Jacobs MJ. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. J Vasc Surg 1999;30:236–44. [DOI] [PubMed] [Google Scholar]
- 22. Spincemaille GH, de Vet HC, Ubbink DT, Jacobs MJ. The results of spinal cord stimulation in critical limb ischemia: a review. Eur J Vasc Endovasc Surg 2001;21:99–105. [DOI] [PubMed] [Google Scholar]
- 23. Horsch S, Schulte S, Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single‐center study of 258 patients. Angiology 2004;55:111–8. [DOI] [PubMed] [Google Scholar]
- 24. Ubbink DT, Vermeulen H. Spinal cord stimulation for critical leg ischemia: a review of effectiveness and optimal patient selection. J Pain Symptom Manage 2006;31(4 Suppl):S30–5. [DOI] [PubMed] [Google Scholar]
- 25. Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT, European Peripheral Vascular Disease Outcome Study SCS‐EPOS . Spinal cord stimulation in the treatment of nonreconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Outcome Study (SCS‐EPOS). Eur J Vasc Endovasc Surg 2003;26:280–6. [DOI] [PubMed] [Google Scholar]
- 26. De Andrés J, Tatay J, Revert A, Valía JC, Villanueva V. The beneficial effect of spinal cord stimulation in a patient with severe cerebral ischemia and upper extremity ischemic pain. Pain Pract 2007;7:135–42. [DOI] [PubMed] [Google Scholar]
- 27. Vallejo R, Kramer J, Benyamin R. Neuromodulation of the cervical spinal cord in the treatment of chronic intractable neck and upper extremity pain: a case series and review of the literature. Pain Physician 2007;10:305–11. [PubMed] [Google Scholar]


