Abstract
Double layer dermal substitute (DS) consist of a 3‐dimensional collagen structures and a superficial silicon layer that are positioned within the defect provide to promote tissue regeneration in skin wounds. DS often have unique physical characteristics due to differences in manufacturing techniques. The aim of this study is the clinical and histological comparison of Nevelia and Integra double layer DSs in patients with post‐traumatic injury wounds. Thirty patients with post‐traumatic wounds localised on the inferior limbs were randomised in 2 groups Nevelia or Integra, followed by autologous dermal epidermal graft (DEG). Clinical results were evaluated through the healing time; Manchester Scar Scale (MSS) and Visual Analog Scale (VAS) at 1, 2, and 3 weeks and after 1 and 3 years. Histological and immunohistochemical evaluation were performed at 0, 2, and 3 weeks. The difference in healing time between groups (P = .467, log‐rank test), pain and self‐estimation was not statistically significant after 35, 42, and 49 days and at 1‐year follow up. Histological data showed evident healing of wound after 2 weeks compared with preoperative with both DSs. At 3 weeks reepithelialisation and dermal regeneration were evident with both substitutes; however Nevelia showed early regenerative properties in terms of epidermal proliferation and dermal renewal compared with Integra. Nevelia showed also a more evident angiogenesis vs Integra evaluated as α‐SMA immunohistochemistry. Differences in the MSS score were statistically significant at 3 years follow up in favour of Nevelia group (P = .001). At long‐term follow up, Nevelia showed a better clinical outcome measured as MSS score vs Integra measured as MSS. Histological and immunohistochemistry data showed that Nevelia allows faster neoangiogenesis and tissue regeneration with neoformed tissue architecture closer to the physiology of the skin.
Keywords: dermal template, long‐term trial, tissue regeneration, wound, wound healing
1. INTRODUCTION
Skin grafts have been used to restore acute and chronic wound deficiencies with different aetiologies. However, the availability of sufficient healthy skin, additional health risks, and deforming donor site morbidity should be issues and have to be considered when opting for skin grafting. Scientists and surgeons have collaborated in the last few years to develop various bioengineered and synthetic alternatives to promote tissue regeneration in skin wounds. A double‐layer dermal substitute (DS) consists of a 3‐dimensional collagen structure and a superficial silicon layer that are positioned within the defect and provide immediate protection against dehydration, micro‐organisms, and toxins.1 The collagen layer then gradually becomes incorporated in the wound bed, a process supported by natural wound‐healing mechanisms such as local inflammation, cell infiltration (neutrophils, macrophages, fibroblasts, and keratinocytes), and neovascularisation of the scaffold.2
The choice of appropriate DS is important to guide cell behaviour, and cytotoxic products or materials that induce extensive scar formation or uncontrolled inflammation should be avoided. DS often has unique physical characteristics due to differences in manufacturing technique. The treatment of full‐thickness post‐traumatic wounds is a multi‐layer approach: orthopaedic, micro‐vascular, and—finally—plastic surgery. In recent years, there have been many exciting developments in products designed to assist wound healing, such as tissue engineering and the use of DS. Debridement and appropriate dressings are often used to accelerate healing. The plastic surgery approach to cover these wounds is bi‐engineering substitute, free flaps, and autologous skin grafting.3 Wound healing is the process of producing tissue, when dermal damage is repaired, that is functionally and cosmetically similar to uninjured skin through complex but coordinated interplay between multiple cell types, bioactive molecules, and extracellular matrix (ECM).1, 3, 4, 5 In adults, physiological skin repair is the development of fibrotic scar tissue, which occurs after nearly all dermal injuries.6, 7 In contrast, foetal wound repair is regenerative, characterised by the absence of fibrosis and restoration of normal skin architecture.8 Inherent differences in cellularity, cutaneous architecture, and bioactive molecular content between adult and foetal tissues are also crucial.9 Dermal skin substitutes represent another method to artificially alter ECM components providing ECM replacement in the form of porous 3‐dimensional dermal templates to stimulate wound healing.10 They represent a relatively new therapeutic option in chronic wound management, with a growing evidence base in diabetic and venous ulcers.11, 12 DS differences are likely to affect multiple wound‐healing processes, including fibroplasia, as ECM characteristics including porosity, elasticity, and biocompatibility strongly influence cellular migration, proliferation, and differentiation during healing.13, 14 Some animal‐derived acellular dermal matrix‐manufactured products have a removable semi‐permeable silicone layer on top acting as a temporary epidermis preventing moisture loss and infection. They can be grouped as acellular bi‐layer substitutes.14, 15 The aim of this study is the clinical and histological randomised comparison of 2 different collagen acellular bi‐layer substitutes Nevelia and Integra in patients with post‐traumatic injury. These DS have similar clinical indications of use but different structural characteristics, such as presence in Integra or absence in Nevelia of chondroitin‐6‐sulphate glycosaminoglycan (GAG).
2. MATERIALS AND METHODS
The authors treated 30 consecutive patients with partial and full‐thickness post‐traumatic skin defects randomised into 2 groups: 15 patients were treated with Nevelia and 15 patients with Integra double layer followed by autologous dermal epidermal graft (DEG) in both groups. All post‐traumatic wounds were localised on the inferior limbs (Table 1A,B). Inclusion criteria were inferior limb post‐traumatic wounds without tendons or bone exposures. After enrolment, participants were randomly assigned to 2 groups on a computer‐generated list. Only an investigator not involved in the evaluations or treatments knew this list. Allocation was revealed from time to time to surgeons, whereas it remained concealed for evaluators.
Table 1.
Patients and wound characteristics in (A) Nevelia‐implanted group and (B) Integra‐implanted group
| (A) Nevelia‐implanted group | |||||
|---|---|---|---|---|---|
| Patient | Age | Gender | Comorbidity | Wound localisation | Wound area (cm) |
| 1 | 21 | M | None | Left foot | 8 × 5 |
| 2 | 67 | F | Cardiopathy | Right foot | 7 × 4 |
| 3 | 70 | M | Cardiopathy | Left limb | 10 × 5 |
| 4 | 53 | F | None | Left thigh | 19 × 11 |
| 5 | 71 | M | Hypertension | Right limb | 17 × 8 |
| 6 | 79 | M | Cardiopathy | Right limb | 5 × 9 |
| 7 | 64 | F | Hypertension | Left limb | 12 × 6 |
| 8 | 67 | F | No | Left foot | 8 × 7 |
| 9 | 73 | M | Cardiopathy | Right limb | 12 × 9 |
| 10 | 58 | F | None | Left thigh | 15 × 7 |
| 11 | 65 | F | Hypertension | Right thigh | 8 × 3 |
| 12 | 67 | F | None | Foot left | 5 × 3 |
| 13 | 38 | M | None | Right limb | 6 × 4 |
| 14 | 18 | F | None | Left limb | 15 × 8 |
| 15 | 72 | M | Hypertension | Right thigh | 19 × 10 |
| (B) Integra‐implanted group | |||||
|---|---|---|---|---|---|
| Patient | Age | Gender | Comorbidity | Wound localisation | Wound area (cm) |
| 1 | 55 | M | None | Right leg | 6 × 9 |
| 2 | 72 | M | Cardiopathy | Left foot | 8 × 7 |
| 3 | 76 | F | None | Right leg | 13 × 6 |
| 4 | 35 | M | None | Left foot | 8 × 5 |
| 5 | 55 | F | Cardiopathy | Left tight | 20 × 10 |
| 6 | 68 | M | Hypertension | Left foot | 8 × 6 |
| 7 | 71 | F | Hypertension | Right leg | 12 × 6 |
| 8 | 55 | F | No | Right leg | 13 × 7 |
| 9 | 67 | M | Cardiopathy | Left thigh | 12 × 5 |
| 10 | 71 | F | None | Right leg | 8 × 9 |
| 11 | 55 | M | None | Left foot | 5 × 4 |
| 12 | 75 | F | None | Right foot | 2 × 5 |
| 13 | 78 | M | Cardiopathy | Right leg | 6 × 2 |
| 14 | 65 | M | None | Left foot | 4 × 2 |
| 15 | 58 | F | None | Right leg | 23 × 15 |
2.1. Dermal substitutes
Integra Dermal Regeneration Template (Integra Life Science, Plainsboro, New Jersey) is a collagen bi‐layer made of bovine type I collagen and shark chondroitin‐6‐sulphate GAG that is bonded to a silicone pseudo‐epidermis.16, 17 Integra is cross‐linked with glutaraldehyde.
Nevelia Bi‐Layer Matrix (Symathese Biomateriaux, Chaponost, France) consists of a porous resorbable matrix of about 2 mm thickness made of stabilised native collagen type I and a silicone sheet of about 200 mm in thickness mechanically reinforced with a polyester fabric.18, 19 For details, see Table 2A,B.
Table 2.
(A) Comparison of main structural characteristics between Nevelia and Integra bi‐layer dermal substitute. (B) Indication of use and standard surgical procedure of both bi‐layer dermal substitutes
| (A) | ||
|---|---|---|
| Nevelia | Integra | |
| Source | Bovine | Bovine |
| Extraction tissue | Calf hide skin | Tendon |
| Type of collagen | Native collagen type I | Collagen I, III |
| Other | components | None |
| Shark | chondroitin‐6‐sulphate | glycosaminoglycan |
| Cross‐linking | Glutaraldehyde | Glutaraldehyde |
| Removal silicon layer | 21 days | 21 days |
| Superior layer | Polyester‐reinforced silicon sheet | Silicon sheet |
| (B) | |
|---|---|
| Nevelia | Integra |
| Porous resorbable matrix of about 2 mm thickness made of stabilised native collagen type I and a silicone sheet of about 200 mm thickness mechanically reinforced with a polyester fabric. No glycosaminoglycan (GAG) added to improve cell attachment and proliferation | A silicone layer on top of a porous matrix comprising a chemically cross‐linked coprecipitate of bovine collagen and shark‐derived chondroitin‐6‐sulphate (GAG) |
| The extraction procedure and the freeze drying process allow the structuring of the collagen into a matrix with optimal hydrophilicity, pore structure, and pore size (20‐125 μm) | The Integra pore size of 20 to 125 μm allows influx of cells |
| It is cellularised by patient own cells in 2 to 3 weeks after implant. The silicone layer is removed after dermal regeneration (21 days) at the time of the thin split‐thickness skin graft | The dermal template usually becomes revascularised within 21 days after grafting; at this point, the silicon sheet can be removed, and a split‐thickness skin graft can be applied |
| Is used for dermal regeneration in skin loss, especially in burns surgery (third‐ and deep second‐degree burns and burns sequelae), chronic wounds surgery (including leg ulcers and diabetic foot), traumatology, skin tumours surgery, reconstructive plastic surgery, and in children. Is used in combination with a thin split‐thickness skin graft | Is used for burns treatment and for full‐thickness burns, chronic ulcers, full‐thickness non‐thermal skin wound management, plastic and reconstructive surgery, in skin tumours surgery, post‐traumatic injury. Is used in combination with a thin split‐thickness skin graft for soft tissues repair |
2.2. Clinical and surgical protocol
Patients’ clinical evaluations have been examined for comorbidities (see Table 1A,B). In our protocol, we performed wound examination, swab culture, and instrumental examination of lower limbs and photographs. We excluded instrumental laser‐Doppler measurement vascular pathology. A short‐term follow up was performed 1, 2, 3, and 4 weeks after DS implant, and a long‐term follow up was performed at 1 and 3 years. Three weeks after DS implant, authors removed the silicon layer and applied DEG in both patients groups.
Photographs were taken with a follow‐up time before and after DEG: 0, 1, 2, 3, and 4 weeks and 1 3 years. Moreover, a long‐term clinical follow up has been recorded 3 years after healing.
Wound bed preparation included debridement, bacterial balance, and management of exudates. Swab culture has been performed to evaluate any microbiological infections and to find appropriate antibiotic therapy if needed. The purpose of optimal preparation of the wound bed is to make it receptive to the DS implant.
2.3. Surgical steps: DS implant and DEG
All procedures were performed in complete asepsis with sedation or epidural and/or loco‐regional anaesthesia. The first surgical step included debridement of damaged tissues and DS application followed by split‐thickness DEG at 28 days after silicon layer removal. Classic skin grafting was performed with a dermatome using a thin split‐thickness depth, meshing all grafts (1:2 ratio), and it was fixed to the wounds by 3/0 nylon sutures or metallic staplers. A moulage compressive dressing with sterile gauze was used to cover the surgical wound and bandage. See Nevelia implants (Figure 1A‐E) for surgical procedure example.
Figure 1.
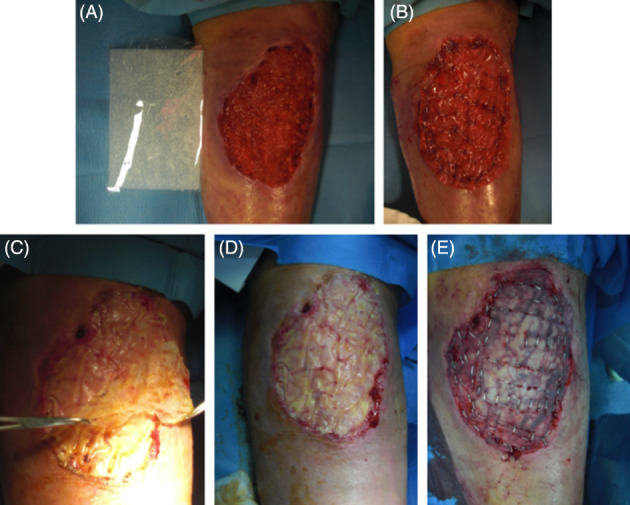
Dermal substitute (DS) standard protocol in surgical procedure: (A, B) preoperative and DSs implant view, (C, D) silicon layer removal (3 weeks after implant), (E) autologous skin graft implant
2.4. Clinical criteria evaluation during treatment and follow up
Clinical results were determined through the evaluation of healing time after DEG and by using the Manchester Scar Scale (MSS) and the Visual Analog Scale (VAS) for pain and patient self‐estimation. Moreover, we evaluated colour shift of the collagen layer of both DS, showing the graft taken, and collagen maturation, showing when the DS was ready for DEG implant. We performed controls 1, 2, 3, and 4 weeks (1‐28 days) after DS implant to evaluate colour shift of both the dermal matrices (photographs comparison). The following controls have been performed after silicon layer removal and DEG implant in both groups 0, 1, 2, 3, and 4 weeks; 1, 2, and 6 months; and 1 and 3 years after healing. Time to complete epithelisation after DEG implant was evaluated to test the effectiveness of the 2 DSs. The aesthetic appearance of the reepithelisation (healing time) was evaluated with the help of 1 plastic surgeon who as unaware of the procedure (A.A.) according to the MSS at healing time and at 3 years follow up. The MSS is based on 5 parameters for scar evaluation: colour, skin texture, contour, distortion, and texture, with a score of 1 (excellent) to 4 (poor) for all parameters except skin texture, which is represented by score 1 or 2 (matte or shiny). Post‐operative pain was evaluated with the VAS (range: 0‐10). Patient self‐estimation was performed in terms of functional and aesthetic outcome. It consisted of grading results from 1 to 4 (1, very disappointed; 2, disappointed; 3, satisfied; and 4, very satisfied).
2.5. Histological evaluation
Incisional punch biopsies (3 mm in diameter) of ulcers were obtained at baseline (T0) and after DS application at T1 (after 2 weeks) and T2 (after 3 weeks). Microscopic evaluation of routinely haematoxylin and eosin‐stained paraffin20, 21 was performed to verify the healing process, and images were acquired using a digital camera (E600 Eclipse, Nikon, Tokyo, Japan). Moreover, to evaluate the presence of elastic and collagen fibres in the dermis, Verhoeff‐Van Gieson staining was also performed.
2.6. Immunohistochemical study
For immunohistochemistry,22 4‐μm thick serial sections were deparaffinised, rehydrated, and—after antigen retrieval and non‐specific peroxidase blocking—incubated with mouse monoclonal anti‐human CD31 (DakoCytomation, Produktionsvej 42, 2600 Glostrup, Denmark) and α‐SMA (DakoCytomation),23 and images were acquired using a digital camera.
2.7. Statistical analysis
Tables reported mean ± SD of the MSS, whereas the figures demonstrate median and quartiles. The Mann‐Whitney U test was performed for the 5 items, and total score of the MSS was combined with the results of patients’ self‐estimation scale, such as the VAS score related to pain. For testing the differences in satisfaction between the 2 groups, the χ 2 test was used. The difference in the prevalence of reepithelialisation percentage (healing time) between the 2 groups was tested using the log‐rank test and reported using Kaplan Meier plot.
3. RESULTS
3.1. Clinical result: healing time, pain, patient self‐estimation
The results of the 2 groups were homogenous for age (Nevelia: 58.9 ± 18.7 years vs Integra: 63.7 ± 11.5 years, P = .624) and size of lesion (Nevelia: 80.9 ± 60.0 cm2 vs Integra: 78.0 ± 87.7 cm2, P = .595).After the DEG implant, complete healing was observed in both groups after 49 days. In particular, in the Nevelia group, complete healing was observed in 7 patients after 35 days, in 5 patients after 42, and in 3 patients after 49; In the Integra group, complete healing was observed in 6 patients after 35 days, in 4 patients after 42 days, and in 5 patients after 49 days (Figure 2). The difference in healing time between groups (P = .467, log‐rank test) was not statistically significant after 35, 42, and 49 days as demonstrated in the Kaplan Meier plot of prevalence of reepithelialisation in Figure 3. Pain‐related VAS scores were not significantly different between the 2 groups (Nevelia: 1.4 ± 1.6 vs Integra: 1.7 ± 1.4; P = .467, Mann‐Whitney U test) at complete healing (49 days). No differences were found in patient self‐estimation at complete healing (1.4 ± 0.6 vs 1.5 ± 0.5, respectively, P = .472, Mann‐Whitney U test).
Figure 2.
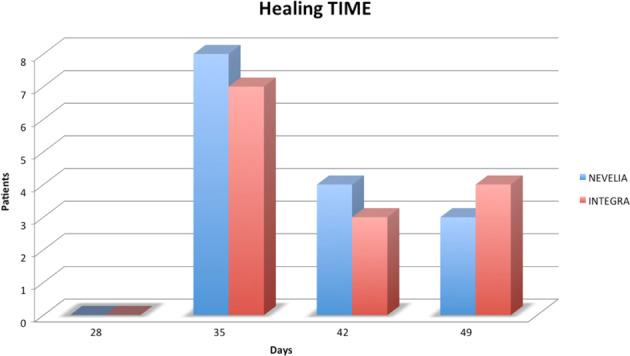
Dermal substitutes (DSs) healing time: number of patients, Nevelia and Integra groups, and days of healing
Figure 3.
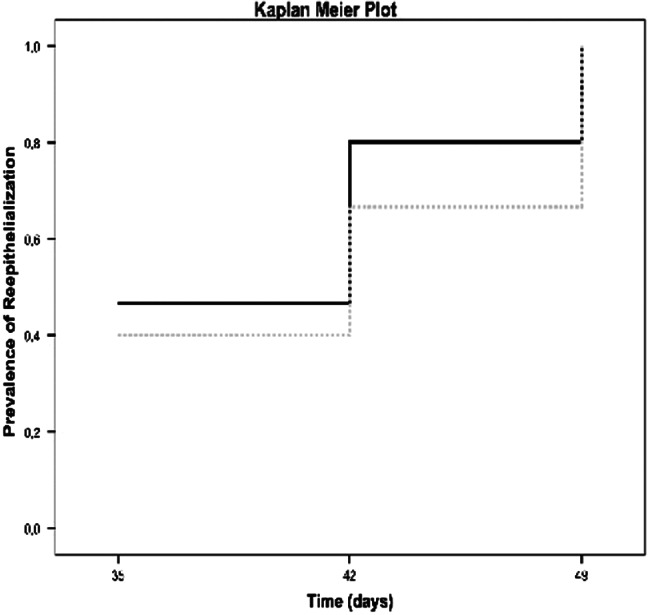
Kaplan Meier plot of prevalence of reepithelialisation. Black line represents Nevelia group, grey line Integra group. No statistically significant differences between the 2 groups at healing time, 49 days (P = .467, log‐rank test)
3.2. Clinical result: Manchester scar scale
At short‐term follow up, at healing time (49 days), differences in the MSS score were not statistically significant between groups, as shown in Table 3 and in the box and whiskers plot of the total scar score (Figure 4). On the contrary, differences in the MSS score were statistically significant at 3 years follow up in the Nevelia group, as shown in Table 4 and Figure 5. Main differences were in terms of contour, distortion, and texture between the 2 groups (Nevelia: P = .001).
Table 3.
Manchester Scar Scale (MSS) at short‐term follow up
| Group | Colour | Matte/Shiny | Contour | Distortion | Texture | Total scar score |
|---|---|---|---|---|---|---|
| Nevelia | 1.7 ± 0.7 | 1.2 ± 0.4 | 1.5 ± 0.7 | 1.7 ± 0.7 | 1.7 ± 0.9 | 7.9 ± 2.0 |
| Integra | 1.8 ± 0.8 | 1.5 ± 0.7 | 1.6 ± 0.6 | 1.7 ± 0.7 | 1.8 ± 0.7 | 8.4 ± 2.0 |
| Group comparison | U = 108, P = .840 | U = 87, P = .188 | U = 95, P = .425 | U = 106, P = .768 | U = 103, P = .467 | U = 99, P = .569 |
Mean ± SD of MSS scores and relevant comparisons between the 2 groups performed using Mann‐Whitney U test (relevant values of U and P were reported) at healing time.
Figure 4.
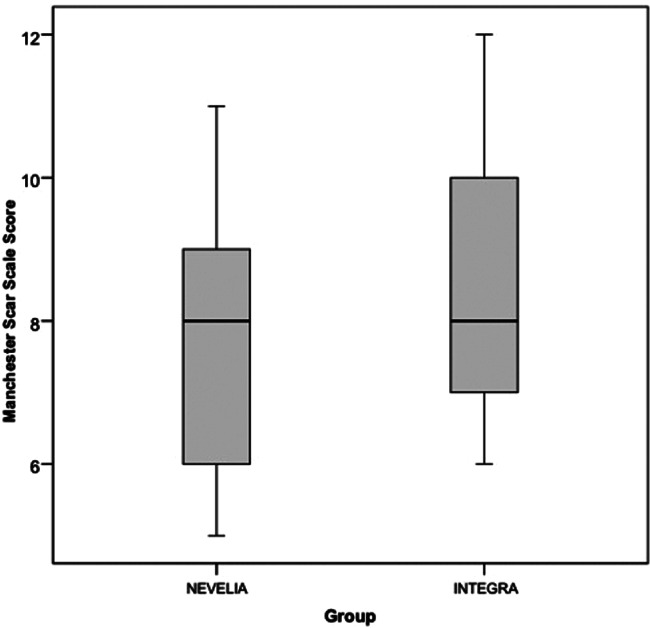
Manchester scar scale (MSS) at short‐term follow up. Box and whiskers plot of total scar score of MSS for Nevelia and Integra groups at short‐term follow up (box represents first and third quartiles, their middle line is the median, whiskers represent minimum and maximum value)
Table 4.
Manchester Scar Scale (MSS) at long‐term follow up
| Group | Colour | Matte/Shiny | Contour | Distortion | Texture | Total scar score |
|---|---|---|---|---|---|---|
| Nevelia | 1.40 ± 0.51 | 1.47 ± 0.52 | 1.27 ± 0.46 | 1.20 ± 0.41 | 1.20 ± 0.41 | 7.53 ± 1.19 |
| Integra | 1.53 ± 0.52 | 1.53 ± 0.64 | 1.73 ± 0.46 | 1.67 ± 0.72 | 1.60 ± 0.51 | 10.07 ± 1.53 |
| Group comparison | .472 | .868 | .012 | .048 | .028 | .001 |
Mean ± SD of MSS at 3‐year follow up. Last row reports the P‐value obtained when applying the Mann‐Whitney U test (in bold if statistically significant).
Figure 5.
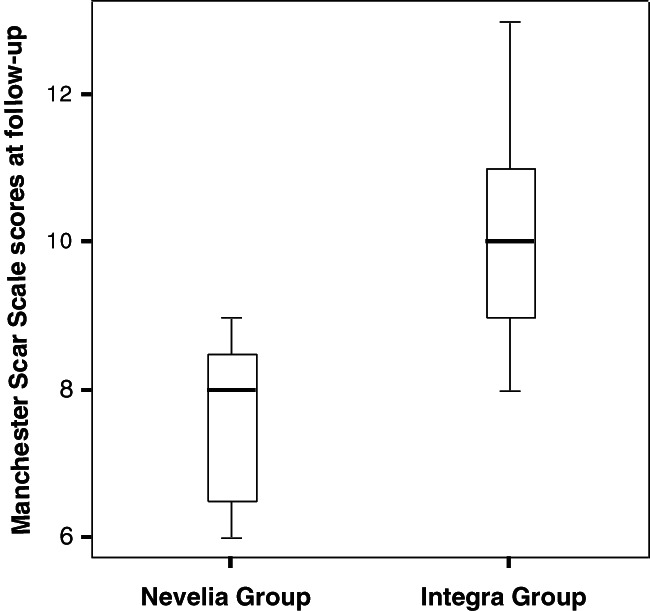
Manchester scar scale (MSS) at long‐term follow up. Box whiskers plot reporting median and quartiles for MSS scores at 3‐year follow up. Significant differences between the 2 groups (Nevelia: P = .001)
3.3. Iconographic clinical results
Both the dermal matrix and skin grafting were integrated successfully in most of treated sites at the end of treatment. The resultant biointegration enabled staged, definitive, and durable soft tissue coverage (Figures 6 and 7). Collagen colour shift evaluation is feasible because of the transparent silicon layer in both DS. Colour shift starts in the first week after implant, indicates the different collagen maturation stages, and ranges from red to vanilla/yellow. Figures 6 and 7 showed a slight difference in the colour shift of Nevelia vs Integra. After 28 days, a skin graft was performed. Figure 8 showed the differences of Nevelia vs Integra after 3 years follow up.
Figure 6.
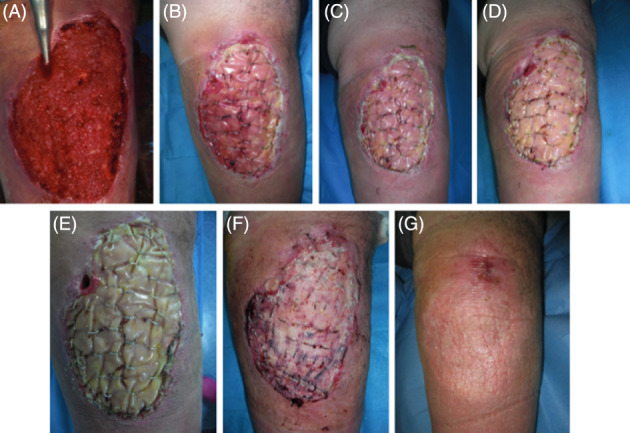
Nevelia short‐term follow up and healing: (A) preoperatory, (B‐E) collagen colour shift at 7‐14‐21‐28 days after Nevelia implant, (F) 7 days after DEG, and (G) healing at 42 days
Figure 7.
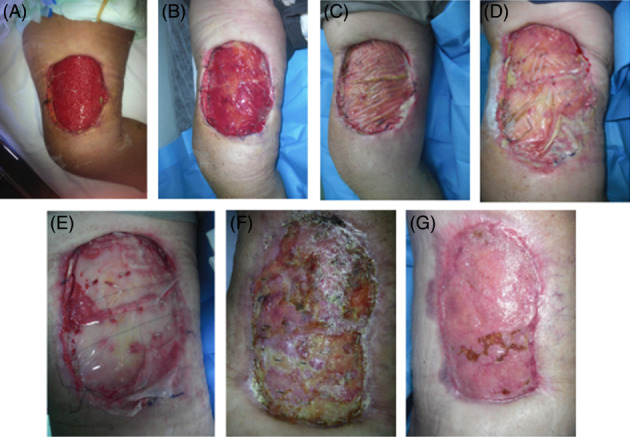
Integra short‐term follow up and healing: (A) preoperatory, (B‐E) collagen colour shift at 7‐14‐21‐28 days after Integra implant, (F) 7 days after DEG, and (g) healing at 42 days
Figure 8.
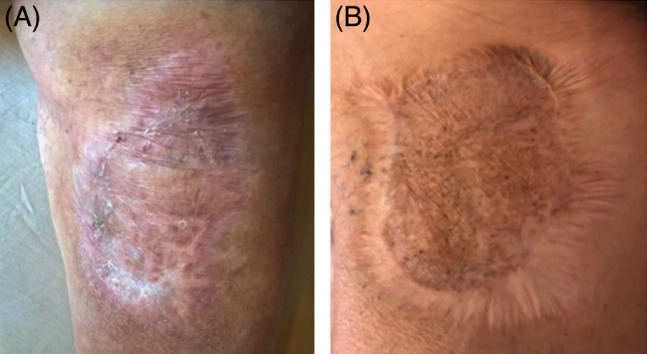
Long‐term follow up at 3 years: (a) Nevelia and (b) Integra
Representative microphotographs of haematoxylin and eosin staining are reported in Figure 9A. Evident wound healing was already documented in T1 (2 weeks after DS implant) compared with T0 with both DSs. T0 biopsies particularly showed cellular debris and dermal inflammatory infiltrate with no evidence of elastic fibres or collagen deposition, as shown by Verhoeff‐Van Gieson staining (Figure 9B). At T1, regenerated skin with reactive epidermal hyperplasia and dermal granulation tissue were observed, along with inflammatory infiltrate, collagen deposition, and newly formed vessels (Figure 10). At T2 (3 weeks after DS implant), reepithelialisation and dermal regeneration (Figure 9A,B) were obvious with both substitutes; however, Nevelia showed early regenerative properties in terms of epidermal proliferation and dermal renewal compared with Integra (see T1, Figure 9A,B), the presence of which was still evident in dermal tissue at T1 and T2 (Figures 9 and 10, arrowheads).
Figure 9.
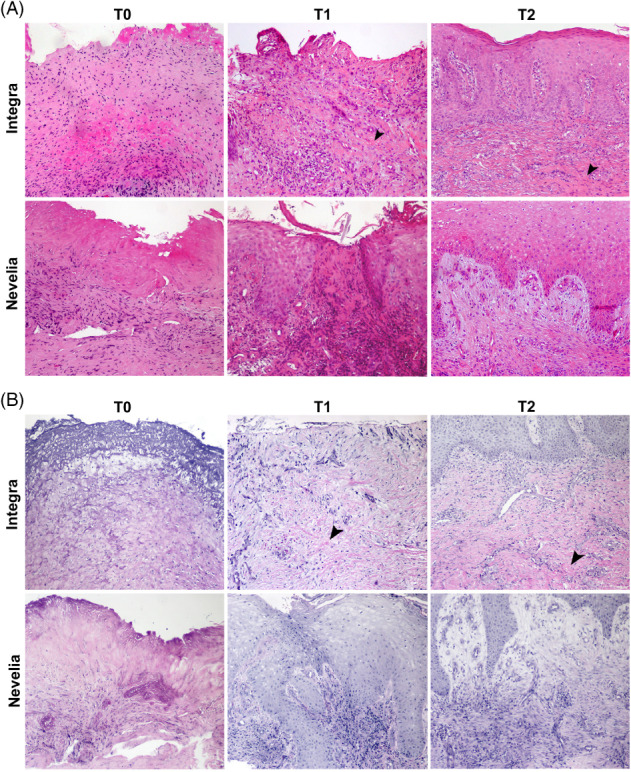
(A, B) Microscopic aspects of wound healing after Integra and Nevelia application. (A) Representative microscopic images of haematoxylin and eosin‐stained paraffin sections of skin biopsies at baseline (T0) showing typical wounds with cellular debris and dermal inflammatory infiltrate. After the DS application at T1 (after 2 weeks) and at T2 (after 3 weeks), skin punch biopsies showed wound healing with reepithelialisation and dermal granulation tissue with (B) collagen and elastic fibre deposition, as shown by Verhoeff‐Van Gieson staining. Presence of Integra is still evident in dermal tissue at T1 and T2 (arrowheads, original magnification: ×100)
Figure 10.
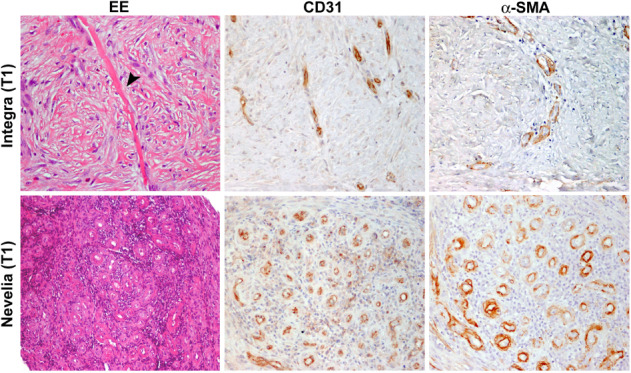
Neoangiogenesis during wound healing after Integra and Nevelia application. Skin punch biopsies at T1 showing wound healing with reepithelialisation and dermal granulation tissue with newly formed vessels, as shown by CD31 and α‐SMA immunohistochemistry. Presence of Integra is still evident in dermal tissue (arrowhead, original magnification: ×200)
4. DISCUSSION
Wound healing is a multi‐cellular process that involves coordinated efforts of several cell types, including keratinocytes, fibroblasts, endothelial cells, macrophages, and platelets. Migration, infiltration, proliferation, and differentiation of these cells will culminate in an inflammatory response, with the formation of new tissue and, ultimately, wound closure. Myers et al24 and Van Zuijlen and coworkers25 demonstrated that this complex process is executed and regulated by an equally complex signalling. It is clear that a biomaterial, which has the purpose of replacing wound tissue, will have to regenerate a tissue that is as close as possible to native tissue.
Engineering of skin substitutes implies deliberate design and fabrication according to specific functional objectives.26 So far, that design specification in skin has relied on the creation of both artificial dermal and epidermal components that, when combined, produce a replacement skin that can be grafted in place.27 To date, materials used as artificial ECM include those derived from naturally occurring materials and those manufactured synthetically. Examples of natural materials include polypeptides, hydroxyapatites, hyaluronic, GAG, fibronectin, collagen, chitosan, and alginates.7Scaffolds often have unique physical characteristics due to differences in manufacturing techniques such as decellularisation, sterilisation, freeze drying, and cross‐linking protocols.2, 4, 28 To resist forces in vivo, such as a wound contraction, scaffold materials are, for example, often frozen dried and/or chemically cross‐linked to enhance strength. However, it has been demonstrated that chemical cross‐linking can alter clinical results.29
Most widely used wound regeneration matrices are preferably resorbable, and their products resulting from reabsorption must be no immunogenic and toxic.7 This process includes 2 fundamental points of skin bio‐suppression, mechanical properties7 and reabsorption, as the 3‐dimensional pores architecture.30 These characteristics are important because they determine behaviour in contact with tissue and cells.
Biological scaffolds, commonly used for regeneration or replacement of damaged tissues, are primarily composed of ECM constituent molecules. Preparation of an ECM scaffold (allogenic or xenogeneic) involves decellularisation of tissue or organ from which ECM is harvested.31, 32 Decellularisation processes are proprietary and differ significantly between products.32 The dermal collagen layer provides a matrix for migration and growth of fibroblasts and other cells involved in wound healing, and the silicon layer acts as a barrier preventing vapour loss and bacterial contamination. Integra is an engineered substrate used in this study, available as a bi‐layer membrane system. The dermal replacement layer is made up of a glutaraldehyde cross‐linked bovine type I collagen with GAG and a superior layer of polysiloxane polymer (silicone) epidermis.33 The Integra dermal component becomes populated with host cells, including fibroblasts, which contribute towards neodermis formation, while the material’s scaffold degrades and the pseudo‐epidermal component protects wounds from vapour loss and bacterial contamination. When the Integra vascularisation and neodermis formation are complete, usually within 15 to 20 days, the silicone layer is peeled off, and the wound can be closed permanently with a DEG. This material was successfully clinically tested in managing burn wounds in 1981 for full‐thickness burns treatment,34, 35, 36 chronic ulcer treatment,37 and full‐thickness non‐thermal skin wound management.38 Advantages of the product include its long shelf life, simple handling, low risks of immunogenic response and disease transmission, and good cosmetic outcomes with reduced rates of contraction and scarring.17, 39 It cannot be used on infected wounds; it requires a relatively long time of 10 to 14 days for vascularisation and also requires a second surgical procedure to achieve permanent wound closure with a DS.
Nevelia Bi‐Layer Matrix consists of a porous resorbable matrix of about 2 mm thickness made of stabilised native collagen type one from calf hides and a silicone sheet of about 200 mm thickness mechanically reinforced with a polyester fabric and does not contain any chondroitin‐6‐sulphate GAG.18 Collagen is purified from calf hides from animal younger than 9 months coming from safe countries and does not contain any chondroitin‐6‐sulphate GAG. The extraction procedure and frozen drying process allow the structure of collagen into a matrix with optimal hydrophilic, pore structure and pore size. Collagen is then cross‐linked with a very low percentage of glutaraldehyde to adjust the collagen degradation rate, while the dermis is regenerated and therefore optimises the neodermis quality. Collagen porous layer promotes and guides regeneration, and a reinforced silicone layer acts as a pseudo‐epidermis. This matrix serves as a support for cell infiltration, thus contributing to the natural tissue regeneration process. It is reabsorbed, becoming a vascularised tissue that is histologically very close to normal dermis, 2 to 3 weeks after it is implanted.18, 40 The silicone layer is removed after dermal regeneration (21 days), at the time of thin split‐thickness skin grafting. Main features are specific native collagen with a large fibrous proportion to retain cell adhesion signals and a mechanical structure to support regeneration, cross‐linking rate for a balanced absorption/regeneration process, and no GAG (chondroitin‐6‐sulphate GAG) added to avoid hydrophilicity in strong exudates lesion.40 Nevelia is used for dermal regeneration in skin loss, especially in burn surgery (third‐ and deep‐second degree burns and burns sequelae) and chronic wounds surgery (including leg ulcers and diabetic foot) traumatology, skin tumour surgery, and reconstructive plastic surgery. Nevelia is used in combination with a thin split‐thickness skin graft to recreate skin close to normal in terms of function and appearance (Table 2A,B).
The Nevelia in vitro study vs the Integra study (data not showed) showed that GAG inhibit fibroblasts and keratinocytes collagen repopulation. Moreover, GAG is highly hydrophilic and may retain lesion inflammatory exudates. Collagen is then cross‐linked with a very low percentage of glutaraldehyde to adjust collagen degradation rate, while the dermis is regenerated and therefore optimises neodermis quality. Nevelia collagen has a large fibrous proportion to retain cell adhesion signals and a mechanical structure to support regeneration, cross‐linking rate for a balanced absorption/regeneration process, and no GAG (chondroitin‐6‐sulphate GAG) added as suggested from in vitro data (not showed). This matrix serves as a support for cell colonisation, thus contributing to natural tissue regeneration process. When Nevelia is reabsorbed, it is replaced by a vascularised tissue that is histologically very close to normal dermis, 2 to 3 weeks after implants.18, 40, 41 The aim of our study was to make comparisons between skin autologous graft application combined and 2 types of dermal collagen matrix, Nevelia and Integra, that had similar indications of use but different structural characteristics to obtain information on the restoration and regeneration of post‐traumatic wounds. In each patient, the DS used showed positive effects of accelerating the improvement of quality and functionality of skin reconstruction. Clinical results at short‐term follow up, particularly at healing time, which was complete at 49 days in both DS, did not show any statistically significant differences in terms of MSS (Table 3, Figure 4), in patient self‐estimation, and pain scores at closure of the wound. Similarly, we did not find any difference at 1‐year follow up between the 2 groups, with clinical satisfactory and stable results (data not showed). On the contrary, at 3‐year follow up Nevelia showed a better MSS score (Table 4, Figure 5) in terms of contour, distortion, and texture between the 2 groups. Of note, the healed tissue was not exposed to solar UV rays in both groups. This result could reflect different tissue interactions of 2 DS in vivo. Accordingly, as shown by microscopic analysis, an evident healing of wounds was already reported at T1 (2 weeks after DS implant, Figure 9) with both DSs. At T0, skin biopsies showed cellular debris and dermal inflammatory infiltration with no evidence of elastic fibres or collagen deposition. At T1, regenerated skin with reactive epidermal hyperplasia and dermal granulation tissue were observed, along with inflammatory infiltration, collagen and elastic fibre deposition, and newly formed vessels. At T2 (3 weeks after implant, Figure 10), reepithelialisation and dermal regeneration were reported in both substitutes; however, Nevelia showed early regenerative properties in terms of epidermal proliferation and dermal renewal compared with Integra, the presence of which was still evident in dermal tissue at T1 and T2. This histological result is coherent with the different colour shift of collagen layer, which is feasible because of transparent silicon layer of both DS, observed between Nevelia and Integra during the first 2 weeks after DS implants. The biomaterial colour shift basically indicates recellularisation and creation of new vessels. This is a crucial step to obtaining a good performance of the 2‐step technique, with subsequent skin grafting. Colour shift starts in the first week after implant, indicates different collagen maturation stages, and ranges from red to vanilla/yellow. Even at this stage, it was possible to note that Nevelia changed colour about 7 days before Integra. This could suggest that Nevelia probably become vascularised and recellularised before Integra (Pictures 2 and 3) as shown and confirmed by CD31 and α‐SMA immunohistochemistry (Figure 10). Moreover, Integra was still evident in dermal tissue at T1 and T2, whereas Nevelia was completely reabsorbed (Figure 9A,B). These observed differences could be explained by the different decellularisation process of 2 DSs or by a different induced macrophage polarisation. Macrophage phenotype can be characterised as pro‐inflammatory (M1) or immunomodulatory and tissue remodelling (M2). Briefly, scaffold materials composed of ECM have been shown to promote a switch from a predominantly macrophage M1 cell population immediately following implantation to a population enriched in macrophage M2 cells by 7 to 14 days post‐implantation.41, 42, 43 The phenotypical profile of the cells that respond to these scaffold materials at early time points has been shown to be a statistical predictor of the downstream outcome associated with their implantation.42 The mechanisms by which ECM‐based scaffold materials promote the M1 to M2 transition remain unknown. However, modification of such scaffold materials with chemical cross‐linking agents that delay or prevent macrophage‐mediated degradation inhibits the formation of the beneficial M2 response and results in downstream scar tissue formation.43 Accordingly, a recent study demonstrated that different collagen matrixes, processed and manufactured using different methods, elicited differing patterns and timeframes of macrophage infiltration.40 Agrawal et al40 demonstrated in an animal model that the host response to Integra on day 7 was predominantly lymphocytes and fibroblasts with some RBCs, neutrophils, and macrophages. By day 14, the collagen strands were fairly uniform but finer than human collagen. By day 21, the macrophage population increased, and many multi‐nucleate giant cells were seen without evidence of granuloma formation. By day 42, the giant cell response grew even further, and there was evidence of collagen destruction at the host‐graft interface, which was visualised as scattered broken fibres in the process of remodelling,. Moreover, Integra showed a mixed M1/M2 population of macrophages at all time points with no significant difference among these cells. The trend for M1:M2 ratio in Integra was skewed towards M2 on day 7, towards M1 on days 14 to 21, and again towards M2 on day 42. Although direct cause‐and‐effect relationships remain to be explored, there appears to be a strong correlation between macrophage subtypes and remodelling responses. Further work to elucidate these findings over longer periods of time and within the context of clinical use and outcomes could lead to rational choices in the use of these materials for differing clinical applications. The use of DS has several advantages: protection of injury site, stimulation of wound bed vascularisation, pain reduction, better scar outcome, and faster healing. An optimal DS should be impermeable to exogenous bacteria, resistant to linear and shear stresses, and have minimal storage requirements and a long shelf life. Importantly, it should incorporate into the patient with minimal scarring and also facilitate angiogenesis.44, 45 Nevelia showed increased angiogenesis, as shown by CD31 and α‐SMA immunohistochemistry, vs Integra DS (Figure 10). Recently, a link between robust angiogenesis and a fibrotic outcome in wounds had been established45: this result can partially explain the better clinical outcome at 3 years in favour of the Nevelia group. Moreover, an optimal DS should evoke a minimal inflammatory response in patients and also have no local or systemic toxicity.46 Truong et al47 showed that Integra elicited the greatest foreign body response to full‐thickness skin wounds in nude mice. In this study, Integra showed numerous fibrinogen biosynthesis (FBG) cells in the healed wound and the least propensity for a fibrous dermal restructuring by day 28; squamous pearls filled the crevices of the Integra “scaffold,” and the hyalinised interstitium was extensively infiltrated by FBG cells. In the healed wound, the Integra matrix was enveloped by fibrous tissue with “islands” of Integra isolated by the in‐growth of fibres into areas that had been cleared by FBG cells. This was a morphology unlike any seen with any of the other DSs tested in the same study. Horizontal fibres, both superficial and deep, arrayed parallel to the tissue interfaces surrounded and infiltrated the other DSs in the healed wound areas. It has been previously reported that 14.4% of human patients who received Integra developed FBG cells and eosinophils.48 This could probably be a reaction to the constituents (ie, denatured bovine collagen and shark chondroitin sulphate) of Integra, which were perceived as foreign bodies. It is possible that this foreign body reaction to Integra components in some patients may impact the generation and vascularisation of the new dermis, especially at later post‐surgical intervals. Some of the other features of a good skin substitute are that it should adhere to the wound surface in a rapid and sustained manner, have appropriate physical and mechanical properties, and undergo controlled degradation.9 In common use, DS is used for the leakage of substances with different aetiologies: diabetes, vascular, burns, or in reconstructive plastic surgery after skin tumour excision or giant nevus. A DS should be easily applicable, flexible, and conformable to plant site as well as having an affordable cost. The choice to compare the use of Integra and Nevelia in post‐traumatic wounds is based on the fact that this type of lesion, tissue regeneration, and healing mechanisms are very similar to physiology. In this way, we have highlighted differences in cellular interaction and behaviour of 2 DSs when implanted both from macroscopic, microscopic, and clinical points of view. For this reason, in our study, we excluded patient with comorbidities such as diabetes, vasculopathy, or autoimmune diseases that could interfere with the physiological regeneration of wounds. In each patient, both the DS were used, showing positive effects of accelerating the improvement of quality and functionality of skin reconstruction. Several questions were raised in this study that require continued comparison of different DSs and their short‐ and long‐term effects on wounds with different aetiologies. It is difficult to explain the differences in clinical outcomes observed only at 3 years of follow up, but it is important to remember that epithelialised wounds remodel continually for more than a year.47, 49 This randomised study demonstrated differences of Integra and Nevelia both in long‐term clinical results and in histological results in post‐traumatic injury, suggesting that biomaterials influence wound microenvironment and tissue regeneration for a long time after implants. Further studies will be necessary to explain that different mechanism of actions could affect clinical outcomes.
5. CONCLUSIONS
Nevelia and Integra can be used for same clinical indications. Their application to post‐traumatic injuries has rendered positive results both in terms of surgical technique and of healing times. Regenerated skin appearance and patient compliance were excellent. Basically, in short time to complete healing, differences between the 2 DSs were found only in histological examinations. In fact, cell interaction was different with regards to both revascularisation times and recellularisation of DSs. From this, it appears that Nevelia is a DS that allows faster neoangiogenesis and tissue regeneration with neoformed tissue architecture closer to skin physiology. At long‐term follow up, Nevelia demonstrated a better clinical outcome, measured as the MSS score. The treatment in 2 steps, DS and SG, guarantees a temporary barrier with multiple functions: haemostatic, reduction of contracture wound, infection, maintenance of skin elasticity and dermal architecture, and better scar appearance. Finally, treatment with DSs and skin grafts results in cost reduction and hospitalisation, improvement of life quality, and cost reduction due to fewer numbers of medications. Nevelia is a new‐generation DS and could be considered an innovative tool in DS technique and in regenerative surgery, a medical‐surgical discipline that has been evolving in recent years and that, along with cellular therapy and other innovative biotechnology, is the future of tissue regeneration.
De Angelis B, Orlandi F, Fernandes Lopes Morais D’Autilio M, et al. Long‐term follow‐up comparison of two different bi‐layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int Wound J. 2018;15:695–706. 10.1111/iwj.12912
REFERENCES
- 1. Clark RA, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018‐1029. [DOI] [PubMed] [Google Scholar]
- 2. Debels H, Hamdi M, Abberton K, Morrison W. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open. 2015;3:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cervelli V, Brinci L, Spallone D, et al. The use of MatriDerm® and skin grafting in post‐traumatic wounds. Int Wound J. 2011;8:400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melman L, Jenkins ED, Hamilton NA, et al. Early biocompatibility of crosslinked and non‐crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia. 2011;15:157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403. [DOI] [PubMed] [Google Scholar]
- 6. Bayat A, McGrouther D, Ferguson M. Skin scarring. BMJ. 2003;326:88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacNeil S. Progress and opportunities for tissue‐engineered skin. Nature. 2007;445:874‐880. [DOI] [PubMed] [Google Scholar]
- 10. Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41:837‐843. [DOI] [PubMed] [Google Scholar]
- 11. Winters CL, Brigido SA, Liden BA, Simmons M, Hartman JF, Wright ML. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care. 2008;21:375‐381. [DOI] [PubMed] [Google Scholar]
- 12. Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen. 2013;21:194‐210. [DOI] [PubMed] [Google Scholar]
- 13. Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment—implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677‐689. [DOI] [PubMed] [Google Scholar]
- 15. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20:493‐508. [DOI] [PubMed] [Google Scholar]
- 16. Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res A. 1980;14:65. [DOI] [PubMed] [Google Scholar]
- 17. Anthony ET, Syed M, Myers S, Moir G, Navsaria H. The development of novel dermal matrices for cutaneous wound repair. Drug Discov Today: Therapeut Strat. 2006;3:81. [Google Scholar]
- 18. Alet JM, Weigert R, Castede JC, Casoli V. Management of traumatic soft tissue defects with dermal regeneration template: A prospective study. Injury. 2014;45:1042‐1048. [DOI] [PubMed] [Google Scholar]
- 19. Leclère FM, Casoli V. Use of bioartificial dermal regeneration template for skin restoration in combat casualty injuries. Regen Med. 2016;11:359‐360. [DOI] [PubMed] [Google Scholar]
- 20. Kotlyar AA, Vered Z, Goldberg I, et al. Insulin‐like growth factor I and II preserve myocardial structure in postinfarct swine. Heart. 2001;86:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cervelli V, Gentile P, De Angelis B, et al. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post‐traumatic lower extremity ulcers. Stem Cell Res. 2011;6:103. [DOI] [PubMed] [Google Scholar]
- 22. Matera MG, Calzetta L, Passeri D, et al. Epithelium integrity is crucial for the relaxant activity of brain natriuretic peptide in human isolated bronchi. Br J Pharmacol. 2011;163:1740‐1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scioli MG, Stasi MA, Passeri D, et al. Propionyl‐l‐carnitine is efficacious in ulcerative colitis through its action on the immune function and microvasculature. Clin Transl Gastroenterol. 2014;20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Myers SR, Navsaria HA, Brain AN, Purkis PE, Leigh IM. Epidermal differentiation and dermal changes in healing following treatment of surgical wounds with sheets of cultured allogeneic keratinocytes. J Clin Pathol. 1995;48:1087‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hakvoort T, Altun V, Van Zuijlen PP, De Boer WI, Van Schadewij WA, van der Kwast T. Transforming growth factor‐beta (1), ‐beta (2), ‐beta (3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burn scars. Eur Cytokine Netw. 2000;11:233. [PubMed] [Google Scholar]
- 26. Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg. 2002;183:445. [DOI] [PubMed] [Google Scholar]
- 27. Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23:403‐412. [DOI] [PubMed] [Google Scholar]
- 28. Parenteau‐Bareil R, Gauvin R, Berthod F. Collagen‐based biomaterials for tissue engineering applications. Materials. 2010;3:1863‐1887. [Google Scholar]
- 29. Delgado LM, Bayon Y, Pandit A, Zeugolis DI. To cross‐link or not to cross‐link? Cross‐linking associated foreign body response of collagen‐based devices. Tissue Eng Part B Rev. 2015;21:298‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostrowska B, Di Luca A, Moroni L, Swieszkowski W. Influence of internal pore architecture on biological and mechanical properties of three‐dimensional fiber deposited scaffolds for bone regeneration. J Biomed Mater Res A. 2016;104:991‐1001. [DOI] [PubMed] [Google Scholar]
- 31. Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233‐3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shevchenko RV, James SL, James SE. A review of tissue‐engineered skin bioconstructs available for skin reconstruction. J Roy Soc Interf. 2009;43:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heitland A, Piatkowski A, Noah EM, Pallua N. Update on the use of collagen/glycosaminoglycate skin substitute—six years of experiences with artificial skin in 15 German burn centers. Burns. 2004;30:471‐475. [DOI] [PubMed] [Google Scholar]
- 36. Heimbach DM, Warden GD, Luterman A, et al. Multicenter post‐approval clinical trial of Integra® dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24:42. [DOI] [PubMed] [Google Scholar]
- 37. Silverstein G. Dermal regeneration template in the surgical management of diabetic foot ulcers: a series of five cases. J Foot Ankle Surg. 2006;45:28‐33. [DOI] [PubMed] [Google Scholar]
- 38. Violas P, Abid A, Darodes P, Galinier P, de Gauzy JS, Cahuzac JP. Integra artificial skin in the management of severe tissue defects, including bone exposure, in injured children. J Pediatr Orthop. 2005;14:381‐384. [DOI] [PubMed] [Google Scholar]
- 39. Kim PJ, Dybowski KS, Steinberg JS. Feature: a closer look at bioengineered alternative tissues. Podiatry Today. 2006;19:38. [Google Scholar]
- 40. Agrawal H, Tholpady SS, Capito AE, Drake DB, Katz AJ. Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices. Open J Regen Med. 2012;1:51‐59. [Google Scholar]
- 41. Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart‐Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835‐1842. [DOI] [PubMed] [Google Scholar]
- 42. Brown BN, Londono R, Tottey S, et al. Macro‐phage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown BN, Valentin JE, Stewart‐Akers AM, McCabe GP, Badylak SF. Macro‐phage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rahimnejad M, Derakhshanfar S, Zhong W. Biomaterials and tissue engineering for scar management in wound care. Burns Trauma. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Pietro LA. Angiogenesis and scar formation in healing wounds. Curr Opin Rheumatol. 2013;25:87. [DOI] [PubMed] [Google Scholar]
- 46. Varkey M, Ding J, Tredget EE. Advances in skin substitutes—potential of tissue engineered skin for facilitating anti‐fibrotic healing. J Funct Biomater. 2015;6:547‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Truong ATN, Kowal‐Vern A, Latenser BA, Wiley DE, Walter RJ. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J Burns Wounds. 2005;4:e4. [PMC free article] [PubMed] [Google Scholar]
- 48. Stern R, McPherson M, Longaker MT. Histologic study of artificial skin used in the treatment of full‐thickness thermal injury. J Burn Care Rehabil. 1990;11:7‐13. [DOI] [PubMed] [Google Scholar]
- 49. Guo SA, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]


