Abstract
Groin wound infections pose a major problem in vascular surgery. Closed‐incision negative pressure therapy (ciNPT) was especially designed for the management of incisions at risk of surgical site infections. The aim of this study was to investigate whether ciNPT is able to reduce the incidence of wound infections after vascular surgery.
Data on 132 consecutive patients, scheduled for vascular surgery with a longitudinal femoral cutdown, were collected prospectively. All patients were randomised either to the ciNPT group (n = 64) or the control group (n = 68) with conventional dressing. In the ciNPT group, the foam dressing was applied intraoperatively and removed after 5 days. The control group received an absorbent dressing. All wounds were evaluated after 5 and 42 days. Infections were graded according the Szilagyi classification (I–III°).
There were no significant differences between both groups considering patient characteristics. Indications for surgery were peripheral arterial disease in 95% (125/132) and aneurysm in 5% (7/132). The overall infection rates were 14% (9/64) in the ciNPT group and 28% (19/68) in the control group (P = 0·055). Early infections were observed in 6% (4/64) of the ciNPT group and 15% (10/68) of the control group (P = 0·125). ciNPT did not reduce infection rates associated with different risk factors for infection.
While the experiences with the ciNPT device were encouraging, the study fails to provide evidence of the efficacy of the device to reduce groin wound infections after vascular surgery. It illustrates far more that larger multicentre studies are required and appear promising to provide further evidence for the use of ciNPT.
Keywords: Closed‐incision negative pressure therapy, Groin wound infection, Peripheral arterial disease, Surgical site infection, Wound complications
Introduction
Surgical site infections (SSI) in the groin pose a major problem in vascular surgery with an incidence after vascular procedures of 6–30% 1, 2. Superficial infections limited to the skin and subcutis can usually be treated by debridement with or without open negative pressure wound therapy (NPWT). However, deep peri‐vascular wound infections pose a life‐ and limb‐threatening complication, especially when the prosthetic graft used for arterial reconstruction is exposed. If the anastomosis is involved, as frequently is the case in groin wounds, these patients require explantation of the synthetic graft and revascularisation with autologous material or an extra‐anatomic bypass, all procedures associated with a high morbidity.
NPWT is generally accepted as a valuable treatment option for highly contaminated or infected open wounds, assisting healing by secondary intention. In 2006, Stannard et al. introduced closed‐incision NPWT (ciNPT) for use on closed surgical incisions to prevent wound dehiscence and to aid healing by primary intention 3. Meanwhile, several case series and retrospective studies have demonstrated that ciNPT is effective in reducing the postoperative infection rate in problematic wounds, especially in cardiac 4, 5, orthopaedic 6, gynaecological 7, 8, 9 and general surgical wounds 10, 11. Grauhan et al. reported that obese patients with median sternotomy for cardiac surgery treated with ciNPT had a significantly lower SSI rate compared to patients treated with conventional wound dressing (4% versus 16%; P = 0·0266) 5. Comparing ciNPT and conventional dressing after femoral cutdown for vascular surgery, Matatov et al. observed a significant reduction of groin wound infections in the ciNPT group compared to historical controls (6% versus 30%; P = 0·0011) 2. However, prospective studies providing evidence for the effectiveness of ciNPT in vascular surgery are still missing.
Therefore, the aim of this study was to prospectively evaluate whether ciNPT is able to reduce the risk of groin wound infections in vascular surgery patients.
Methods
The study was conducted in a single academic vascular surgical centre in Germany. Appropriate approval from the Ethic Committee of the University Ulm, Germany, was secured, and all patients provided written informed consent.
Patient selection and randomisation
All consecutive patients scheduled for vascular surgery with a femoral cutdown were eligible for the study. Pertinent clinical and demographic data were collected prospectively. Eligibility criteria included age > 18 years and the need for an open, non‐emergency surgical procedure for peripheral arterial disease (PAD) or aneurysm involving the femoral artery using a longitudinal femoral cutdown in the groin. Exclusion criteria were dementia (not capable of informed consent) and declining to participate.
Patients were randomised in two groups, a ciNPT group and a non‐ciNPT group (control group). Random assignment of the patients to the two treatment groups was performed according to an external randomisation sequence. Sealed randomisation envelopes were provided by an external institution (Institute of Epidemiology and Medical Biometry, University Ulm, Germany). On eligibility confirmation, the sequential randomisation envelope was opened, and the assignment was allocated.
Procedural conduct
All patients routinely received perioperative antibiotics (Cefuroxim 1·5 g) as a single shot before operation and again after 4 hours of surgery, if appropriate. After arterial reconstruction, a subfascial suction drainage was placed, and primary wound closure was performed with running sutures for the fascia and the subcutaneous tissue (Vicryl®, Ethicon Inc., Somerville, NJ) and single stitch sutures of the skin (Ethilon®, Ethicon Inc.) in both groups. In the ciNPT group, a negative pressure wound dressing (Prevena™ System, KCI Medical, San Antonio, TX) was applied on the closed skin intraoperatively. The system is comprised of a therapy unit containing a pump with a 45‐ml canister delivering a continuous negative pressure of 125 mmHg and a self‐adhesive dressing with a foam bolster that manifolds the negative pressure to the incision area. A special polyester interface layer protects the skin from direct contact with the foam bolster, while at the same time allowing delivery of negative pressure and fluid removal. In the control group, all wounds were covered with an absorbent adhesive dressing (ABE®, Meditrade GmbH, Kiefersfelden, Germany) under the same conditions as in the ciNPT group. The drainage was removed at the second or third day at the discretion of the surgeon. The ciNPT dressing was removed after 5 days. The absorbent dressing was left in place until gross soiling impaired wound hygiene, and there was a clinical need to remove the dressing, or until postoperative day 2, whichever came first. On the fifth postoperative day, all wounds were documented by photography and classified according to the Szilagyi classification 12. Grade I infections only involved the skin (dermal infection), grade II extended to the subcutaneous tissue without reaching the vessels, and grade III finally involved the artery or bypass. Six weeks after surgery, all wounds were evaluated once more in the same manner.
Statistical analysis
The primary end point of the study was the occurrence of SSIs in both groups. As a secondary end point, the incidence of SSI was analysed according to different risk factors for the development of wound infections: age > 70 years, body mass index (BMI) ≥ 30, diabetes mellitus, smoking, PAD stage IV, duration of the operation ≥3 hours and the use of prosthetic graft.
Data were collected and analysed using SPSS (Version 22·0) statistical software (SPSS, Munich, Germany). Data are given as median [inter quartile range (IQR)]. The Mann–Whitney U‐test was used for comparison of groups. Logistic regression was used for multivariable analysis of risk factors for SSI. A P‐value <0·05 was considered statistically significant.
Results
Enrolment and allocation
Figure 1 shows randomisation and allocation. Among the 150 patients screened, 141 consenting patients were eligible and were randomised between January 2012 and October 2014. In total, 9 patients (6%) did not complete follow up due to urgent re‐operation or because of death during follow up, leaving 132 patients to be analysed.
Figure 1.
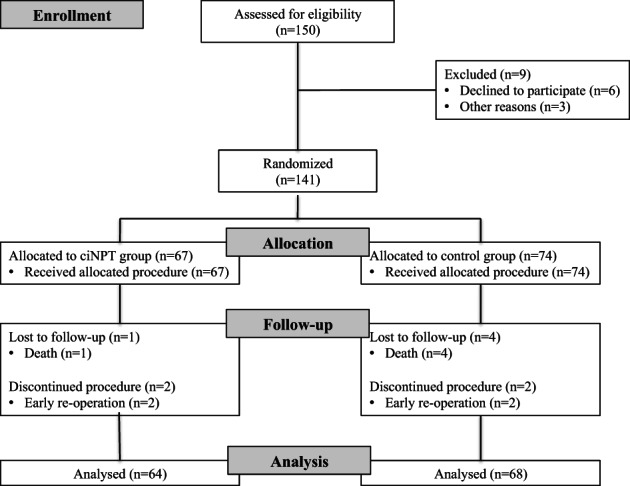
Patient flow diagram.
Patient characteristics
Demographic and clinical data are given in Table 1. Patient characteristics, comorbidities and procedures did not differ between both groups. Indications for surgery were primarily related to PAD (95%), with intermittent claudication being the most common diagnosis. In 37 patients (28%), prosthetic grafts were used for arterial reconstruction.
Table 1.
Patient demographics, risk factors and comorbidities
| ciNPT group | Control group | P | |
|---|---|---|---|
| Patient (n) | 64 (48%) | 68 (52%) | |
| Male | 48 (75%) | 57 (84%) | 0·211 |
| Age (years) | 72 (64–75) | 70 (60–78) | 0·567 |
| Diabetes | 19 (30%) | 20 (29%) | 0·972 |
| Smoker | 48 (75%) | 54 (79%) | 0·580 |
| BMI | 27 (25–29) | 27 (24–30) | 0·893 |
| ESRD | 1 (2%) | 1 (2%) | 0·935 |
| Operation time (>3 hours) | 27 (42%) | 30 (44%) | 0·938 |
| Diagnosis | |||
| PAD II | 43 (67%) | 49 (72%) | 0·544 |
| PAD III | 7 (11%) | 3 (4%) | 0·158 |
| PAD IV | 11 (17%) | 12 (18%) | 0·945 |
| Aneurysm | 3 (5%) | 4 (6%) | 0·760 |
| Procedure | |||
| Profundaplasty | 18 (28%) | 17 (25%) | 0·548 |
| Profundaplasty + angioplasty | 14 (22%) | 13 (19%) | 0·696 |
| Retrograde Iliac‐TEA | 16 (25%) | 13 (19%) | 0·416 |
| Iliaco‐femoral bypass | 3 (5%) | 6 (9%) | 0·348 |
| Inguinal interposition graft | 2 (3%) | 2 (3%) | 0·951 |
| Y‐prosthesis | 1 (2%) | 4 (6%) | 0·196 |
| Femoro‐distal bypass | 10 (16%) | 12 (18%) | 0·756 |
| EVAR | — | 1 (2%) | 0·332 |
| Graft material | |||
| Autolog | 3 (5%) | 4 (6%) | 0·760 |
| Xenogen (bovine) | 46 (72%) | 42 (62%) | 0·256 |
| Alloplastic | 15 (23%) | 22 (32%) | 0·220 |
BMI, body mass index; ciNPT, closed‐incision negative pressure therapy; ESRD, end‐stage renal disease; PAD, peripheral arterial disease; EVAR, endovascular aortic repair; TEA, thrombendarterectomy.
Early wound infection
There were no specific complications with either dressing. At the fifth postoperative day, 14 SSIs were observed: 4 in the ciNPT group and 10 in the control group (Table 2). While all wound infections were graded Szilagyi I in the control group, in the ciNPT group, I and II infections were observed. No patient developed a severe SSI III.
Table 2.
Primary end point results: surgical site infections after 5 days and 42 days
| ciNPT group | Control group | |||
|---|---|---|---|---|
| (n = 64) | (n = 68) | P | ||
| Early (5th day) | Szilagyi I | 3 (5%) | 10 (15%) | |
| Szilagyi II | 1 (2%) | — | ||
| Szilagyi III | — | — | ||
| Overall SSI | 4 (6%) | 10 (15%) | 0·125 | |
| Late (42nd day) | Szilagyi I | 5 (8%) | 12 (18%) | |
| Szilagyi II | 4 (6%) | 7 (10%) | ||
| Szilagyi III | — | — | ||
| Overall SSI | 9 (14%) | 19 (28%) | 0·055 |
ciNPT, closed‐incision negative pressure therapy.
Late wound infection
At the end of follow up, 9 infections were observed in the ciNPT group and 19 in the control group (Table 2). In the ciNPT group, 5 grade I infections and 4 grade II infections occurred, while the control group showed 12 grade I and 7 grade II SSIs. Again, no severe grade III infection was observed in either group.
Risk factors associated with infection
In univariate analysis, only increasing BMI proved to be a risk factor for SSIs (odds ratio 1·13; P = 0·033). Table 3 gives the infection rates in both groups according to the different risk factors. Although the numbers of infections observed in the control group exceeded those in the ciNPT group, none of the analysed risk factors in the use of ciNPT could reduce the infection rate significantly.
Table 3.
Secondary end point results: surgical site infections after 42 days depending on risk factors for infection
| Risk factor | n | ciNPT group (n = 64) | Control group (n = 68) | P |
|---|---|---|---|---|
| Age | ≤70 years | 5 (7%) | 10 (15%) | 0·053 |
| >70 years | 4 (6%) | 9 (14%) | ||
| BMI | <30 | 6 (6%) | 11 (11%) | 0·060 |
| ≥30 | 3 (10%) | 8 (27%) | ||
| Diabetes | No | 7 (8%) | 12 (13%) | 0·367 |
| Yes | 2 (5%) | 7 (18%) | ||
| Smoker | No | 3 (10%) | 2 (7%) | 0·059 |
| Yes | 6 (6%) | 17 (17%) | ||
| PAD IV | No | 7 (6%) | 12 (11%) | 0·052 |
| Yes | 2 (9%) | 7 (30%) | ||
| OP time | <3 hours | 7 (9%) | 9 (12%) | 0·055 |
| ≥3 hours | 2 (4%) | 10 (18%) | ||
| Prosthesis | No | 8 (8%) | 11 (12%) | 0·060 |
| Yes | 1 (3%) | 8 (22%) |
BMI, body mass index; ciNPT, closed‐incision negative pressure therapy; OP time, operation time; PAD, peripheral arterial disease.
Discussion
The use of ciNPT as postoperative dressing in the groin was not able to reduce the incidence of early and late groin wound infections after vascular surgery. Neither could ciNPT reduce the infection rate in different patient groups with risk factors for SSI.
Current attempts to prevent SSIs include perioperative systemic antibiotics, aseptic incision site surgical preparation, meticulous surgical technique, antibiotic‐coated sutures, silver‐impregnated dressings, antiseptic wound irrigation and iodine‐impregnated skin draps 13. However, none of these techniques has won widespread recognition so far. A decade ago, Stannard et al. introduced NPWT as protection for problematic closed surgical incisions as well 3. Since then, ciNPT has been applied on a variety of problematic surgical wounds, including sternotomy for cardiac surgery 4, 5, abdominal hernia repair 10, 11, caesarean section 7, 8, 9, trauma 6 and vascular surgery 2, 14, 15. In general, most of the case series and retrospective studies observed a decreased rate of surgical site occurrences (SSO), including SSIs and wound dehiscence. The most recently published meta‐analysis calculated a 30% relative reduction in surgical SSI rate for all wounds 16. Patients with additional risk factors for SSI, such as obesity 7, 17, 18, diabetes 19 and oncology patients 20, were reported to benefit from ciNPT. Recently, Willy et al. published a multidisciplinary consensus paper providing evidence for the effectiveness of ciNPT and giving recommendations for its use in different surgical specialties 21.
Very few studies have described the use of ciNPT in patients undergoing vascular surgery. In a case report, Haghshenasskashani et al. described ciNPT to be a useful tool in patients prone to post‐reconstructive oedema after popliteo‐tibial bypass grafting 22. In a retrospective study with a historical cohort, Matatov et al. observed significantly fewer SSIs in the ciNPT group (n = 52) compared to the control group (n = 63) (6% versus 30%) after longitudinal or transverse groin incisions for vascular surgery 2. Furthermore, the infections occurring in the group with ciNPT were less severe than in the controls. In a small intra‐individual case–control study (n = 8) with bilateral femoral cutdown, one side treated with ciNPT and the other with absorbent dressing, Weir et al. observed three SSOs (seroma, deep wound necrosis) requiring surgical re‐intervention on the control side, whereas the ciNPT side needed no further surgery for two minor SSOs 14. Karl et al. used ciNPT in vascular surgery patients with high risk for SSIs (n = 14), and they observed less infections in the ciNPT group compared to a historical cohort 15.
To our knowledge, the present study is the first randomised controlled trial comparing ciNPT with normal absorbent dressing in patients undergoing arterial reconstructive surgery. Although the reduction of groin wound infections using ciNPT did not reach statistical significance, the significance levels indicate that there might be a correlation in favour of the ciNPT group and that there is at least a trend towards reduction of SSI rates using ciNPT as the postoperative dressing (Figure 2). Stannard et al. reported on a multicentre RCT comparing ciNPT (n = 144) with standard care (n = 122) after lower‐extremity osteosynthesis 23. The authors found a significant decrease in the incidence of SSIs (19·0% versus 10·0%; P = 0·049) and wound dehiscence (16·5% versus 8·6%; P = 0·044) in favour of ciNPT, providing evidence for the use of negative pressure therapy devices for problematic surgical wounds.
Figure 2.
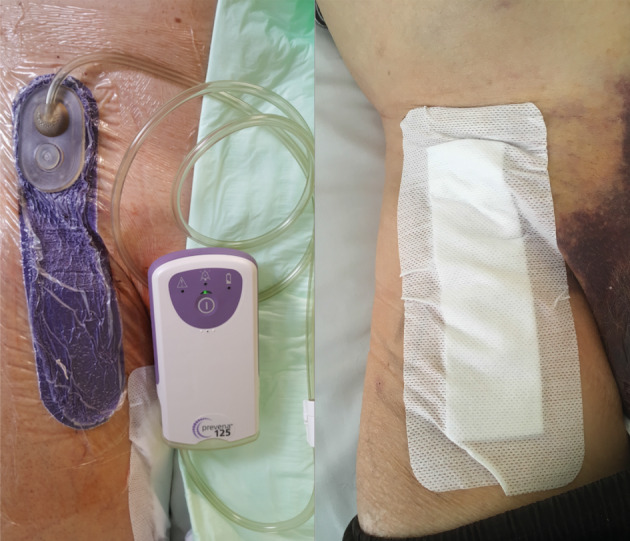
Application of closed‐incision negative pressure therapy (ciNPT) device (left) and absorbent dressing (right) after femoral cutdown for arterial reconstruction in the right groin.
Obesity, diabetes mellitus, advanced age, American Society of Anesthesiologists (ASA) score > 3 and prolonged surgical operation time are recognised as additional risk factors for wound infections 24, 25. However, the present study was unable to prove a beneficial effect of ciNPT on infection rates in patients with different risk factors for SSI.
Reports on adverse effects of ciNPT are rare. One study was stopped prematurely due to skin blister formation in 63% in the ciNPT group, although the self‐made dressing was used for a comparatively short time period of only 48 hours 26. This complication was probably due to the lack of a non‐adherent film dressing to protect the skin from direct contact with the foam bolster and possibly a too‐high tension when applying the foil. In the present study, no adverse effects of the ciNPT system were observed. With a little practice, the self‐adhesive system could be easily applied on the closed wound without air leakage. The patients tolerated the device well and were mobilised regularly on the first postoperative day, carrying the therapy unit in their pocket. A potential risk is the failure of the suction device, leading to oedema, maceration, skin breakdown and subsequent increased risk of SSI under the adhesive dressing as described for open trauma wounds 27. Early replacement of the ciNPT device can avoid further adverse effects in these cases.
The suction drainage and its removal did not affect the negative pressure of the ciNPT. The drain can easily be removed without tearing the self‐adhesive dressing. The subfascial drain removed fluid from the deep, while at the same time, the ciNPT drained the more superficial, subcutaneous layers. As all three tissue layers – fascia, subcutaneous tissue and skin – were closed separately, it appears unlikely that the two suction devices affected each other.
The mechanism of action for ciNPT is not yet explained sufficiently. The reduction of seroma and haematoma 23, the increase in tissue perfusion 28 and the reduction of lateral tension across the closed surgical incision 29 appear to play a major role in avoiding wound dehiscence and infections. Furthermore, postoperative contamination of the surgical wound is reduced by ciNPT as the device is applied under sterile conditions in the Operating Room (OR) and remains as protection on the wound for 5 days. In this context, Turtiainen et al. reported that a high bacterial load in the surgical wound on the second day after lower limb vascular surgery is a risk factor for SSIs 30.
Given the considerably higher costs of ciNPT compared to standard absorbent dressings, it remains controversial whether this new treatment is cost effective despite its potential benefit in reducing SSIs. Lewis et al. found ciNPT to be a cost‐effective treatment in obese women undergoing laparotomy for endometrial cancer provided the wound complication rate can be reduced by 30%, as in their study 31. Based on the available evidence, Tuffaha et al. calculated ciNPT to be cost effective in obese women undergoing caesarean section 9. However, the authors point out that the evidence is poor, and a high uncertainty still surrounds this issue. Using a computer model, Echebiri et al. provided evidence that ciNPT might be cost effective after caesarean delivery in patients with a high risk of SSIs but not for low‐risk patients 8. Consistently, Karl et al. calculated from the data of their small case study on vascular surgery patients that the use of ciNPT is cost effective in high‐risk patients prone to SSIs 15. In contrast to these reports, the present study could not demonstrate a benefit of ciNPT in any of the observed risk groups.
This study has some limitations. The number of patients, although higher than in most series on ciNPT, was still comparatively small, possibly leading to a type 2 statistical error, explaining the lack of statistical evidence despite the observed differences between both study groups. In accordance with other similar trials 2, we used the Szilagyi classification as a wound scoring system, and no additional objective scoring system was used. Therefore, although the classification was always determined by the same author (CM), a subjective error in judgement of the wound could not be excluded completely.
While the experiences with the ciNPT device were encouraging, the study fails to provide evidence of the efficacy of the device to reduce groin wound infections after vascular surgery. It illustrates far more that larger multicentre studies are required and appear promising to provide further evidence for the use of ciNPT and to determine possible cost savings in various groups of patients.
References
- 1. Newington DP, Houghton PWJ, Baird RN, Horrocks M. Groin wound infection after arterial surgery. Br J Surg 1991;78:617–9. [DOI] [PubMed] [Google Scholar]
- 2. Matatov T, Reddy KN, Doucet LD, Zhao CX, Zhang WW. Experience with a new negative pressure incision management system in prevention of groin wound infection in vascular surgery patients. J Vasc Surg 2013;57:791–5. [DOI] [PubMed] [Google Scholar]
- 3. Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 4. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009;16:140–6. [DOI] [PubMed] [Google Scholar]
- 5. Grauhan O, Navasardyan A, Tutkun B, Henning F, Muller P, Hummel M, Hetzer R. Effect of surgical incision management on wound infections in a poststernotomy patient population. Int Wound J 2014;11:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reddix RN Jr, Leng XI, Woodall J, Jackson B, Dedmond B, Webb LX. The effect of incisional negative pressure therapy on wound complications after acetabular fracture surgery. J Surg Orthop Adv 2010;19:91–7. [PubMed] [Google Scholar]
- 7. Mark KS, Alger L, Terplan M. Incisional negative pressure therapy to prevent wound complications following caesarean section in morbidly obese women: a pilot study. Surg Innov 2014;21:345–9. [DOI] [PubMed] [Google Scholar]
- 8. Echebiri NC, McDoom MM, Aalto MM, Fauntleroy J, Nagappan N, Barnabei VM. Prophylactic use of negative pressure wound therapy after caesarean delivery. Obstet Gynecol 2015;125:299–307. [DOI] [PubMed] [Google Scholar]
- 9. Tuffaha HW, Gillespie BM, Chaboyer W, Gordon LG, Scuffham PA. Cost‐utility analysis of negative pressure wound therapy in high‐risk caesarean section wounds. J Surg Res 2015;195:612–22. [DOI] [PubMed] [Google Scholar]
- 10. Soares KC, Baltodano PA, Hicks CW, Cooney CM, Olorundare IO, Cornell P, Burce K, Eckhauser FE. Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg 2015;209:324–32. [DOI] [PubMed] [Google Scholar]
- 11. Conde‐Green A, Chung TL, Holton LH III, Hui‐Chou HG, Zhu Y, Wang H, Zahiri H, Singh DP. Incisional negative‐pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg 2013;71:394–7. [DOI] [PubMed] [Google Scholar]
- 12. Szilagyi DE, Smith RF, Elliott JP, Vrandecic MP. Infection in arterial reconstruction with synthetic grafts. Ann Surg 1972;176(3):321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willy C, Engelhardt M, Stichling M, Grauhan O. The impact of surgical site occurrence and the role of closed incision negative pressure therapy. Int Wound J 2016;13 (S3 Suppl):35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weir G. The use of a surgical incision management system on vascular surgery incisions: a pilot study. Int Wound J 2014;11(1 Suppl):10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karl T, Woeste S. Prevention of inguinal wound healing disorders in vascular surgery. Results of using an epidermal negative pressure system (Prevena™). Gefässchirurgie 2013;18:120–5. [Google Scholar]
- 16. Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. Closed incision negative‐pressure therapy is associated with decreased surgical‐site infections: a meta‐analysis. Plast Reconstr Surg 2015;136:592–602. [DOI] [PubMed] [Google Scholar]
- 17. Anglim B, O'Connor H, Daly S. Prevena negative pressure wound therapy applied to closed Pfannenstiel incisions at time of caesarean section in patients deemed at high risk for wound infection. J Obstet Gynaecol 2015;35:255–8. [DOI] [PubMed] [Google Scholar]
- 18. Grauhan O, Navasardyan A, Hofmann M, Müller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 2013;145:1387–92. [DOI] [PubMed] [Google Scholar]
- 19. Zhou ZY, Liu YK, Chen HL, Liu F. Prevention of surgical site infection after ankle surgery using vacuum‐assisted closure therapy in high‐risk patients with diabetes. J Foot Ankle Surg 2016;55:129–31. [DOI] [PubMed] [Google Scholar]
- 20. Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high‐risk patients with laparotomy incisions using negative‐pressure therapy. Am J Surg 2013;205:647–54. [DOI] [PubMed] [Google Scholar]
- 21. Willy C, Agarwal A, Andersen CA, De Santis G, Gabriel A, Grauhan O, Guerra OM, Lipsky BA, Malas MB, Mathiesen LL, Singh DP, Reddy VS. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J 2017;14:385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haghshenasskashani A, Varcoe RL. A new negative pressure dressing (Prevena™) to prevent wound complications following lower limb distal arterial bypass. Br J Diabetes Vasc Dis 2011;11:21–4. [Google Scholar]
- 23. Stannard JP, Gabriel A, Lehner B. Use of negative pressure wound therapy over clean, closed surgical incisions. Int Wound J 2012;9(1 Suppl):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neumayer L, Hosokawa P, Itani K, El‐Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1178–87. [DOI] [PubMed] [Google Scholar]
- 25. Imai E, Ueda M, Kanao K, Kubota T, Hasegawa H, Omae K, Kitajima M. Surgical site infection risk factors identified by multivariate analysis for patients undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control 2008;36:727–31. [DOI] [PubMed] [Google Scholar]
- 26. Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressing after total knee arthroplasty. Curr Orthop Pract 2011;22:176–9. [Google Scholar]
- 27. Collinge C, Reddix R. The incidence of wound complications related to negative pressure wound therapy power outage and interruption of treatment in orthopaedic trauma patients. J Orthop Trauma 2011;25:96–100. [DOI] [PubMed] [Google Scholar]
- 28. Borgquist O, Anesäter E, Hedström E, Lee CK, Ingemansson R, Malmsjö M. Measurements of wound edge microvascular blood flow during negative pressure wound therapy using thermodiffusion and transcutaneous and invasive laser Doppler velocimetry. Wound Repair Regen 2011;19:727–33. [DOI] [PubMed] [Google Scholar]
- 29. Meeker J, Weinhold P, Dahners L. Negative pressure therapy on primarily closed wounds improves wound healing parameters at 3 days in a porcine model. J Orthop Trauma 2011;25:756–61. [DOI] [PubMed] [Google Scholar]
- 30. Turtiainen J, Hakala T, Hakkarainen T, Karhukorpi J. The impact of surgical wound bacterial colonization on the incidence of surgical site infection after lower limb vascular surgery: a prospective observational study. Eur J Vasc Endovasc Surg 2014;47:411–7. [DOI] [PubMed] [Google Scholar]
- 31. Lewis LS, Convery PA, Bolac CS, Valea FA, Lowery WJ, Havrilesky LJ. Cost of care using prophylactic negative pressure wound vacuum on closed laparotomy incisions. Gynecol Oncol 2014;132:684–9. [DOI] [PubMed] [Google Scholar]


