Abstract
Extremely limited data are available for the treatment of helical rim keloids. The purpose of the present study was to demonstrate the successful treatment of helical rim keloids using surgical exicison followed by a newly designed pressure therapy device. We treated 40 pure helical rim keloids in 36 patients with surgical excisions followed by pressure therapy using a combination of magnets and silicone gel sheeting for 12 hours over a period of 2 years, from May 2012 to February 2014, at tertiary medical centre. The follow‐up period was 18 months. Primary outcome was recurrence of keloids. Secondary outcome was patient satisfaction as assessed by the Patient Observer Scar Assessment Scale (POSAS). The overall recurrence‐free rate was 95·0% after a follow‐up period of 18 months. Scores obtained from the POSAS showed that most items were reported to be improved. This adjuvant therapy protocol resulted in excellent outcomes in cases of helical rim keloids compared to previously published protocols.
Keywords: Helix, Keloid, Pressure, Silicone
Introduction
Auricular keloids can appear months or even years after the ear piercing and tend not to spontaneously regress and consequently cause various patient morbidities such as cosmetic disfigurement and psychological trauma.
Because of treatment challenges, numerous treatment modalities including pressure therapy, cryotherapy, intralesional steroid injection, radiation therapy, topical silicone‐gel sheeting application and various laser therapies have been performed for auricular keloids.
Postsurgical pressure therapy has evolved as an important adjuvant treatment for auricular keloids, and numerous pressure earrings have also been tried in many institutions with their own rationale 1, 2, 3, 4, 5, 6, 7.
To date, however, there have been limited studies regarding keloids of helical rim compared to earlobe keloids 8, 9, 10. As there have been increasing trends for multiple piercings, incidence of helical rim keloids are expected to increase as well. Compared to those of earlobes, overlying soft tissue of helical rim is relatively thin, and soft tissue itself is closely adherent to auricular cartilage.
Although we previously reported successful postsurgical use of magnets for the treatment of auricular keloids, some patients were presented with helical rim necrosis following excessive application of magnets. For this reason, we came up with new adjuvant pressure tools, ‘Magsil’, using magnets and silicone gel sheeting. The purpose of the current study is to demonstrate our successful postsurgical use of ‘Magsil’ for the treatment of helical rim keloids and analyse the long‐term efficacy of this new device to prevent keloid recurrence following surgical excision.
Patients and methods
Forty patients with helical rim keloids were treated with surgical excision followed by Magsil therapy over a period of 2 years, from May 2012 to February 2014, at tertiary medical centre.
Inclusion criteria
(i) Primary keloids that were limited to a helical rim area caused by ear piercings; the helical rim keloid was elevated and extended beyond the dimensions of the initiating injury lesion and the lesion was not treated previously and other areas of the ear was not affected; (ii) female patients older than 18 years; (iii) patients for whom surgical excision with primary closure was scheduled; and (iv) patients who did not undergo additional ear piercings during treatment before final outcome measurement.
Patients were excluded from the study if they were unavailable for follow‐up, and all patients who were included agreed to comply with the treatment protocol. Patients were also excluded from the study if they received additional adjuvant therapy during treatment or histological confirmation was not obtained. All included patients consented to the requirement for final follow‐up after 18 months.
Intra and postoperative care, and follow up and outcome assessment
All procedures were performed under local anaesthesia. We excised the keloidal cores as completely as possible leaving marginal skin to cover the defect. We closed wounds with appropriate approximation using nylon 5‐0 interrupted sutures, and compressive hydrocolloid wound dressing was applied. All keloids were sent out for histological examination to confirm clinical diagnoses.
Patients were instructed to use the Magsil for approximately 12 hours per day for 4 months until the therapy was completed as shown in Figure 1. During the adjuvant pressure therapy, patients were frequently seen for follow‐up for recurrence and evaluation of the success of the therapy (Figures 1 and 2). In all patients, a follow‐up period of 18 months was applied to assess treatment outcome, which was recorded as recurrence or non‐recurrence. The Patient and Observer Scar Assessment Scale (POSAS) was used to evaluate overall patient satisfaction.
Figure 1.

Adjuvant pressure therapy using a combination of silicone gel sheeting and magnets.
Statistical analysis
All statistical analyses were conducted using SPSS version 20.0 (SPSS, Inc., Chicago, IL). Descriptive statistics are presented as means with standard deviations or as numbers and percentages. A paired t‐test was used to analyse the results of the POSAS at baseline and 18 months, postoperatively. Two‐tailed hypothesis tests were considered statistically significant if P < 0·05.
Results
Primary outcome
All patients completed the treatment protocol with a follow‐up interval of 18 months. Of these patients, 95·0% experienced successful eradication of their helical rim keloids, whereas 5·0% had recurrences. The postoperative course was uneventful without exception. The representative pre‐ and postoperative views are shown in Figures 2 and 3.
Figure 2.
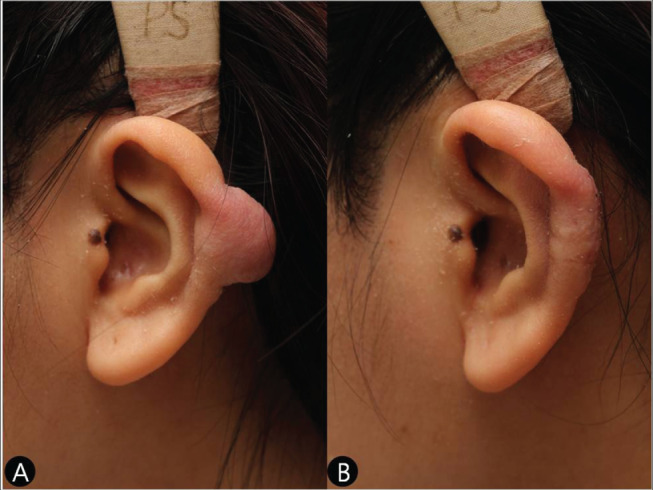
(A) A preoperative view of a sessile pattern keloid on the left helical rim in a 21‐year‐old girl. (B) A postoperative view of the same patient (Case 1).
Figure 3.
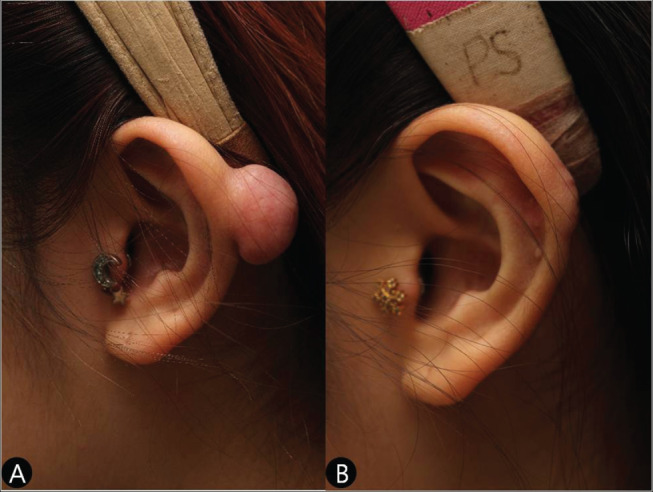
(A) A preoperative view of a pedunculated‐pattern keloid on the left helical rim in a 23‐year‐old girl. (B) A postoperative view of the same patient (Case 2).
Secondary outcome
Treatment with Magsil resulted in a statistically significant improvement of most of the item scores of the POSAS in terms of pain, itchiness, color, stiffness, thickness and irregularity of patient scale along with pigmentation, thickness, relief and pliability of observer scale. The results are shown in Tables 1 and 2.
Table 1.
Results of patient scar assessment scale
| Patient scar assessment scale | Baseline | Month 18 | Statistical difference | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P‐value | |||
| Total Score | 32·65 | 2·38 | 20·83 | 2·40 | <0·001* |
| Pain | 5·10 | 0·63 | 3·45 | 0·75 | <0·001* |
| Itchiness | 4·90 | 0·78 | 3·43 | 0·71 | <0·001* |
| Color | 5·45 | 1·11 | 3·68 | 1·10 | <0·001 |
| Stiffness | 6·15 | 1·00 | 3·40 | 0·98 | <0·001* |
| Thickness | 6·58 | 1·11 | 3·45 | 1·11 | <0·001* |
| Irregularity | 4·48 | 1·34 | 3·43 | 0·81 | <0·001* |
Statistically significant if P < 0·05.
Table 2.
Results of observer scar assessment scale
| Observer scar assessment scale | Baseline | Month 18 | Statistical difference | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P‐value | |||
| Total Score | 28·5 | 2·86 | 17·93 | 2·37 | <0·001* |
| Vascularisation | 4·28 | 1·06 | 3·98 | 0·97 | 0·103 |
| Pigmentation | 5·80 | 1·56 | 3·58 | 1·48 | <0·001* |
| Thickness | 7·03 | 1·39 | 3·13 | 1·07 | <0·001* |
| Relief | 4·68 | 1·21 | 3·68 | 0·83 | <0·001* |
| Pliability | 6·73 | 1·04 | 3·58 | 1·08 | <0·001* |
Discussion
Keloids are a proliferative ailment of fibrous tissue secondary to trauma, infection, surgery and other various causes 11. Keloids have a tendency to recur after surgical excision alone, with rates of 80–100% 12. Simple excision is thought to promote additional collagen synthesis resulting in regrowth 13.
In a current study, we adopted the POSAS that was developed in 2004 for burn scar assessment and has since been validated for several conditions such as linear scars and, more recently, keloid scars 14, 15, 16. This scale has the benefit of incorporating patient opinion and can better assess symptoms such as pain, itchiness and thickness 17. Nicholas et al. concluded that the POSAS is a reliable and valid method of assessing keloid scars in a clinical context according their study in 2012 15. Our POSAS results revealed that our protocol, using a combination of magnets and silicone gel sheeting, is very effective in terms of pain, itchiness, color, stiffness, thickness and irregularity of patient scale along with pigmentation, thickness, relief and pliability of observer scale. This improved subjective and objective scar characteristics is also evidenced by an excellent overall recurrence‐free rate of 95·0% after a follow‐up period of 18 months.
Keloids of the helical rim are less frequently seen than those on the earlobe but are equally disfiguring 8. Therefore, successful treatment of keloids of this anatomical location is very important as well. However, extremely limited data are available for the treatment of helical rim keloids. Sand et al. reported a case of a large keloid on the helical rim that was successfully treated with surgery, intralesional injection of a combination of 0·5 ml triamcinolonacetonid and 2% scandicain and a custom‐designed silicon pressure clip 18. Rasheed and Malachy reported their experience of six helical rim keloids in five patients using surgical excision followed by split‐thickness skin grafting and postoperative steroid injection therapy at 2 weeks, postoperatively 9. Burm and Hansen demonstrated their successful experience in seven helical keloids in seven patients using surgical excision followed by full‐thickness skin grafting that was followed by multivitamins' administration and topical creams and sunscreen application 8.
As suggested by previous studies, huge helical defects following surgical excision of keloids can be a great reconstructive challenge to many plastic surgeons. For this reason, Burm and Hansen reported their successful experiences using full‐thickness skin grafting with marginal de‐epithelialization to reconstruct the defect following the excision of helical rim keloids 8, while Rasheed and Malachy reported their split‐thickness skin grafting experiences as full‐thickness skin grafting is generally considered too unreliable over a poorly vascularised bed such as a helical defect with exposed cartilage 9. However, as already suggested in our previous study, using skin grafts and neglecting the initial step on the reconstructive ladder can expose patients to more risk than necessary. In addition, we think that closing defects with a skin graft using distant tissue would make keloids vulnerable to recurrence as well as poor color and texture match, which consequently contributes to poor patient satisfaction 19.
Different from the surgical approach to earlobe keloids, we did not excise all the skin covering the keloidal core. Rather, we left some marginal skin that would mange to cover the resultant defect. This conservative surgical approach is based on our previous histopathological collagen architecture of auricular keloids as shown in Figure 4. Part C was probably the main cause of keloid recurrence, and ‘WHFN’ (whirling hypercellular fibrous micronodules) consisted of densely compacted activated young fibroblasts, more than twice the rate in other parts, so that these might serve as a proliferating centre of keloid collagen structure 20. If surgeons can eradicate this Part C, proliferating the centre of the keloid collagen structure, and leave some of part A to cover the defect, recurrence might not be a great issue. The application of silicone gel sheeting to the ears is inconvenient and bothersome. Additionally, they are easily dislocated, and they are not conventionally used for the treatment of auricular keloids despite their known mechanism of actions such as greater occlusion and hydration, higher temperature and changes of collagenase kinetics. However, by manually inserting magnets into silicone gel sheeting, the sheeting serves as effective padding between magnets and intervening soft tissue. With this padding effect, we do not see any dermatological problems or skin necrosis in the current case series.
Figure 4.
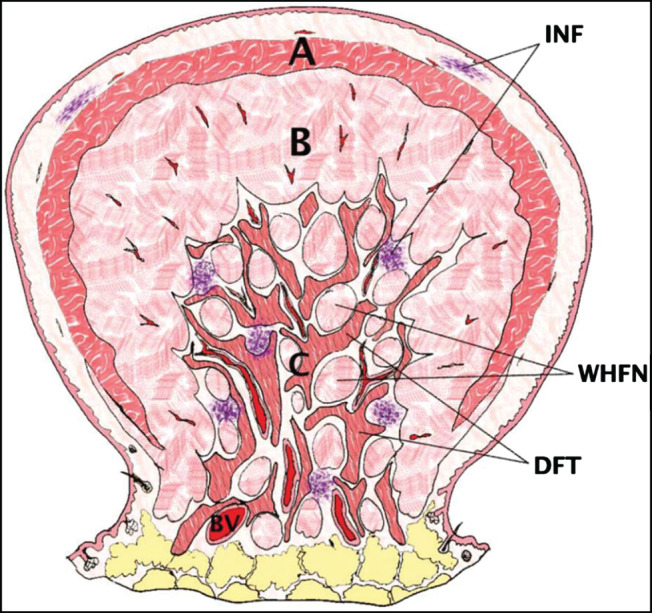
Schematic illustration of the microarchitecture of auricular keloids. (A) keloidal collagen fibers; (B) haphazardly arranged dense fibrous fascicles; (C) mixed dense fibrous collagen tissue with whorling hypercelluar fibrous micronodules/fascicles and intervening loose connective tissue with abundant vasculature; INF, inflammatory cell infiltration; WHFN, whorling hypercellular fibrous micronodules; DFT, dense fibrous collagen tissue; BV, blood vessel (Reprinted with permission from dermatological surgery) 20.
Acknowledgements
We convery our sincere thanks to Dr. Jang, affiliated with Ivy plastic surgery clinic in Jamwon‐dong (Sinsa‐station), for providing devotion and support for this work. The authors declare that they have no conflict of interest.
Correction added on 27 January 2017, after first online publication: Tae Hwan Park's affiliation and correspondence address have been changed.
References
- 1. Vachiramon A, Bamber MA. A U‐loop pressure clip for earlobe keloid. J Prosthet Dent 2004;92:389–91. [DOI] [PubMed] [Google Scholar]
- 2. Yencha MW, Oberman JP. Combined therapy in the treatment of auricular keloids. Ear Nose Throat J 2006;85:93–4, 96‐97. [PubMed] [Google Scholar]
- 3. Akoz T, Gideroglu K, Akan M. Combination of different techniques for the treatment of earlobe keloids. Aesthetic Plast Surg 2002;26:184–8. [DOI] [PubMed] [Google Scholar]
- 4. Sela M, Taicher S. Prosthetic treatment of earlobe keloids. J Prosthet Dent 1984;52:417–8. [DOI] [PubMed] [Google Scholar]
- 5. Chang CH, Song JY, Park JH, et al. The efficacy of magnetic disks for the treatment of earlobe hypertrophic scar. Ann Plast Surg 2005;54:566–9. [DOI] [PubMed] [Google Scholar]
- 6. Chrisostomidis C, Konofaos P, Chrisostomidis G, et al. Pressure therapy with a round rod for hypertrophic scars: reply. Plast Reconstr Surg 2008;121:692. [DOI] [PubMed] [Google Scholar]
- 7. Park TH. New pressure device, “Magsil,” as an adjuvant pressure therapy for ear keloids. Arch Facial Plast Surg 2012;14:298–9. [DOI] [PubMed] [Google Scholar]
- 8. Burm JS, Hansen JE. Full‐thickness skin grafting with marginal deepithelialization of the defect for reconstruction of helical rim keloids. Ann Plast Surg 2010;65:193–6. [DOI] [PubMed] [Google Scholar]
- 9. Rasheed IA, Malachy AE. The management of helical rim keloids with excision, split thickness skin graft and intralesional triamcinolone acetonide. J Cutan Aesthet Surg 2014;7:51–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuzbasioglu E. Reverse activated hyrax pressure appliance for treatment of a keloid located at auricula helix. J Prosthodont 2013;22:509–12. [DOI] [PubMed] [Google Scholar]
- 11. Park TH. Keloid on scapular area secondary to therapeutic dry cupping. Int Wound J 2015 Oct;12(5):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park TH, Seo SW, Kim JK, et al. Outcomes of surgical excision with pressure therapy using magnets and identification of risk factors for recurrent keloids. Plast Reconstr Surg 2011;128:431–9. [DOI] [PubMed] [Google Scholar]
- 13. Niessen FB, Spauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999;104:1435–58. [DOI] [PubMed] [Google Scholar]
- 14. Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004;113:1960–5; discussion 1966–1967. [DOI] [PubMed] [Google Scholar]
- 15. Nicholas RS, Falvey H, Lemonas P, et al. Patient‐related keloid scar assessment and outcome measures. Plast Reconstr Surg 2012;129:648–56. [DOI] [PubMed] [Google Scholar]
- 16. van de Kar AL, Corion LU, Smeulders MJ, et al. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg 2005;116:514–22. [DOI] [PubMed] [Google Scholar]
- 17. Lorenz P, Bari AS. Scar prevention, treatment, and revision. In: Neligan P, editor. Plastic Surgery, 3rd edn. Elsevierhealth, 2013:299. [Google Scholar]
- 18. Sand M, Sand D, Boorboor P, et al. Combination of surgical excision and custom designed silicon pressure splint therapy for keloids on the helical rim. Head Face Med 2007;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park TH, Seo SW, Kim JK, et al. Earlobe keloids: classification according to gross morphology determines proper surgical approach. Dermatol Surg 2012;38:406–12. [DOI] [PubMed] [Google Scholar]
- 20. Chong Y, Park TH, Seo SW, et al. Histomorphometric analysis of collagen architecture of auricular keloids in an Asian population. Dermatol Surg 2015;31:415–522. [DOI] [PubMed] [Google Scholar]


